Abstract
MicroRNAs are short non-coding RNAs, expressed in humans and involved in sequence-specific post-transcriptional regulation of gene expression. They have emerged as key players in a wide array of biological processes, and changes in their expression and/or function have been associated with plethora of human diseases. Atherosclerosis and its related clinical complications, such as myocardial infarction or stroke, represent the leading cause of death in the western world. Accumulating experimental evidence has revealed a key role for microRNAs in regulating cellular and molecular processes related to atherosclerosis development, ranging from risk factors, to plaque initiation and progression, up to atherosclerotic plaque rupture. In this review, we will focus on how microRNAs can influence atherosclerosis biology, as well as the potential clinical applications of microRNAs which are being developed as both targets and therapeutics for a growing industry hoping to harness the power of RNA-guided gene regulation to fight disease and infection.
MicroRNA: discovery and function
Since the initial discovery of microRNAs as essential regulators of development in the nematode Caenorhabditis elegans,1 thousands of microRNA genes have been identified in animal and plant genomes.2 Around 60% of the human genome and nearly every major gene cascade is predicted to be under microRNA regulation3, suggesting that non-coding RNAs may match or even rival proteins in their regulatory capacity. Because of this, it is not surprising that the disruption of microRNA function contributes to many human disorders, such as cancer, cardiovascular diseases and neurological dysfunctions.4 MicroRNAs are processed in a two-step mechanism from primary transcripts (pri-microRNAs) by RNA polymerase II-generated characterized by a hairpin structure with a double stranded stem.5 In the nucleus, the RNase III Drosha cleaves the pri-microRNAs at the 5′ and 3′ end into microRNA precursors (pre-microRNAs) that contain a 2-nt overhang at the 3′ end. The GTP-dependent Exportin 5 transporter translocates the pre-microRNAs into the cytoplasm, where they are further processed. The RNase III enzyme Dicer recognizes the 2-nt overhang, and cleaves the pre-microRNAs near the terminal loop of the hairpin into 19–24 nt long mature microRNA duplexes. Dicer then assembles with RNA binding proteins and Argonaute (Ago) proteins to form a RNA-induced silencing complexes (RISC) (Table I).6 The separation of the microRNA duplexes into corresponding 5′ and 3′ strands (denoted −5p and −3p, respectively) occurs through a process called unwinding in which the N-terminal end of the Ago proteins wedges into the microRNA double strand, and thereby releases one of the strands (the passenger strand) from the RISC,7 thus allowing the mature RISC to bind to target mRNA(s). The canonical target sites interact through complementary pairing with the nucleotides 2–8 at the 5′ end of microRNAs (termed the “seed” sequence) and are usually located in the 3′ untranslated region (UTR) in the target mRNA. In the final step, the RISC complex recruits the GW182 family of proteins to the 3′UTR region enable the repression of mRNA translation or promote mRNA deadenylation and degradation, leading to the repression of protein expression.8 Whether microRNAs remain in the cells that produced them or are transferred to other recipient cells through extracellular vesicles, their predominant biological role is closely linked to their ability to guide RISC complexes in mediating mainly translational repression of specific mRNAs and thus silencing gene expression. MicroRNAs have the unique ability to simultaneously target multiple genes within the same or related pathway, reducing the expression of functionally related genes. MicroRNAs act generally in concert (a mRNA may contain >40 binding sites for different microRNAs), and each microRNA may regulate up to 200 to 300 different mRNAs, thus explaining their ability to control ~60% of the human genome and their involvement in nearly every biological pathway.
Table I.
Summary of important microRNA-related terms.
| 3′UTR | The 3′UTR controls many aspects of mRNA metabolism, such as transport, localization, efficiency of translation and stability. 3′UTRs can extend over several kilobases and generally contain binding sites for various regulatory proteins and microRNAs allowing dynamic and combinatorial regulation |
| MicroRNA-3p | The mature miRNA strand derived from the 3′ arm of the precursor duplex. |
| MicroRNA-5p | The mature miRNA strand derived from the 5′ arm of the precursor duplex. |
| RISC | A ribonucleoprotein complex that consists of a small RNA guide strand bound to an Argonaute protein. RISC mediates all RNA-silencing pathways, and it can also include auxiliary proteins that extend or modify its function |
| Seed sequence | Nucleotides at position 2–8 at the 5′ end of microRNAs, which interact through complementary pairing with the target mRNA in the 3′UTR |
| Antagomirs | Oligonucleotides of about 22 nucleotides that are complementary to a given microRNA sequence and can specifically inhibit microRNA activity |
Atherosclerosis: a cholesterol-driven inflammatory disease
The development of atherosclerotic vascular disease is promoted by the presence of concomitant risk factors, including dyslipidemia, which drives a chronic inflammatory reaction in the vessel wall. In the circulation, cholesterol is carried on lipoproteins, which can both deliver (eg, low-density lipoprotein (LDL)) and remove (eg, high-density lipoprotein (HDL)) cholesterol from cells and tissues to maintain cholesterol homeostasis.9 Excess LDL-cholesterol (LDL-C) is retained in the subendothelial space, causing endothelial cells (ECs) to become activated and recruit monocytes into the area to clear away the accumulated cholesterol. Monocyte-derived macrophages engulf the modified LDL (oxidized or aggregated) and become inflammatory foam cells, and recruit further inflammatory cells (T-cells, B-cells) into the growing intimal layer of the vessel. Over time, lesions progress from early fatty streaks to complex vulnerable plaques that are responsible for the clinical consequences of the disease, namely myocardial infarction or stroke. Imbalances that favor the accumulation of cellular cholesterol, such as high levels of LDL-C and low levels of HDL-cholesterol (HDL-C), promote atherosclerosis.
In addition to inflammatory cells, ECs and vascular smooth muscle cells (VSMCs) participate in the atherogenic process. At sites with disturbed laminar blood flow, ECs express adhesion molecules that promote inflammatory cell recruitment.10 VSMCs proliferate and engulf lipid and transform into macrophage-like foam cells, further promoting lipid accumulation and lesion expansion. Because the mechanisms and cellular involvement in the development of atherosclerosis are so diverse, microRNAs have unsurprisingly been identified as regulatory players that govern virtually every step in the development of atherosclerosis. We will discuss the major microRNA players below and summarize the pre-clinical findings of these atherosclerosis-targeting microRNAs in Table II.
Table II.
Summary of microRNA-based therapies tested in pre-clinical models of atherosclerosis.
