Abstract
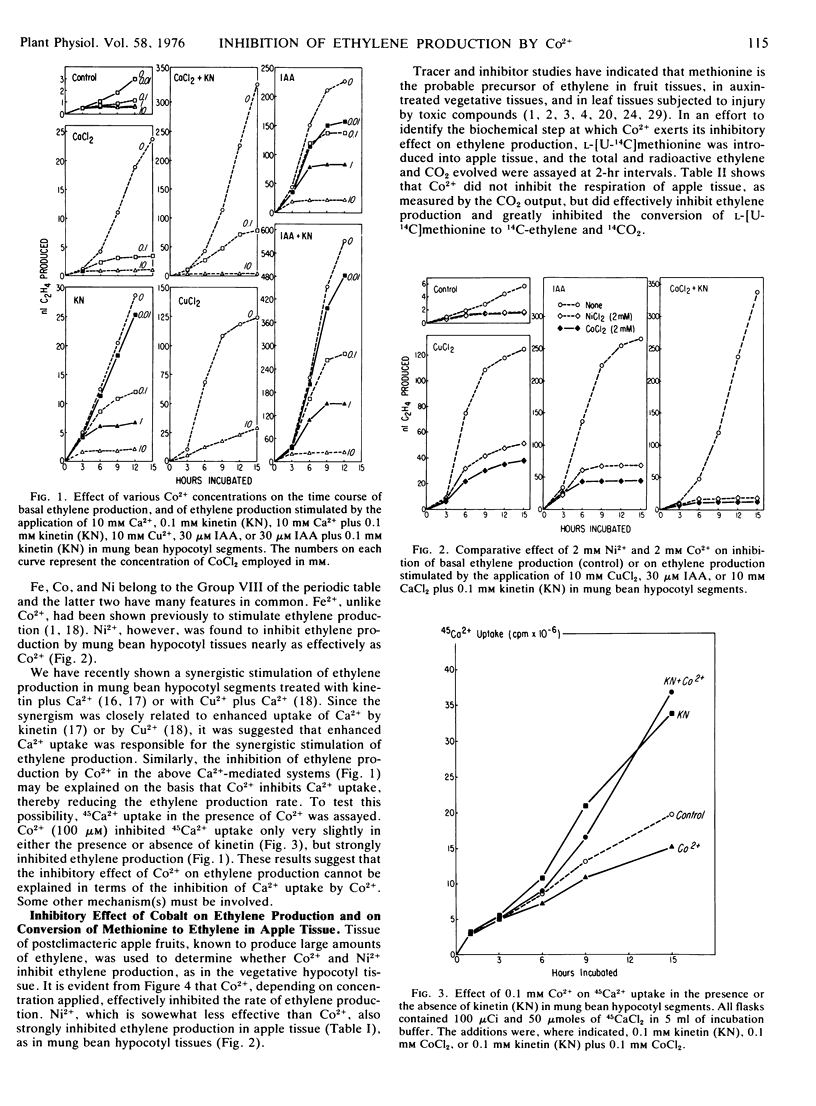
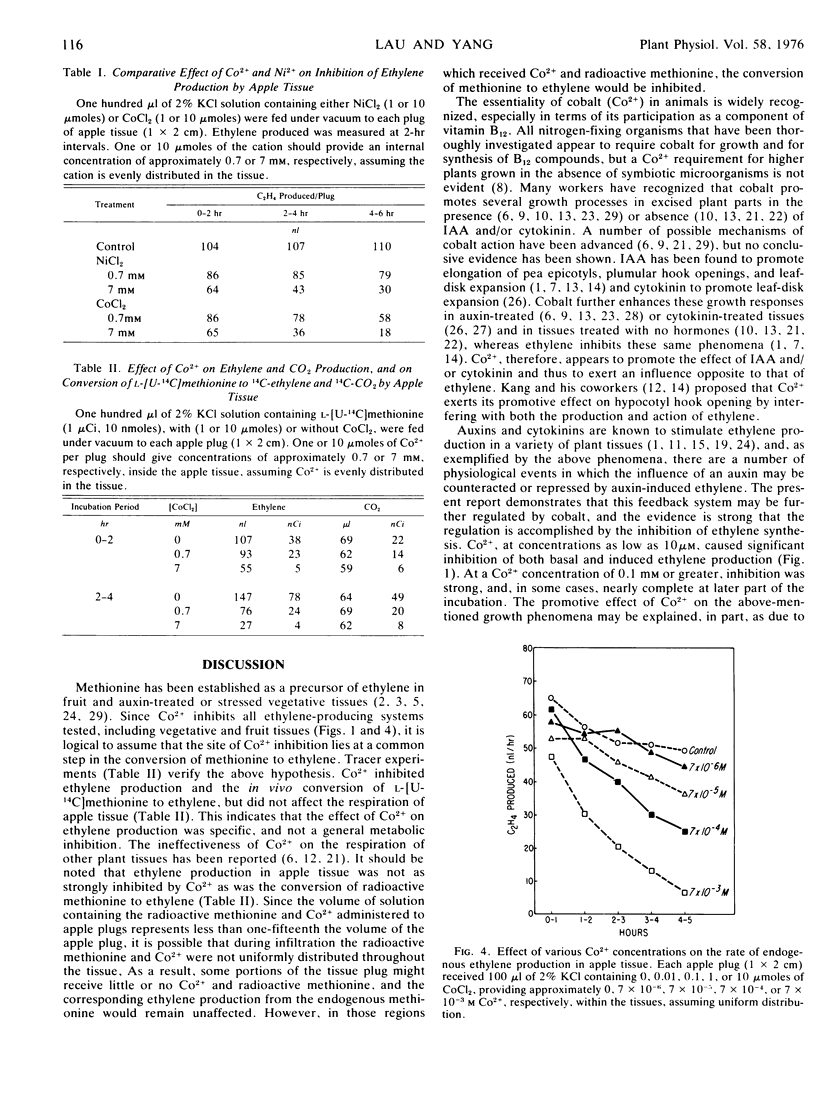
The effect of Co2+ on ethylene production by mung bean (Phaseolus aureus Roxb.) and by apple tissues was studied. Co2+, depending on concentrations applied, effectively inhibited ethylene production by both tissues. It also strongly inhibited the ethylene production induced by IAA, kinetin, IAA plus kinetin, Ca2+, kinetin plus Ca2+, or Cu2+ treatments in mung bean hypocotyl segments. While Co2+ greatly inhibited ethylene production, it had little effect on the respiration of apple tissue, indicating that Co2+ does not exert its inhibitory effect as a general metabolic inhibitor. Ni2+, which belongs to the same group as Co2+ in the periodic table, also markedly curtailed both the basal and the induced ethylene production by apple and mung bean hypocotyl tissues.
In a system in which kinetin and Ca2+ were applied together, kinetin greatly enhanced Ca2+ uptake, thus enhancing ethylene production. Co2+, however, slightly inhibited the uptake of Ca2+ but appreciably inhibited ethylene production, either in the presence or in the absence of kinetin. Tracer experiments using apple tissue indicated that Co2+ strongly inhibited the in vivo conversion of l-[U-14C]methionine to 14C-ethylene. These data suggest that Co2+ inhibited ethylene production by inhibiting the conversion of methionine to ethylene, a common step which is required for ethylene formation by higher plants.
