Multidrug-resistant (MDR), extensively drug-resistant (XDR), and pan-drug-resistant (PDR) strains of Acinetobacter baumannii have frequently been characterized. The ability of A. baumannii to develop resistance to antibiotics is a key reason this organism has been difficult to study using genetic and molecular biology approaches. Here we report selectable markers that are not only useful but necessary for the selection of drug-resistant transformants in the setting of drug-resistant backgrounds. Use of these selectable markers can be applied to a variety of genetic and molecular techniques such as mutagenesis and transformation. These selectable markers will help promote genetic and molecular biology studies of otherwise onerous drug-resistant strains, while avoiding the generation of pathogenic organisms that are resistant to clinically relevant antibiotics.
KEYWORDS: Acinetobacter, antibiotic resistance, genetics, Gram-negative bacteria, molecular biology
ABSTRACT
Acinetobacter baumannii is one of the most antibiotic-resistant pathogens in clinical medicine, and extensively drug-resistant (XDR) strains are commonly isolated from infected patients. Such XDR strains are already resistant to traditional selectable genetic markers, limiting the ability to conduct pathogenesis research by genetic disruption. Optimization of selectable markers is therefore critical for the advancement of fundamental molecular biology techniques to use in these strains. We screened 23 drugs that constitute a broad array of antibiotics spanning multiple drug classes against HUMC1, a highly virulent and XDR A. baumannii clinical blood and lung isolate. HUMC1 is resistant to all clinically useful antibiotics that are reported by the clinical microbiology laboratory, except for colistin. Ethical concerns about intentionally establishing pan-resistance, including to the last-line agent, colistin, in a clinical isolate made identification of other markers desirable. We screened additional antibiotics that are in clinical use and those that are useful only in a lab setting to identify selectable markers that were effective at selecting for transformants in vitro. We show that supraphysiological levels of tetracycline can overcome innate drug resistance displayed by this XDR strain. Last, we demonstrate that transformation of the tetA (tetracycline resistance) and Sh ble (zeocin resistance), but not pac (puromycin resistance), resistance cassettes allow for selection of drug-resistant transformants. These results make the genetic manipulation of XDR A. baumannii strains easily achieved.
IMPORTANCE Multidrug-resistant (MDR), extensively drug-resistant (XDR), and pan-drug-resistant (PDR) strains of Acinetobacter baumannii have frequently been characterized. The ability of A. baumannii to develop resistance to antibiotics is a key reason this organism has been difficult to study using genetic and molecular biology approaches. Here we report selectable markers that are not only useful but necessary for the selection of drug-resistant transformants in the setting of drug-resistant backgrounds. Use of these selectable markers can be applied to a variety of genetic and molecular techniques such as mutagenesis and transformation. These selectable markers will help promote genetic and molecular biology studies of otherwise onerous drug-resistant strains, while avoiding the generation of pathogenic organisms that are resistant to clinically relevant antibiotics.
INTRODUCTION
Infections due to Acinetobacter baumannii have been identified by the Infectious Diseases Society of America (IDSA) and the Centers for Disease Control and Prevention (CDC) as a significant public health concern (1, 2). Of particular concern regarding A. baumannii is the exceptionally high frequency of extensively drug-resistant (XDR) strains (2–6). New prophylactic and therapeutic strategies are needed to combat such strains. The key to development of such novel approaches is a better understanding of pathogenesis of these infections (2, 4, 5, 7–9).
The prevailing dogma espouses that a fitness cost is always associated with the acquisition of antibiotic resistance (10–12). On the contrary, recent reports suggest that a fitness advantage exists for some specific antibiotic resistance mutations in Salmonella enterica serotype Typhi, Pseudomonas aeruginosa, and A. baumannii (13, 14). Thus, given the remarkable rise in frequency of XDR A. baumannii clinical strains, the use of an XDR strain is needed to best model clinically relevant infection dynamics in pathogenesis studies.