| microRNA-based therapies | Effect(s) | References |
|---|---|---|
| Anti-miR-148a | Reduction of LDL-C, and increase of LDLR, ABCA1 and HDL-C levels in transgenic mice | 13 |
| Anti-miR-122 | Reduction of plasma triglycerides and cholesterol levels in mice | 16 |
| MiR-30c overexpression | Reduction of hyperlipidemia and atherosclerosis development in ApoE−/− mice | 21 |
| Anti-miR-33 | Increase in HDL levels, enhancement of cholesterol efflux, and reduction of atherosclerosis in LDLR−/− and ApoE−/− mice and primates. | 23, 24, 31, 33 |
| Anti-miR-33 | No alteration of atherosclerosis development in LDLR−/− mice | 34 |
| MiR-126-5p overexpression | Increase of endothelial cell proliferation and reduction of atherosclerosis in ApoE−/− mice | 56 |
| Anti-miR-92a | Reduction of endothelial inflammation and atherosclerosis development in LDLR−/− mice | 59 |
| Anti-miR-33 | Increase of anti-inflammatory M2 macrophages, and reduction of atherosclerosis in LDLR−/− mice | 67 |
| Anti-miR-155 | Reduction of atherosclerotic lesion formation in ApoE−/− mice | 68–70 |
| Anti-miR-155 | Enhancement of atherosclerotic development in LDLR−/− mice | 71 |
1. MicroRNAs and cholesterol homeostasis
Recent clinical and preclinical studies have shown that microRNAs control LDL and HDL abundance and function, thus improving our understanding of the regulatory circuits governing plasma lipoprotein levels. Thanks to their ability to modulate expression and function of transcription factors, enzymes and receptors governing lipoprotein metabolism, microRNAs control the genesis and metabolism of lipoproteins. Recently, a genome-wide association study investigated the association between single nucleotide polymorphisms (SNPs) and abnormalities of plasma lipids and found an interesting association with 69 microRNAs and blood lipids.11 Several of them (eg, miR-148a, miR-128-1, miR-130b, and miR-301b) were identified as effective modulators of key genes in lipoprotein metabolism, such as LDL-receptor (LDLR) and ATP-binding cassette A1 (ABCA1). LDLR expression in the liver promotes the clearance of circulating LDL particles and is a major determinant of plasma cholesterol levels, whereas ABCA1 mediates unidirectional efflux of phospholipids and cholesterol to lipid-free apolipoproteins and HDL.12 Notably, miR-148a is predominantly expressed in liver and able to regulate both expression and function of these gene in vivo.11,13 Experimental inhibition of miR-148a by antisense oligonucleotides resulted in an increase in hepatic LDLR and ABCA1 expression with marked modification in plasma lipoprotein profile (decreased LDL-C levels and increased HDL-C) and without abnormalities in markers of liver damage, suggesting that altered expression of this microRNA may contribute to dyslipidemia.13 MiR-223, whose expression is dependent on intracellular cholesterol levels, has also been found to play a role in lipid metabolism.14 Indeed, miR-223 inhibits cholesterol synthesis via posttranscriptional regulation of 3-hydroxy-3-methylglutaryl(HMG)-CoA synthase 1 (HMGS1) and methylsterol mono-oxygenase 1, and by affecting cholesterol efflux through repression of scavenger receptor class B member 1 (SR-BI) and up-regulation of ABCA1, whereas miR-223−/− mice display increased HDL-C levels, as well as hepatic and plasma total cholesterol.14 Moreover, miR-223 is also transferred from cells to HDL particles via an SR-BI-dependent process. Its levels are higher in patients with familiar hypercholesterolemia and in ApoE- and LDLR-deficient mice under high-fat diet, and might play a role as intercellular communication system regulating liver gene expression.15
The liver plays a major role in both the production and the clearance of lipoproteins, and many hepatic-enriched microRNAs have been identified as regulators of lipoprotein metabolism. MiR-122, which account for about 70% of the total liver microRNAs, was shown to regulate plasma triglycerides and cholesterol levels, as the inhibition of miR-122 by both anti-sense interference or genetic deletion in transgenic mice leads to a strong reduction of both lipid pools.16–18 Targets of this miR-122 include HMGCoA reductase (HMGCR), the rate-limiting enzyme for intracellular cholesterol biosynthesis, and microsomal triglyceride transfer protein (MTTP), a protein required for the hepatic assembly of VLDL and ApoB-containing lipoproteins.16,18 Notably, miR-122 function seems to be broadly required for the expression of hepatocyte-specific genes, rather than the specific targeting of lipid metabolism pathways19. In contrast, miR-27b and miR-30c act as direct post-transcriptional regulators of cholesterol and lipoprotein metabolism networks.14,20,21 MiR-27b is a cholesterol-responsive hepatic microRNA that represses many targets involved in lipid metabolism and lipoprotein remodeling, such as ANGPTL3 and GPAM.20 Finally, miR-30c was shown to have a potent effect on the production of VLDL and LDL. Indeed, miR-30c reduces de novo lipogenesis by targeting lysophosphatidylglycerol acyltransferase 1 (LPGAT1) responsible for de novo hepatic lipid biosynthesis, and miR-30c overexpression reduced hyperlipidemia and attenuated atherosclerosis in ApoE−/− mice by targeting MTTP. 21 A large body of literature has focused on the role of microRNAs in the regulation of HDL biogenesis and cholesterol efflux, which control levels of plasma HDL-C and the reverse cholesterol transport pathway through which excess cholesterol is removed to the liver for excretion. Indeed, epidemiological studies have shown an atheroprotective role for HDL, which is thought to be associated with its ability to mediate reverse cholesterol transport. Among the relevant effectors, ABCA1 plays a central role in these processes by controlling cholesterol efflux across the cell membrane onto lipid-poor apoA1 to mediate both hepatic HDL biogenesis and the removal of excess cholesterol from peripheral cells, particularly macrophages in atherosclerotic plaques.12 Of note, the sequence of the ABCA1 3′UTR is among the longest in the human genome and include numerous conserved binding sites for microRNAs including miR-33,22–24 miR-758,25 miR-128,13 miR-144,26 miR-148a,11 miR-106,27 and others.28 Of these, inhibition of miR-33, miR-144, miR-128, and miR-148a has also been tested in vivo and shown to increase plasma levels of HDL-C in mice or monkeys. The final step of reverse cholesterol transport is the selective uptake of HDL cholesterol into the liver, and is mediated by SR-BI. In this context, miR-223,29 miR-455-5p,30 miR-96,29 miR-185,29 and miR-125a30 have been demonstrated to repress hepatic SR-BI, providing an additional mechanism that modulates HDL cholesterol transport and may drive atherosclerosis development.
MiR-33 is the most extensively investigated microRNA in the regulation of ABCA1 and ABCG1. In humans, miR-33a and miR-33b are encoded within introns of the sterol regulatory element-binding protein (SREBP) 2 and SREBP1 genes, the main transcription factors controlling cholesterol and fatty acid metabolism.31 Thus, the concomitant transcription of SREBP2, which promotes intracellular cholesterol synthesis and LDL uptake, and miR-33a, which represses cholesterol efflux via down-regulation of ABCA1 (in humans and mice) and ABCG1 (in mice), plays a synergistic effect in increasing intracellular cholesterol levels in macrophages and hepatocytes.31 As a result, in vivo short term antagonism of miR-33 in rodents and in non-human primates provides a significant increase in plasma HDL and enhanced cholesterol transport from macrophages to the plasma, liver, and feces by up to 80%.23,24,31 This increase in reverse cholesterol transport is likely the combined effects of de-repression of ABCA1 and additional miR-33 targets such as ABCB11 and ATP8B1 that promote cholesterol excretion into bile.32 In fact, miR-33 has become one of the most attractive microRNA targets for drug development to treat dyslipidemia and atherosclerosis (Figure 1). Although initial studies showed that inhibition of miR-33 reduced atherosclerosis in LDL receptor −/− or apoE −/− mice,24,33 other studies have reported that inhibition of this microRNA did not attenuate the progression of atherosclerosis in LDLR−/− mice.34,35 Indeed, in some mouse models, long-term treatment with anti-miR-33 in mice fed a high-fat diet was associated with increased plasma triglycerides levels and moderate liver steatosis.36 These outcomes could be explained by the concomitant overexpression of genes involved in fatty acid synthesis, namely acetyl-CoA carboxylase and fatty acid synthase, though this was not observed in non-human primates or in some mouse models.34,36 Therefore, although miR-33 represents a promising target for dyslipidemia and atherosclerosis, the potential side-effects of long-term treatment mandate further investigation.
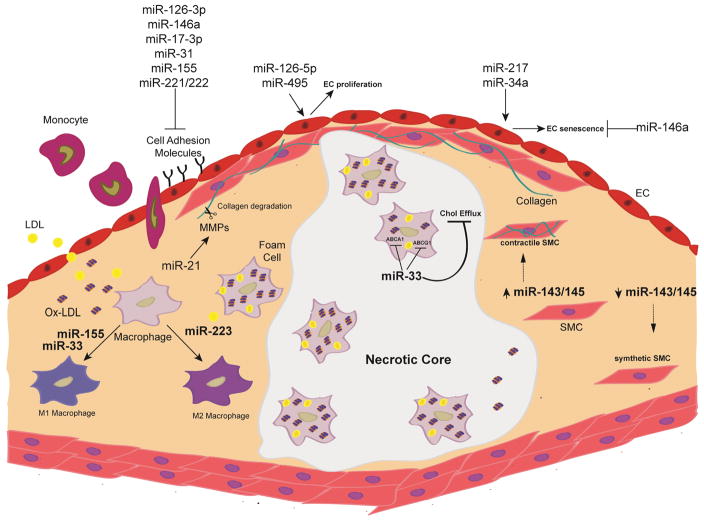
Figure 1.