Co2+ is known to promote elongation, leaf expansion, and hook opening in excised plant parts in response to applied auxins or cytokinins. Since ethylene is known to inhibit these growth phenomena, it is suggested that Co2+ exerts its promotive effect, at least in part, by inhibiting ethylene formation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles A. L. Biochemical Pathway of Stress-induced Ethylene. Plant Physiol. 1972 Oct;50(4):496–498. doi: 10.1104/pp.50.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur A. H., Yang S. F., Pratt H. K. Ethylene biosynthesis in fruit tissues. Plant Physiol. 1971 May;47(5):696–699. doi: 10.1104/pp.47.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Clagett C. O. Conversion of methionine to ethylene in vegetative tissue and fruits. Biochem Biophys Res Commun. 1967 Apr 20;27(2):125–130. doi: 10.1016/s0006-291x(67)80050-0. [DOI] [PubMed] [Google Scholar]
- Burg S. P., Thimann K. V. Studies on the Ethylene Production of Apple Tissue. Plant Physiol. 1960 Jan;35(1):24–35. doi: 10.1104/pp.35.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick A. V., Burg S. P. Regulation of root growth by auxin-ethylene interaction. Plant Physiol. 1970 Feb;45(2):192–200. doi: 10.1104/pp.45.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS H. J., KLIEWER M. VITAMIN B12 COMPOUNDS IN RELATION TO THE REQUIREMENT OF COBALT FOR HIGHER PLANTS AND NITROGEN-FIXING ORGANISMS. Ann N Y Acad Sci. 1964 Apr 24;112:735–755. doi: 10.1111/j.1749-6632.1964.tb45052.x. [DOI] [PubMed] [Google Scholar]
- GALSTON A. W., SIEGEL S. M. Antiperoxidative action of the cobaltous ion and its consequences for plant growth. Science. 1954 Dec 24;120(3130):1070–1071. doi: 10.1126/science.120.3130.1070. [DOI] [PubMed] [Google Scholar]
- Kang B. G., Newcomb W., Burg S. P. Mechanism of Auxin-induced Ethylene Production. Plant Physiol. 1971 Apr;47(4):504–509. doi: 10.1104/pp.47.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B. G., Yocum C. S., Burg S. P., Ray P. M. Ethylene and carbon dioxide: mediation of hypocotyl hook-opening response. Science. 1967 May 19;156(3777):958–959. doi: 10.1126/science.156.3777.958. [DOI] [PubMed] [Google Scholar]
- Lau O. L., Yang S. F. Interaction of kinetin and calcium in relation to their effect on stimulation of ethylene production. Plant Physiol. 1975 Apr;55(4):738–740. doi: 10.1104/pp.55.4.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau O. L., Yang S. F. Mechanism of a Synergistic Effect of Kinetin on Auxin-induced Ethylene Production: Suppression of Auxin Conjugation. Plant Physiol. 1973 Jun;51(6):1011–1014. doi: 10.1104/pp.51.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau O. L., Yang S. F. Stimulation of ethylene production in the mung bean hypocotyls by cupric ion, calcium ion, and kinetin. Plant Physiol. 1976 Jan;57(1):88–92. doi: 10.1104/pp.57.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Kunishi A. Stimulation of ethylene production in apple tissue slices by methionine. Plant Physiol. 1966 Mar;41(3):376–382. doi: 10.1104/pp.41.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loercher L., Liverman J. L. Influence of Cobalt on Leaf Expansion and Oxidative Phosphorylation. Plant Physiol. 1964 Sep;39(5):720–725. doi: 10.1104/pp.39.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER C. O. Promoting effect of cobaltous and nickelous ions on expansion of etiolated bean leaf disks. Arch Biochem Biophys. 1951 Jun;32(1):216–218. doi: 10.1016/0003-9861(51)90256-1. [DOI] [PubMed] [Google Scholar]
- Miller C. O. The Influence of Cobalt and Sugars upon the Elongation of Etiolated Pea Stem Segments. Plant Physiol. 1954 Jan;29(1):79–82. doi: 10.1104/pp.29.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury F. B. Growth Regulators and Flowering. II. The Cobaltous Ion. Plant Physiol. 1959 Nov;34(6):598–604. doi: 10.1104/pp.34.6.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. A., Liverman J. L. Promotion of Leaf Expansion by Kinetin and Benzylaminopurine. Plant Physiol. 1956 Jul;31(4):321–322. doi: 10.1104/pp.31.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]