Unfortunately, our understanding of A. baumannii pathogenesis has been greatly hampered by a lack of available genetic manipulation techniques for highly resistant and clinically relevant strains (15–17). Advances in microbial genetics have provided tools such as transposon and site-directed mutagenesis that have rapidly improved our ability to study and manipulate organisms of interest (18–22).
However, such techniques require the use of a selectable marker to allow outgrowth of a desired mutant (23–27). Selectable markers take advantage of antibiotic resistance cassettes to allow for selection of mutants when grown under antibiotic selective pressure (28). The conundrum is that XDR A. baumannii strains are already resistant to commonly used selectable markers, precluding effective selection of such strains with most traditionally used selectable markers (17, 28–32). Thus, optimization of selectable markers is critical for the fundamental advancement of molecular biology research with XDR strains.
We have previously published that HUMC1, an XDR A. baumannii clinical blood and lung isolate resistant to all clinically reported antibiotics except colistin, is hypervirulent in murine models of infection (15, 16, 33, 34). Given its virulence and near-pan-drug-resistant status, intentional induction of colistin resistance in this strain, for example by inserting the MCR gene, would raise ethical concerns. Thus, while HUMC1 is a very useful model strain for studying pathogenesis, its intrinsic antibiotic resistance has made genetic manipulation challenging. To identify suitable selectable markers for such a resistant strain, we screened 23 compounds that constitute a broad array of antibiotics spanning multiple drug classes. Despite its intrinsic antibiotic resistance, we successfully identified selectable markers that are effective in vitro against HUMC1. Last, we show that supraphysiological levels of a drug, irrelevant to clinical use but achievable in vitro for selection of transformants, can overcome innate drug resistance displayed by an XDR strain.
RESULTS
MIC testing.
Based on results generated in the clinical microbiology laboratory at the hospital at which HUMC1 was isolated, A. baumannii HUMC1 was resistant to all clinical antibiotics except for colistin (Table 1). However, we noted that the tetracycline MIC of 12.5 µg/ml, while clinically defined as resistant due to an inability to achieve drug levels this high in vivo, was well within the range of concentrations achievable in vitro to enable selection of more-resistant clones. Furthermore, when we tested the related antibiotic doxycycline, we found a lower MIC (Table 1). Finally, two antibacterial agents that are not used clinically, puromycin and zeocin, also had activity against HUMC1 (Table 1).
TABLE 1 .
MIC results for drugs against A. baumannii HUMC1 and ATCC 17978a
| Drug(s) | MIC(s) (µg/ml) of drug(s) against strain: |
Method | |
|---|---|---|---|
| HUMC1 | ATCC 17978 | ||
| Amikacin | >128 | 8 | Vitek 2 |
| Gentamicin | >128 | 8 | Vitek 2 |
| Aztreonam | 64 | 16 | Vitek 2 |
| Ampicillin-sulbactam | 16/8 | 1/0.5 | Vitek 2 |
| Piperacillin-tazobactam | >128/4 | 0.06/4 | Vitek 2 |
| Cefepime | 32 | 2 | Vitek 2 |
| Meropenem | 32 | 0.25 | Vitek 2 |
| Imipenem | 16 | 0.25 | Vitek 2 |
| Ertapenem | 128 | 4 | Vitek 2 |
| Doripenem | 16 | 0.5 | Vitek 2 |
| Ciprofloxacin | >128 | 0.125 | Vitek 2 |
| Colistin | 2 | 2 | Vitek 2 |
| Tigecycline | 4 | 0.25 | Vitek 2 |
| Tellurite | 62.5 | Resazurin | |
| Actinomycin D | >500 | >500 | Resazurin |
| Blasticidin S HCl | >2,500 | >2,500 | Resazurin |
| Doxycycline hydrochloride | 0.25 | <0.03125 | Resazurin |
| Geneticin | >1,000 | >1,000 | Resazurin |
| Kanamycin | >50 | >50 | Resazurin |
| Puromycin | 78.125 | <39.06 | Resazurin |
| Streptomycin | >50 | >50 | Resazurin |
| Tetracycline hydrochloride | 12.5 | 0.125 | Resazurin |
| Zeocin | 12.5 | 6.25 | Resazurin |
A. baumannii HUMC1 is sensitive to colistin, doxycycline, tetracycline (supraphysiological concentrations but attainable in vitro), puromycin, and zeocin.