Overview of the major microRNAs and their impacts on the cellular mechanisms that drive atherosclerosis. Arrow (
 ) denotes promotion and line (
) denotes promotion and line (
 ) denotes inhibition. EC=endothelial cell, SMC=smooth muscle cell, LDL=low-density lipoprotein, MMP=matrix metalloproteinases,
) denotes inhibition. EC=endothelial cell, SMC=smooth muscle cell, LDL=low-density lipoprotein, MMP=matrix metalloproteinases,
Although miR-33 has been the most extensively studied in vivo, several other microRNAs have also been shown to modulate cholesterol efflux in macrophages and other cell types. Recently, miR-302a was found to target ABCA1, and blocking miR-302a using antagomirs reduced atherosclerosis progression in mice.37 MiR-10b also targets ABCA1 and ABCG1 and thus reduces cholesterol efflux from lipid-loaded macrophages.38 MiR-26 can also target ABCA1, as well as other genes involved in cholesterol mobilization, such as ADP-ribosylation factor-like 7, an intracellular transport protein that acts in concert with ABCA1 to mobilize cholesterol to the cell surface for removal.39 Recently, coenzyme Q10 was demonstrated to reduce miR-378 expression, which increased the expression of ABCG1 in mouse and human macrophages, thus facilitating macrophage cholesterol efflux in vitro and in vivo.40 Taken together, the available evidence strongly supports a relevant contribution of microRNAs in lipid metabolism and shed light on new potential therapeutic targets for treatment of dyslipidemia and for cardiovascular prevention.
2. MicroRNAs in initiation of atherosclerosis
MicroRNAs in endothelial activation
When dyslipidemia occurs, especially under disturbed patterns of blood flow, the endothelium becomes activated by pro-inflammatory cytokines and by oxidized lipoproteins. The activation of ECs promotes leukocyte recruitment from the blood into extravascular tissues, thus contributing to the pathogenesis of atherosclerosis.41 Several signaling pathways participate in this process, prominently promoting the transcription factor NF-κB. In the endothelium, NF-κB signal transduction modulates the expression of numerous pro-inflammatory genes involved in cell adhesion such as E- and P-selectin, vascular cell adhesion molecule 1 (VCAM-1), and intercellular adhesion molecule 1 (ICAM-1), as well as various chemokines and cytokines.42,43 As one of the most highly expressed microRNAs in ECs, miR-126-3p interferes with this process by inhibiting the expression of VCAM-1 and hampering in vitro leukocyte adhesion to ECs (Figure 1).44 MiR-31 and miR-17-3p directly target E-selectin and ICAM-1 expression, respectively, thus acting as a brake on TNFα-induced EC activation.45 Finally, miR-155 and miR-221/222 inhibit the angiotensin II-induced inflammatory response in ECs by targeting the transcription factor Ets-1 and its downstream genes, such as VCAM-1 and monocyte chemoattractant protein-1.46 MiR-10a is highly expressed at predilection sites of the aorta exposed to disturbed flow, and his inhibition by antagomirs enhances NF-κB– dependent adhesion molecule expression in ECs.47 Moreover, let-7g has been shown to act as a suppressor of endothelial inflammatory activation through inhibition of both TGF-β and SIRT1, whereas ECs acquire a pro-inflammatory-activated phenotype in its absence.48 Likewise, hyperlipidemic patients have lower let-7g levels with increased circulating levels of PAI-1, a marker of endothelial dysfunction and thrombogenic activation.48 Studies in mice and humans demonstrated that miR-181b acts as a suppressor of EC inflammatory responses in vascular disease states by targeting Importin-a3, a protein required for nuclear translocation of NF-kB, thus inhibiting a set of NF-kB-responsive genes including VCAM-1 and E-selectin.49,50 Systemic delivery of miR-181b mimic reduces NF-kB activity and atherosclerotic lesion development in mice, which was in part due to decreased numbers of lesional macrophages and CD4+ T cells.49 MiR-146a plays also an important role in dampening endothelial activation by promoting eNOS expression through targeting the RNA-binding protein HuR, and by repressing the induction of adhesion molecules through targeting TRAF6 and IRAK1/2, once again acting as a negative feedback loop for NFkB.51 Atheroprotective flow patterns are associated with a reduced level of miR-92a and an increase in KLF-2, which promotes an anti-inflammatory phenotype in ECs, thus supporting a pivotal role for miR-92a in regulating ECs response to shear stress.52 On the other hand, KLF-2 is able to regulate the expression of specific microRNAs, such as miR-145, which can repress the expression of junctional adhesion molecule-A and thus inhibits monocyte adhesion to endothelium.53
MicroRNAs in endothelial proliferation
Even if ECs growth occurs rather sporadically in the adult vasculature, it has recently been established that microRNAs can influence endothelial proliferation. Increased ECs turnover mainly takes place at predilection sites of atherosclerosis in response to increased levels of stress-induced ECs death and detachment.54,55 In this setting, miR-126 has been shown to regulate endothelial proliferation. Although both strands of this microRNA are present and functional in ECs, only the miR-126-5p strand impacts ECs proliferation improving re-endothelialization after vascular injury by targeting Delta-like 1 homolog (DLK1).56 Reduced miR-126-5p levels at predilection sites upregulated DLK1 expression, thus controlling and limiting ECs proliferation. In contrast, at non-predilection sites, laminar blood flow protects EC function and limits EC turnover and provides a higher proliferative capacity by inducing miR-126-5p expression.56 Oxidized LDL has been shown to inhibit ECs replication in vitro and hyperlipidemia significantly reduces ECs proliferation at predilection sites in vivo.56,57 Under hyperlipidemic conditions, the DLK1-mediated anti-proliferative response turns out disadvantageous at the predilection sites and results in decreased EC regeneration, whereas at the non-predilection sites, high levels of miR-126-5p reduce DLK1 expression, thereby enabling EC regeneration.56 Indeed, miR-126-5p mimic therapy under these condition was shown to improve EC proliferative reserve at predilection sites, thereby limiting atherogenesis.56 MiR-495 is also able to promote ECs proliferation by targeting CCL2, but additional studies need to be performed to elucidate his potential role during atherogenesis58. MiR-92a, which is preferentially up-regulated by the combination of disturbed flow and oxidized LDL, was also shown to be able to modulate endothelial proliferation in atheroprone regions and mouse models.59 Indeed, in vivo blockade of miR-92a expression reduced endothelial inflammation, decreased atherosclerotic plaque size and promoted a more stable lesion phenotype. Hence, anti-miR-92a therapy may serve as another atheroprotective therapeutic strategy.
MicroRNAs in endothelial senescence
As proliferating cells, ECs can also react to stress by undergoing a phenotypically distinctive and permanent form of growth arrest called “cellular senescence” which is a driver of vascular dysfunction. MiR-217 was shown to negatively regulate the expression of silent information regulator 1 (SirT1), an atheroprotective flow-induced anti-inflammatory deacetylase that activates eNOS and inactivates NF-kB.60 Indeed, overexpression of miR-217 in young ECs induced a premature senescent-like phenotype and decreased angiogenesis, whereas its inhibition in old ECs reduced senescence and increased angiogenesis. SirT1 can also be targeted by miR-34a, inducing EC senescence and suppressing cell proliferation by inhibiting cell cycle progression.61 On the other hand, aging, which is related to an increase of reactive oxygen species (ROS) and promoted senescence, decreases the expression of miR-146 in ECs, which negatively regulate the expression of NOX4, the main endothelial isoform of the NADPH oxidases complex.62 Thus, miR-146 overexpression appears to be a novel therapeutic tool for vascular disorders by targeting the production of ROS and therefore regulating senescence. That being said, all these studies were performed in vitro and further in vivo studies are needed to determine microRNAs ability to regulate endothelial senescence and potentially impact atherogenesis through this path.
3. MicroRNAs regulation of inflammatory pathways
Inflammation is a major contributor to atherosclerosis. In this context, macrophages are crucial in maintaining vessel-wall lipid homeostasis and coordinating inflammatory responses, thus playing a central role in the pathophysiology of atherosclerosis.63 MicroRNAs are key players in macrophage biology by regulating their inflammatory capacity which can profoundly affect plaque evolution.64 Several studies revealed an essential role for microRNAs in regulating macrophage polarization, a crucial component of the inflammatory response (Figure 1). For example, miR-124 inhibits macrophage activation and polarization from a pro-inflammatory M1 toward an anti-inflammatory M2 phenotype, by targeting the transcription factor C/EBP-α.65 Similarly, miR-223 was shown to have a suppressive effect on macrophage pro-inflammatory activation by targeting the protein Pknox1, and miR-223-deficient macrophages exhibited increases in M1 and decreases in M2 polarization biomarkers.66 MiR-33 is also involved in fate of atherosclerotic macrophages as miR-33 inhibition in hypercholesterolemic mice was shown to result in accumulation of inflammation-suppressing M2 macrophages, thus reducing atherosclerosis progression.67 MiR-342-5p, expressed in lesional macrophages, is the most prominently induced microRNA during early atherosclerosis. Notably, miR-342-5p enhances pro-inflammatory macrophage mediators, such as inducible nitric oxide synthase and IL-6 via up-regulation of miR-155, and his inhibition was shown to reduce atherosclerotic lesion formation in ApoE−/− mice.68 MiR-155, is induced in macrophages in atherosclerotic regions of ApoE−/− mice where it targets B cell leukemia/lymphoma 6 (BCL6), a transcription factor that attenuates NF-kB activation.69 ApoE−/− mice with hematopoietic miR-155 deficiency showed a reduction in macrophage inflammatory responses, increased macrophage cholesterol efflux, and reduced lesion size.70 In contrast, bone marrow miR-155 deficiency in LDLR−/− mice enhanced atherosclerosis by recruiting more myeloid inflammatory cells to the plaque and inducing a more pro-inflammatory macrophage phenotype.71 Together, these data reflect the stage-specific and lipid-dependent roles for endogenous miR-155 in regulating the inflammatory signaling pathways in atherosclerotic macrophages, which can differ in the different mouse models of atherosclerosis. Other microRNAs have been shown as regulators of macrophage response to inflammatory stimuli. For example, overexpression of miR-125b potentiates the responsiveness of macrophages to interferon (IFN-γ) via the miR-125b suppression of IFN regulatory factor 4,72. The related microRNA, miR-125a-5p, diminished the M1 phenotype but promoted M2 marker expression induced by IL-4.73
Because lipoprotein uptake by macrophages is one of the earliest pathogenic events in nascent atherosclerotic plaques to induce the formation of foam cells, miR-125a-5p and miR-146a may play a protective role against the development of atherosclerosis as they were found to decrease lipid uptake and cytokine release in oxLDL-stimulated macrophages, notably through the targeting of oxysterol binding protein-like 9 and TLR4, respectively.74,75 Likewise, miR-155 overexpression reduced lipid uptake in monocytic cell lines and primary monocyte-derived dendritic cells in vitro,76 but in primary macrophages from atherosclerotic ApoE−/− mice, miR-155 was found to enhance oxLDL-induced foam cell formation by targeting HMG box-transcription protein 1, implying that miR-155 also has cell-specific functions.77 Anti-miR-155 injection in ApoE−/− mice effectively decreased lipid-laden macrophage accumulation in lesions as well as the formation of aortic atherosclerotic plaques.77 However, as mentioned above, it is possible that miR-155 exerts stage-specific effects during atherogenesis, depending on which microRNA targets are expressed. Finally, miR-155 expression was significantly higher in CD14+ monocytes from patients with coronary artery disease than from healthy controls, pointing this microRNA as a potentially relevant myeloid target for atherosclerosis. Indeed, miR-155 was shown to mediate inflammatory mediators in macrophages to promote atherosclerotic plaque formation and rupture via the SOC1S-STAT3-PDCD4 axis.78
Other immune cells, such as dendritic cells and T cells, are present and play an important role during atherosclerosis development, and microRNAs were shown to regulate their function. miR-181a targets the c-Fos transcription factor, and attenuates the oxLDL-induced inflammatory response by blocking the secretion of pro-inflammatory cytokines (TNF-α, IL-6), and reducing dendritic maturation cell surface molecules CD40 and CD83.79 The activation of the T cell receptor increases the expression of miR-146a, which protects T cells by reducing apoptosis by targeting the pro-apoptotic factor Fas-associated death domain (FADD) and the NF-kB mediators TRAF6/IRAK1.80 Indeed, T cells lacking miR-146a are hyperactivated by antigen stimulation, however, more studies will be necessary to understand the role of dendritic- and T cell specific microRNA-mediated effects in the context of atherosclerosis.
MicroRNAs in smooth muscle cell phenotype
VSMCs play an important role in atherosclerosis development, notably through their shift from a “contractile” to a “synthetic” phenotype. Indeed, beside the higher rates of migration and proliferation, synthetic VSMCs express higher proportions of lipoprotein and scavenger receptors that could promote pathological lipid uptake.81 These last few years, microRNAs were shown to be involved in the phenotypic switch of VSMCs, especially the miR-143/-145 cluster which can direct VSMCs fate and regulate their quiescent versus proliferative phenotype (Figure 1).82 MicroRNA-143/-145 are abundantly expressed in VSMCs and can also be taking up by them from ECs-derived microvesicles.82,83 Following VSMC cholesterol loading, miR-143/-145 was shown to be involved in their conversion into a dysfunctional macrophage-like phenotype.84 MiR-143/-145 effects on VSMCs differentiation and response to injury are a consequence of targeting several genes, such as KLF-4, KLF-5, or ACE.82,85,86. However, the role of miR-143/-145 in atherosclerosis is still under ongoing debate, as both overexpression and genetic deletion of miR-143/-145 reduces neointima formation and prevents atherosclerosis.86–88 As miR-143/-145, miR-133a prevents VSMCs from the switch toward a synthetic phenotype and its levels are decreased after vascular injury.89 Moreover, miR-663 has been shown to promote VSMCs differentiation by targeting the transcription factor JunB and its downstream molecules such as myosin light chain 9 and matrix metalloproteinase 9, while local overexpression of miR-663 reduces neointima formation after carotid artery ligation injury.90 Finally, miR-15b and miR-16 have also been described as important players in maintaining the contractile phenotype of VSMCs through targeting of the oncogene yes-associated protein.91 On the other hand, some microRNAs can also promote “dedifferentiation” of VSMCs. Indeed, miR-26a induces both VSMCs proliferation and migration by interfering with the TGF-β signaling pathway,92 while after a vascular injury, miR-21 contributes to neointima formation by promoting VSMCs proliferation through the targeting of Phosphatase and TENsin homolog protein and Bcl-2.93 Similarly, miR-221 and miR-222 increase the proliferative rate of VSMCs by silencing Kip1 and Kip2. In fact, inhibition of miR-221 and miR-222 results in decreased VSMCs proliferation and reduced neointima formation after angioplasty.94
4. MicroRNAs in atherosclerotic plaque rupture
Rupture-prone vulnerable plaques are typically associated with the presence of highly inflammatory cell content and a large necrotic core covered by a thin fibrous cap.95 Accumulating evidence demonstrate various roles for microRNAs in processes related to the risk of atherosclerotic plaque rupture. Due to the fact that animal models poorly mimic the process of plaque rupture in humans, it has been difficult to directly assess the contribution of individual microRNAs in this process. Nonetheless, each step in the formation of a stable plaque is affected by microRNAs.