Tetracycline resistance.
Tetracycline resistance is conferred by the tetA gene from pBR322 and commonly found on many plasmids used for molecular biology. The fact that doxycycline retained activity against the strain despite tetracycline resistance suggested that the resistance observed was not due to the tetA gene. We confirmed that tetracycline resistance in the HUMC1 isolate was not due to the presence of the tetA gene. A BLAST search for tetA against the HUMC1 genome did not return any hits, and PCR for tetA using purified HUMC1 genomic DNA (gDNA) was negative as well. Colonies were successfully isolated by plating on agar plates supplemented with 50, 75, or 100 µg/ml of tetracycline, and no growth was observed for the nontransformed HUMC1 control, indicating the ability of the tetA gene to be used as a selectable marker in HUMC1, despite clinically defined tetracycline resistance.
The purified pABBR_GFP plasmid was transformed into HUMC1 isolate, and transformants were selected by plating on tryptic soy agar (TSA) plate with 100 µg/ml of tetracycline. Expression of green fluorescent protein (GFP) was confirmed in transformed HUMC1 with nontransformed HUMC1 as a negative control using a fluorescence microscope (Fig. 1).
FIG 1 .
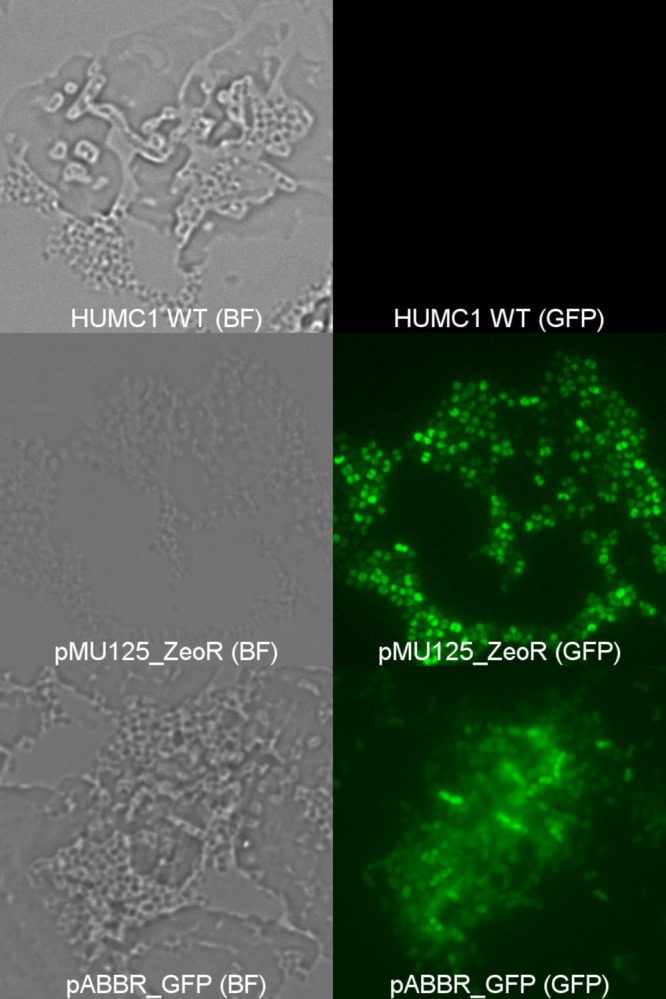
Successful transformation and expression of GFP in A. baumannii HUMC1 using plasmids containing zeocin (pMU125_GFP) or tetracycline (pABBR_GFP) resistance gene. The wild-type (WT) A. baumannii HUMC1 alone or carrying plasmid pMU125_GFP or pABBR_GFP is shown by bright-field microscopy (BF) or fluorescence microscopy (GFP). Magnification, ×1,000.