Fibrous cap thinning
Thickness of the fibrous cap is determined by the balance between collagen synthesis and breakdown. VSMCs are likely the main source of collagen and, as discussed above, microRNAs are key regulators of VSMCs phenotype with effects on atherosclerosis progression. Moreover, several microRNAs are able to regulate collagen synthesis and fibrosis, including miR-133a, miR-145, miR-192, miR-21, and miR-29b.87,96–99 In addition to its ability to regulate VSMCs differentiation, overexpression of miR-145 enhances collagen type I and III expression in human aortic VSMCs and drives atherosclerotic plaques toward the acquisition of features typical of stable phenotype, such as increased collagen content and fibrous cap area.87 As a result, miR-143/145 may lead to decreased VSMCs proliferation in during atherosclerotic plaque initiation, but also stabilize the fibrous cap in advanced plaques. Overexpression of miR-21 inhibits ROS-induced VSMCs apoptosis and death,100 while delivery of vascular miR-126 in mice promoted an increase in the number of intimal VSMCs, higher collagen content, and reduced apoptotic cells, consistent with a more stable plaque phenotype.101 Notably, miR-29 might play a role in plaque stability as it was shown to directly suppress IFN-γ production by targeting IFN-γ transcript, which normally block the expression of pro-collagens by VSMCs.102 Collagens are stable proteins and their degradation relies on the activity of matrix-metalloproteinases (MMPs). In atherosclerotic plaques, macrophages are able to synthesize an array of MMPs including MMP-1, -2, -3, -8, -9, -13, -14.103 MMP9 is one of the most highly expressed MMPs in unstable plaques and was shown to be targeted by miR-133a, but also indirectly upregulated by miR-21 via repression of its inhibitor, RECK, in macrophages.104,105 Moreover, anti-miR-24 therapy was shown to enhance MMP-14 proteolytic activity, thus promoting atherosclerotic plaque progression and characteristics associated with plaque instability, such as decreased collagen content and increased macrophage infiltration.106 In summary, therapeutic stabilization of existing plaques could by achieved though direct targeting VSMC-derived microRNAs to prevent VSMC death, reduction in collagen production and breakdown in the fibrous cap.
Necrotic core
Death of foam cells can lead to sub-endothelial accumulation of cell debris, lipoproteins and cholesterol crystals, thus forming a lipid-rich area with low cell content, termed the necrotic core. Indeed, defective apoptotic cell clearance through impaired efferocytosis leads to apoptotic cell accumulation, delayed resolution of inflammation, and expansion of the necrotic core. MiR-155 inhibits efferocytosis by targeting BCL6, thus mir-155 deficiency reduces necrotic core formation and the deposition of apoptotic cell debris,107 making the interaction between miR-155 and BCL6 as a promising target to inhibit atherosclerosis progression. By contrast, elevated macrophage miR-21 was shown to promote efferocytosis and suppress the innate immune response.108 Interestingly, ABCA1 and ABCG1 are also involved in preserving macrophage cell viability during efferocytosis,109 thus suggesting that macrophage microRNAs involved in the regulation of these transporters might affect efferocytosis and impact atherosclerosis evolution in advanced stages. Finally, in addition to breaching the fibrous cap in the necrotic core, cholesterol crystals also activate the NLRP3 inflammasome that induces the release of active IL-1β and other cytokines to further promotes the instability of atherosclerotic plaque.110 In this context, miR-223 negatively regulates the inflammatory response by blocking NLRP3 inflammasome and IL-1β production.111
5. Other risk factors for atherosclerosis & microRNA targeting
In addition to their role in cholesterol homeostasis, microRNAs are also involved in regulation of other risk factors that are linked to the development of atherosclerosis. Indeed, since the discovery of microRNAs, an increasing number of them have been found to be involved in diabetes mellitus pathogenesis, and their dysregulation can lead to profound impairment of glucose metabolism.112 MicroRNAs can particularly affect pancreatic β-cells through the regulation of cell survival and apoptosis, proliferation, differentiation, or insulin secretion.113 Besides, they are also implicated in the insulin resistance regulation by targeting insulin receptors and their substrates.113 MicroRNAs have been also shown to be important regulators of hypertension and blood pressure. Notably, a murine models of Dicer deletion displayed a drop in blood pressure,114 as well as miR-143/-145 KO mice,115 and plethora of microRNAs were shown to be involved in hypertension in both human studies and animal models.116 Taken together, these studies demonstrated that microRNAs can regulate atherosclerosis development through a lot of different ways by affecting and regulating numerous related-diseases and risk factors.
6. Potential clinical applications of microRNAs
Unique and reliable biomarkers for atherosclerosis and plaque stability are greatly needed. MicroRNAs, especially circulating microRNAs, are currently investigated as biomarkers in a wide range of cardiovascular conditions including atherosclerosis. Indeed, circulating microRNAs are remarkably stable and easy to detect in body fluids. Moreover, changes in their expression are often tissue- or disease-specific. For instance, several studies revealed a distinct microRNA expression signature in the blood of patients with acute coronary syndrome.117,118 Combining multiple microRNAs in a microRNA profile may provide greater accuracy than assessment of a single one, thus making large-scale studies indispensable to establish the real potential of circulating microRNAs as biomarkers for atherosclerosis-related diseases.
A huge jump forward was made in the field of RNA therapeutics with the recent Food and Drug Administration approval of the Mipomersen, an antisense oligonucleotide drug targeting apoB to treat familial hypercholesterolemia.119 This will likely pave the way for other oligonucleotide-based drugs, including microRNAs therapies. The multiplicity of microRNA targets enables them to regulate several steps in a disease pathway by fine-tuning downstream signaling mediators in parallel. Thus, regulating specific stages of atherosclerosis through delivery of a pool of microRNA mimics or inhibitors might be an attractive therapeutic approach. Therapies based on microRNA inhibitors or microRNA mimics are now being developed to repress pathological microRNAs or over-express protective microRNAs, respectively. Indeed, numerous microRNAs therapeutics are in pre-clinical development, with two of them that have reached clinical trials. The first is a locked nucleic acid directed against miR-122 (Miravirsen), which targets hepatitis C virus RNA.120 In non-human primates miR-122 inhibition resulted in long-lasting suppression of hepatitis C virus viremia with no evidence of viral resistance or side effects.121 In a human phase II study, Miravirsen demonstrated dose-dependent antiviral activity. Remarkably, in several patients treated with the highest doses of Miravirsen, hepatitis C virus RNA became undetectable during the study.17 The second microRNA therapeutic in clinical trial is a double-stranded microRNA mimic of miR-34 (MRX34), which has entered a clinical Phase I trial in patients with advanced liver cancer. MRX34 is a miR-34 mimic encapsulated in a liposomal nanoparticle which acts as a tumor suppressor by inhibiting multiple oncogenic pathways and stimulating antitumor immune responses.122 Systemic delivery of microRNA-derived therapeutics are mostly taken up in the liver, the kidney, and the spleen,123 but many additional peripheral tissues, such as vessels and heart, have also been successfully targeted using currently available delivery methods.49 MicroRNA mimetic therapy using synthetic microRNAs offers the ability to reconstitute a microRNA that is downregulated during disease or to decrease gene pathways involved in disease pathology, but the delivery of therapeutic microRNA molecules faces many challenges because of their double-stranded nature. Drug delivery vehicles such as liposomes, polymeric micelles, and lipoprotein-based drug carriers are being developed to deliver these oligonucleotides to cells. Conversely, single-stranded anti-miR oligonucleotides can be formulated in saline for subcutaneous or intravenous delivery and do not require lipid-based delivery systems. However, some of the current challenges associated with microRNAs therapeutics include the ability to target the drug to a specific tissue, and the potential requirement of multiple doses to achieve sustained target repression or microRNAs enrichment.
Also, whether therapeutic manipulation of microRNA may represent an efficient and safe atherosclerosis treatment in humans is still, however, an open debate. Although it is obvious that several microRNAs have an important regulatory impact on atherosclerosis in vivo, it is unclear what the contribution of individual microRNAs may be. Moreover, in most animal studies so far, the phenotypic effects of microRNAs therapeutics have only been studied in the targeted tissue of interest, and this might omit potential off-target effects of microRNAs in different tissues, stages, or environment. Indeed, because microRNAs can simultaneously alter expression of multiple target genes and often disrupt entire signaling networks, resulting in efficient changes in the activity and phenotype of target cells, this broad functionality can be a liability that results in difficult-to-control off-target effects and toxicities. Lentiviruses (LVs), adenoviruses (AVs), and adeno-associated viruses (AAVs) can transfer microRNA sequences,124 which after viral entry into target cells are transcribed and processed into mature microRNA. Despite efficient cellular delivery, viral vectors raise concerns over safety of genomic integration of LVs, which may trigger expression of oncogenes, or excessive immunogenicity and the transient nature of microRNA expression in the case of AVs and AAVs. Nonviral methods of microRNA delivery instead rely on lipid and polymeric nanoparticles to protect the microRNA from degradation by nucleases and increase their half-life in the circulation, but the cell-specific delivery is still a major concern. Recently, a new strategy was developed for the targeted delivery of microRNA mimics. The tumor suppressor let-7g microRNA mimic was conjugated in the form of an unformulated oligonucleotides to an aptamer specific for the oncogenic tyrosine kinase receptor, Axl, which is expressed by various types of solid tumors.125 The resulting aptamer–microRNA conjugate retained the cellular specificity, binding affinity, and inhibitory function and was able to successfully target let-7g in target tissues.126. Although this has not yet been extended to chronic and multi-cellular diseases like atherosclerosis, the combined targeting of a disease-specific microRNA with a cell-specific receptor shows the promise of this approach.