Zeocin resistance.
Zeocin is an antibiotic that is not used clinically. Resistance to zeocin is conferred by the Sh ble gene. Unfortunately, plasmids that contain the Sh ble gene with an Acinetobacter origin of replication are not readily available, so we developed pMSG360Zeo_AB and pCR-Blunt II-TOPO_AB (Fig. 2). Successful transformants were selected by plating on low-salt Luria-Bertani broth (LB) agar supplemented with 250 µg/ml zeocin. The presence of the plasmid was further verified in the transformants by PCR.
FIG 2 .
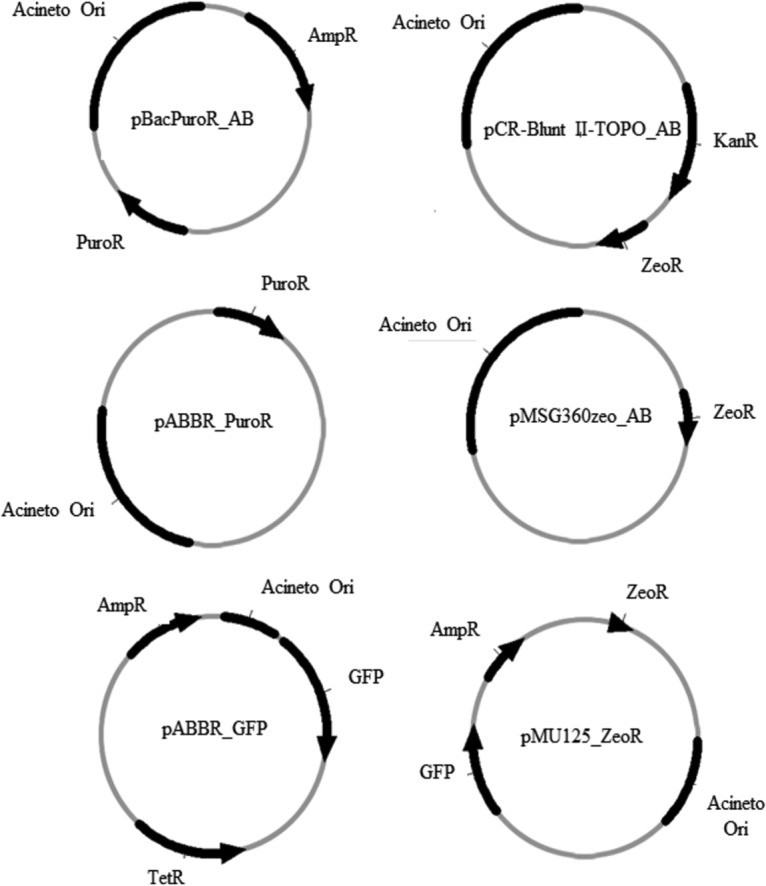
Plasmid constructs developed for this study. Constructs were developed by linearizing the vector backbone and insert by PCR, and assembly of the linear parts was performed by Gibson assembly.
In order to demonstrate efficacy of zeocin selection to maintain HUMC1 transformants, the Sh ble resistance gene from pCR-Blunt II-TOPO was cloned into pMU125 to form pMU125_ZeoR and was transformed into HUMC1 isolate. Transformants were selected for on low-salt LB agar supplemented with 250 µg/ml zeocin. Successful transformation of pMU125_ZeoR into HUMC1 was confirmed by fluorescence microscopy (Fig. 1).
Puromycin resistance.