Conclusion
In conclusion, there is little doubt that microRNAs are an important biological player in the development and progression of atherosclerosis. To enable to therapeutic path forward, it will be critical to understand the full complement of targets of specific microRNAs and understand what microRNA pathways may cross-talk. As RNA-based therapeutics move into the clinic, targeting microRNAs for chronic diseases like atherosclerosis remain not far off the horizon.
Summary.
Atherosclerosis is a multifactorial and slowly progressing disease responsible for most of the cardiovascular morbidity and mortality in industrialized countries. MicroRNAs are non-coding RNAs that are predicted to regulate translational repression of ~60% of the human genome, and thus involved in regulation of every cellular processes. Accumulating studies unveiled a prominent role for microRNAs in regulating atherogenesis, making them promising candidate for microRNAs-based therapies and clinical applications.
Acknowledgments
Research relating to microRNAs and atherosclerosis in the Rayner laboratory is supported by funding to K.J.R from the Canadian Institutes for Health Research, The Heart and Stroke Foundation of Canada, and the National Institutes of Health. B.L was supported by a postdoctoral fellowship from the University of Ottawa Heart Institute Foundation and the Strategic Research Endowed Funds.
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reinhart BJ, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 2.Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic acids research. 2011;39:D152–157. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ardekani AM, Naeini MM. The Role of MicroRNAs in Human Diseases. Avicenna journal of medical biotechnology. 2010;2:161–179. [PMC free article] [PubMed] [Google Scholar]
- 5.Ha M, Kim VN. Regulation of microRNA biogenesis. Nature reviews. Molecular cell biology. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 6.Kawamata T, Seitz H, Tomari Y. Structural determinants of miRNAs for RISC loading and slicer-independent unwinding. Nature structural & molecular biology. 2009;16:953–960. doi: 10.1038/nsmb.1630. [DOI] [PubMed] [Google Scholar]
- 7.Kwak PB, Tomari Y. The N domain of Argonaute drives duplex unwinding during RISC assembly. Nature structural & molecular biology. 2012;19:145–151. doi: 10.1038/nsmb.2232. [DOI] [PubMed] [Google Scholar]
- 8.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nature reviews. Genetics. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 9.Daniels TF, Killinger KM, Michal JJ, Wright RW, Jr, Jiang Z. Lipoproteins, cholesterol homeostasis and cardiac health. International journal of biological sciences. 2009;5:474–488. doi: 10.7150/ijbs.5.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross R. Atherosclerosis--an inflammatory disease. The New England journal of medicine. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 11.Wagschal A, et al. Genome-wide identification of microRNAs regulating cholesterol and triglyceride homeostasis. Nature medicine. 2015;21:1290–1297. doi: 10.1038/nm.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yvan-Charvet L, Wang N, Tall AR. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:139–143. doi: 10.1161/ATVBAHA.108.179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goedeke L, et al. MicroRNA-148a regulates LDL receptor and ABCA1 expression to control circulating lipoprotein levels. Nature medicine. 2015;21:1280–1289. doi: 10.1038/nm.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vickers KC, et al. MicroRNA-223 coordinates cholesterol homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:14518–14523. doi: 10.1073/pnas.1215767111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nature cell biology. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esau C, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell metabolism. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Janssen HL, et al. Treatment of HCV infection by targeting microRNA. The New England journal of medicine. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 18.Tsai WC, et al. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. The Journal of clinical investigation. 2012;122:2884–2897. doi: 10.1172/JCI63455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elmen J, et al. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic acids research. 2008;36:1153–1162. doi: 10.1093/nar/gkm1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vickers KC, et al. MicroRNA-27b is a regulatory hub in lipid metabolism and is altered in dyslipidemia. Hepatology. 2013;57:533–542. doi: 10.1002/hep.25846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soh J, Iqbal J, Queiroz J, Fernandez-Hernando C, Hussain MM. MicroRNA-30c reduces hyperlipidemia and atherosclerosis in mice by decreasing lipid synthesis and lipoprotein secretion. Nature medicine. 2013;19:892–900. doi: 10.1038/nm.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horie T, et al. MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17321–17326. doi: 10.1073/pnas.1008499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rayner KJ, et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature. 2011;478:404–407. doi: 10.1038/nature10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rayner KJ, et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. The Journal of clinical investigation. 2011;121:2921–2931. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramirez CM, et al. MicroRNA-758 regulates cholesterol efflux through posttranscriptional repression of ATP-binding cassette transporter A1. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:2707–2714. doi: 10.1161/ATVBAHA.111.232066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Aguiar Vallim TQ, et al. MicroRNA-144 regulates hepatic ATP binding cassette transporter A1 and plasma high-density lipoprotein after activation of the nuclear receptor farnesoid X receptor. Circulation research. 2013;112:1602–1612. doi: 10.1161/CIRCRESAHA.112.300648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J, et al. MiR-106b impairs cholesterol efflux and increases Abeta levels by repressing ABCA1 expression. Experimental neurology. 2012;235:476–483. doi: 10.1016/j.expneurol.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price NL, Ramirez CM, Fernandez-Hernando C. Relevance of microRNA in metabolic diseases. Critical reviews in clinical laboratory sciences. 2014;51:305–320. doi: 10.3109/10408363.2014.937522. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, et al. MicroRNAs 185, 96, and 223 repress selective high-density lipoprotein cholesterol uptake through posttranscriptional inhibition. Molecular and cellular biology. 2013;33:1956–1964. doi: 10.1128/MCB.01580-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu Z, Shen WJ, Kraemer FB, Azhar S. MicroRNAs 125a and 455 repress lipoprotein-supported steroidogenesis by targeting scavenger receptor class B type I in steroidogenic cells. Molecular and cellular biology. 2012;32:5035–5045. doi: 10.1128/MCB.01002-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rayner KJ, et al. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen RM, et al. miR-33 controls the expression of biliary transporters, and mediates statin- and diet-induced hepatotoxicity. EMBO molecular medicine. 2012;4:882–895. doi: 10.1002/emmm.201201228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horie T, et al. MicroRNA-33 deficiency reduces the progression of atherosclerotic plaque in ApoE−/− mice. Journal of the American Heart Association. 2012;1:e003376. doi: 10.1161/JAHA.112.003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marquart TJ, Wu J, Lusis AJ, Baldan A. Anti-miR-33 therapy does not alter the progression of atherosclerosis in low-density lipoprotein receptor-deficient mice. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:455–458. doi: 10.1161/ATVBAHA.112.300639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naar AM. Anti-atherosclerosis or No Anti-atherosclerosis: That is the miR-33 question. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:447–448. doi: 10.1161/ATVBAHA.112.301021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goedeke L, et al. Long-term therapeutic silencing of miR-33 increases circulating triglyceride levels and hepatic lipid accumulation in mice. EMBO molecular medicine. 2014;6:1133–1141. doi: 10.15252/emmm.201404046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meiler S, Baumer Y, Toulmin E, Seng K, Boisvert WA. MicroRNA 302a is a novel modulator of cholesterol homeostasis and atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:323–331. doi: 10.1161/ATVBAHA.114.304878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang D, et al. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circulation research. 2012;111:967–981. doi: 10.1161/CIRCRESAHA.112.266502. [DOI] [PubMed] [Google Scholar]
- 39.Sun D, et al. MiR-26 controls LXR-dependent cholesterol efflux by targeting ABCA1 and ARL7. FEBS letters. 2012;586:1472–1479. doi: 10.1016/j.febslet.2012.03.068. [DOI] [PubMed] [Google Scholar]
- 40.Wang D, et al. Coenzyme Q10 promotes macrophage cholesterol efflux by regulation of the activator protein-1/miR-378/ATP-binding cassette transporter G1-signaling pathway. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:1860–1870. doi: 10.1161/ATVBAHA.113.302879. [DOI] [PubMed] [Google Scholar]
- 41.d’Audigier C, et al. Thrombin receptor PAR-1 activation on endothelial progenitor cells enhances chemotaxis-associated genes expression and leukocyte recruitment by a COX-2-dependent mechanism. Angiogenesis. 2015;18:347–359. doi: 10.1007/s10456-015-9471-8. [DOI] [PubMed] [Google Scholar]
- 42.Cuhlmann S, et al. Disturbed blood flow induces RelA expression via c-Jun N-terminal kinase 1: a novel mode of NF-kappaB regulation that promotes arterial inflammation. Circulation research. 2011;108:950–959. doi: 10.1161/CIRCRESAHA.110.233841. [DOI] [PubMed] [Google Scholar]
- 43.Collins T, et al. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1995;9:899–909. [PubMed] [Google Scholar]
- 44.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1516–1521. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suarez Y, Wang C, Manes TD, Pober JS. Cutting edge: TNF-induced microRNAs regulate TNF-induced expression of E-selectin and intercellular adhesion molecule-1 on human endothelial cells: feedback control of inflammation. Journal of immunology. 2010;184:21–25. doi: 10.4049/jimmunol.0902369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu N, et al. Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis. 2011;215:286–293. doi: 10.1016/j.atherosclerosis.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 47.Fang Y, Shi C, Manduchi E, Civelek M, Davies PF. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13450–13455. doi: 10.1073/pnas.1002120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liao YC, et al. Let-7g improves multiple endothelial functions through targeting transforming growth factor-beta and SIRT-1 signaling. Journal of the American College of Cardiology. 2014;63:1685–1694. doi: 10.1016/j.jacc.2013.09.069. [DOI] [PubMed] [Google Scholar]
- 49.Sun X, et al. Systemic delivery of microRNA-181b inhibits nuclear factor-kappaB activation, vascular inflammation, and atherosclerosis in apolipoprotein E-deficient mice. Circulation research. 2014;114:32–40. doi: 10.1161/CIRCRESAHA.113.302089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun X, et al. MicroRNA-181b regulates NF-kappaB-mediated vascular inflammation. The Journal of clinical investigation. 2012;122:1973–1990. doi: 10.1172/JCI61495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng HS, et al. MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO molecular medicine. 2013;5:949–966. doi: 10.1002/emmm.201202318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu W, et al. Flow-Dependent Regulation of Kruppel-Like Factor 2 Is Mediated by MicroRNA-92a. Circulation. 2011;124:633–641. doi: 10.1161/CIRCULATIONAHA.110.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmitt MM, et al. Endothelial junctional adhesion molecule-a guides monocytes into flow-dependent predilection sites of atherosclerosis. Circulation. 2014;129:66–76. doi: 10.1161/CIRCULATIONAHA.113.004149. [DOI] [PubMed] [Google Scholar]
- 54.Foteinos G, Hu Y, Xiao Q, Metzler B, Xu Q. Rapid endothelial turnover in atherosclerosis-prone areas coincides with stem cell repair in apolipoprotein E-deficient mice. Circulation. 2008;117:1856–1863. doi: 10.1161/CIRCULATIONAHA.107.746008. [DOI] [PubMed] [Google Scholar]
- 55.Zeng L, et al. Sustained activation of XBP1 splicing leads to endothelial apoptosis and atherosclerosis development in response to disturbed flow. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8326–8331. doi: 10.1073/pnas.0903197106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schober A, et al. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nature medicine. 2014;20:368–376. doi: 10.1038/nm.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen CH, et al. Oxidized low-density lipoproteins inhibit endothelial cell proliferation by suppressing basic fibroblast growth factor expression. Circulation. 2000;101:171–177. doi: 10.1161/01.cir.101.2.171. [DOI] [PubMed] [Google Scholar]
- 58.Liu D, et al. MicroRNA-495 regulates the proliferation and apoptosis of human umbilical vein endothelial cells by targeting chemokine CCL2. Thrombosis research. 2015;135:146–154. doi: 10.1016/j.thromres.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 59.Loyer X, et al. Inhibition of microRNA-92a prevents endothelial dysfunction and atherosclerosis in mice. Circulation research. 2014;114:434–443. doi: 10.1161/CIRCRESAHA.114.302213. [DOI] [PubMed] [Google Scholar]
- 60.Menghini R, et al. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation. 2009;120:1524–1532. doi: 10.1161/CIRCULATIONAHA.109.864629. [DOI] [PubMed] [Google Scholar]
- 61.Ito T, Yagi S, Yamakuchi M. MicroRNA-34a regulation of endothelial senescence. Biochemical and biophysical research communications. 2010;398:735–740. doi: 10.1016/j.bbrc.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 62.Vasa-Nicotera M, et al. miR-146a is modulated in human endothelial cell with aging. Atherosclerosis. 2011;217:326–330. doi: 10.1016/j.atherosclerosis.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 63.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wei Y, Schober A. MicroRNA regulation of macrophages in human pathologies. Cellular and molecular life sciences: CMLS. 2016 doi: 10.1007/s00018-016-2254-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-alpha-PU.1 pathway. Nature medicine. 2011;17:64–70. doi: 10.1038/nm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhuang G, et al. A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation. Circulation. 2012;125:2892–2903. doi: 10.1161/CIRCULATIONAHA.111.087817. [DOI] [PubMed] [Google Scholar]
- 67.Ouimet M, et al. MicroRNA-33-dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. The Journal of clinical investigation. 2015;125:4334–4348. doi: 10.1172/JCI81676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei Y, et al. The microRNA-342-5p fosters inflammatory macrophage activation through an Akt1- and microRNA-155-dependent pathway during atherosclerosis. Circulation. 2013;127:1609–1619. doi: 10.1161/CIRCULATIONAHA.112.000736. [DOI] [PubMed] [Google Scholar]
- 69.Nazari-Jahantigh M, et al. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. The Journal of clinical investigation. 2012;122:4190–4202. doi: 10.1172/JCI61716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Du F, et al. MicroRNA-155 deficiency results in decreased macrophage inflammation and attenuated atherogenesis in apolipoprotein E-deficient mice. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:759–767. doi: 10.1161/ATVBAHA.113.302701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Donners MM, et al. Hematopoietic miR155 deficiency enhances atherosclerosis and decreases plaque stability in hyperlipidemic mice. PloS one. 2012;7:e35877. doi: 10.1371/journal.pone.0035877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chaudhuri AA, et al. MicroRNA-125b potentiates macrophage activation. Journal of immunology. 2011;187:5062–5068. doi: 10.4049/jimmunol.1102001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Banerjee S, et al. miR-125a-5p regulates differential activation of macrophages and inflammation. The Journal of biological chemistry. 2013;288:35428–35436. doi: 10.1074/jbc.M112.426866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen T, et al. MicroRNA-125a-5p partly regulates the inflammatory response, lipid uptake, and ORP9 expression in oxLDL-stimulated monocyte/macrophages. Cardiovascular research. 2009;83:131–139. doi: 10.1093/cvr/cvp121. [DOI] [PubMed] [Google Scholar]
- 75.Yang K, et al. MiR-146a inhibits oxidized low-density lipoprotein-induced lipid accumulation and inflammatory response via targeting toll-like receptor 4. FEBS letters. 2011;585:854–860. doi: 10.1016/j.febslet.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 76.Chen T, et al. MicroRNA-155 regulates lipid uptake, adhesion/chemokine marker secretion and SCG2 expression in oxLDL-stimulated dendritic cells/macrophages. International journal of cardiology. 2011;147:446–447. doi: 10.1016/j.ijcard.2010.10.133. [DOI] [PubMed] [Google Scholar]
- 77.Tian FJ, et al. Elevated microRNA-155 promotes foam cell formation by targeting HBP1 in atherogenesis. Cardiovascular research. 2014;103:100–110. doi: 10.1093/cvr/cvu070. [DOI] [PubMed] [Google Scholar]
- 78.Ye J, et al. miR-155 Regulated Inflammation Response by the SOCS1-STAT3-PDCD4 Axis in Atherogenesis. Mediators of inflammation. 2016;2016:8060182. doi: 10.1155/2016/8060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu C, et al. microRNA-181a represses ox-LDL-stimulated inflammatory response in dendritic cell by targeting c-Fos. Journal of lipid research. 2012;53:2355–2363. doi: 10.1194/jlr.M028878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang L, et al. miR-146a controls the resolution of T cell responses in mice. The Journal of experimental medicine. 2012;209:1655–1670. doi: 10.1084/jem.20112218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bennett MR, Sinha S, Owens GK. Vascular Smooth Muscle Cells in Atherosclerosis. Circulation research. 2016;118:692–702. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cordes KR, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hergenreider E, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nature cell biology. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 84.Vengrenyuk Y, et al. Cholesterol loading reprograms the microRNA-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:535–546. doi: 10.1161/ATVBAHA.114.304029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boettger T, et al. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. The Journal of clinical investigation. 2009;119:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheng Y, et al. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circulation research. 2009;105:158–166. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lovren F, et al. MicroRNA-145 targeted therapy reduces atherosclerosis. Circulation. 2012;126:S81–90. doi: 10.1161/CIRCULATIONAHA.111.084186. [DOI] [PubMed] [Google Scholar]
- 88.Xin M, et al. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes & development. 2009;23:2166–2178. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Torella D, et al. MicroRNA-133 controls vascular smooth muscle cell phenotypic switch in vitro and vascular remodeling in vivo. Circulation research. 2011;109:880–893. doi: 10.1161/CIRCRESAHA.111.240150. [DOI] [PubMed] [Google Scholar]
- 90.Li P, et al. MicroRNA-663 regulates human vascular smooth muscle cell phenotypic switch and vascular neointimal formation. Circulation research. 2013;113:1117–1127. doi: 10.1161/CIRCRESAHA.113.301306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu F, et al. MicroRNA-15b/16 Attenuates Vascular Neointima Formation by Promoting the Contractile Phenotype of Vascular Smooth Muscle Through Targeting YAP. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:2145–2152. doi: 10.1161/ATVBAHA.115.305748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leeper NJ, et al. MicroRNA-26a is a novel regulator of vascular smooth muscle cell function. Journal of cellular physiology. 2011;226:1035–1043. doi: 10.1002/jcp.22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ji R, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circulation research. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 94.Liu X, et al. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circulation research. 2009;104:476–487. doi: 10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boon RA, et al. MicroRNA-29 in aortic dilation: implications for aneurysm formation. Circulation research. 2011;109:1115–1119. doi: 10.1161/CIRCRESAHA.111.255737. [DOI] [PubMed] [Google Scholar]
- 97.Castoldi G, et al. MiR-133a regulates collagen 1A1: potential role of miR-133a in myocardial fibrosis in angiotensin II-dependent hypertension. Journal of cellular physiology. 2012;227:850–856. doi: 10.1002/jcp.22939. [DOI] [PubMed] [Google Scholar]
- 98.Kato M, et al. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3432–3437. doi: 10.1073/pnas.0611192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thum T, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 100.Lin Y, et al. Involvement of MicroRNAs in hydrogen peroxide-mediated gene regulation and cellular injury response in vascular smooth muscle cells. The Journal of biological chemistry. 2009;284:7903–7913. doi: 10.1074/jbc.M806920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zernecke A, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Science signaling. 2009;2:ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 102.Ma F, et al. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-gamma. Nature immunology. 2011;12:861–869. doi: 10.1038/ni.2073. [DOI] [PubMed] [Google Scholar]
- 103.Newby AC. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:2108–2114. doi: 10.1161/ATVBAHA.108.173898. [DOI] [PubMed] [Google Scholar]
- 104.Cipollone F, et al. A unique microRNA signature associated with plaque instability in humans. Stroke; a journal of cerebral circulation. 2011;42:2556–2563. doi: 10.1161/STROKEAHA.110.597575. [DOI] [PubMed] [Google Scholar]
- 105.Fan X, Wang E, Wang X, Cong X, Chen X. MicroRNA-21 is a unique signature associated with coronary plaque instability in humans by regulating matrix metalloproteinase-9 via reversion-inducing cysteine-rich protein with Kazal motifs. Experimental and molecular pathology. 2014;96:242–249. doi: 10.1016/j.yexmp.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 106.Di Gregoli K, et al. MicroRNA-24 regulates macrophage behavior and retards atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:1990–2000. doi: 10.1161/ATVBAHA.114.304088. [DOI] [PubMed] [Google Scholar]
- 107.Wei Y, et al. Regulation of Csf1r and Bcl6 in macrophages mediates the stage-specific effects of microRNA-155 on atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:796–803. doi: 10.1161/ATVBAHA.114.304723. [DOI] [PubMed] [Google Scholar]
- 108.Das A, Ganesh K, Khanna S, Sen CK, Roy S. Engulfment of apoptotic cells by macrophages: a role of microRNA-21 in the resolution of wound inflammation. Journal of immunology. 2014;192:1120–1129. doi: 10.4049/jimmunol.1300613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yvan-Charvet L, et al. ABCA1 and ABCG1 protect against oxidative stress-induced macrophage apoptosis during efferocytosis. Circulation research. 2010;106:1861–1869. doi: 10.1161/CIRCRESAHA.110.217281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rajamaki K, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PloS one. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Haneklaus M, et al. Cutting edge: miR-223 and EBV miR-BART15 regulate the NLRP3 inflammasome and IL-1beta production. Journal of immunology. 2012;189:3795–3799. doi: 10.4049/jimmunol.1200312. [DOI] [PubMed] [Google Scholar]
- 112.Dey N, et al. MicroRNA-21 orchestrates high glucose-induced signals to TOR complex 1, resulting in renal cell pathology in diabetes. The Journal of biological chemistry. 2011;286:25586–25603. doi: 10.1074/jbc.M110.208066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Feng J, Xing W, Xie L. Regulatory Roles of MicroRNAs in Diabetes. International journal of molecular sciences. 2016:17. doi: 10.3390/ijms17101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sequeira-Lopez ML, et al. The microRNA-processing enzyme dicer maintains juxtaglomerular cells. Journal of the American Society of Nephrology: JASN. 2010;21:460–467. doi: 10.1681/ASN.2009090964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xin M, et al. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes & development. 2009;23:2166–2178. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Santulli G. MicroRNAs and Endothelial (Dys) Function. Journal of cellular physiology. 2016;231:1638–1644. doi: 10.1002/jcp.25276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Karakas M, et al. Circulating microRNAs strongly predict cardiovascular death in patients with coronary artery disease-results from the large AtheroGene study. European heart journal. 2016 doi: 10.1093/eurheartj/ehw250. [DOI] [PubMed]
- 118.Fichtlscherer S, et al. Circulating microRNAs in patients with coronary artery disease. Circulation research. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 119.Bell DA, Hooper AJ, Burnett JR. Mipomersen, an antisense apolipoprotein B synthesis inhibitor. Expert opinion on investigational drugs. 2011;20:265–272. doi: 10.1517/13543784.2011.547471. [DOI] [PubMed] [Google Scholar]
- 120.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 121.Lanford RE, et al. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nature reviews. Drug discovery. 2013;12:847–865. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Broderick JA, Zamore PD. MicroRNA therapeutics. Gene therapy. 2011;18:1104–1110. doi: 10.1038/gt.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kota J, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cerchia L, et al. Targeting Axl with an high-affinity inhibitory aptamer. Molecular therapy: the journal of the American Society of Gene Therapy. 2012;20:2291–2303. doi: 10.1038/mt.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Esposito CL, et al. Multifunctional aptamer-miRNA conjugates for targeted cancer therapy. Molecular therapy: the journal of the American Society of Gene Therapy. 2014;22:1151–1163. doi: 10.1038/mt.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]