Resistance to puromycin is conferred by the pac gene encoding puromycin N-acetyltransferase (PAC). The pac open reading frame was cloned from pBacPuroR-NeoR by PCR and inserted into pABBR_MCS by the Gibson assembly method to form pABBR_PuroR (Table 2 and Fig. 2). The plasmid was sequenced, and it was confirmed that the pac open reading frame was in frame with the promoter and no mutations were present. The plasmid allowed for selection of puromycin-resistant colonies in Escherichia coli, but transformation of the plasmid was unable to confer puromycin resistance in A. baumannii.
TABLE 2 .
Description of plasmids used in this study
| Plasmid | Resistance gene(s)a | Source and/or reference |
|---|---|---|
| pMol130-TelR | Tellurite | Addgene plasmid no. 50799 (30) |
| pBacPuroR-NeoR | Amp, neomycin, puromycin | Addgene plasmid no. 34921 (41) |
| pMSG360zeo | Zeocin | Addgene plasmid no. 27154 (42) |
| pCR-Blunt II-TOPO | Zeocin | ThermoFisher catalog no. K2800J10 |
| pWH1266 | Amp, Tet | 43 |
| pABBR_MCS | Amp, Tet | 43 |
| pBacPuroR_AB | Puro | This study |
| pABBR_PuroR | Puro | This study |
| pCR-Blunt II_AB | Zeocin | This study |
| pMSG360zeo_AB | Zeocin | This study |
| pABBR_GFP | Amp, Tet | This study |
| pMU125_ZeoR | Amp, zeocin | This study |
| pMU125 | Amp | 44 |
Amp, ampicillin; Tet, tetracycline; Puro, puromycin.
We attempted a second plasmid construct design in which the pac resistance cassette from pBacPuroR-NeoR vector was left intact. pBacPuroR_AB was formed by cloning the A. baumannii ori region from pABBR_MCS and assembling it into a linearized pBacPuroR-NeoR using the Gibson assembly method (Table 2 and Fig. 2). Sanger sequencing was done to confirm the proper assembly of the construct. This second construct version allowed for selection of E. coli transformants, but once again we were unable to select A. baumannii transformants.
DISCUSSION
Standard clinical definitions and classifications of drug sensitivity for microbes are based on achievable levels of antibiotics in the body (35). However, these definitions can be unnecessarily conservative when considering in vitro use as a selectable marker for genetic manipulation. It is possible to achieve significantly higher drug concentrations in vitro than in vivo (plasma, serum, bone, tissue, etc.). Here we have demonstrated that concentrations of tetracycline unachievable in vivo can be easily used in vitro for selection of “highly drug-resistant” mutants in a clinically drug-resistant strain. Furthermore, we found that the XDR strain was susceptible to several selectable markers that are not used as clinical antibiotics and also to a drug (doxycycline) that is used clinically but was not reported by the clinical microbiology laboratory. Thus, we emphasize the need to conduct systematic screens of potential selectable markers not limited by presumptions based on resistance profiles reported clinically.
Previous efforts have attempted to introduce and optimize standard genetic and molecular biology techniques in A. baumannii such as transformation, gene knockout, and transposon libraries (27, 32, 36). However, there are still relatively few molecular tools that have been validated for use in A. baumannii compared to other bacterial species such as E. coli, Bacillus subtilis, Staphylococcus aureus, and Mycobacterium tuberculosis. For example, there were no Acinetobacter plasmids available through Addgene.org (a nonprofit plasmid repository) (Cambridge, MA) at the time of this publication. Validation and standardization of these basic tools will benefit the research community in general and make Acinetobacter research more accessible.
We attempted to develop our constructs conferring resistance in one of two ways. First, the coding sequence (CDS) region of the antibiotic resistance gene (tetA, Sh ble, or pac) was cloned in frame with the bla promoter which is recognized by the highly conserved sigma-70 (rpoD) “constitutive housekeeping” promoter. The sigma-70 sigma factor is highly conserved in E. coli and A. baumannii so it was reasonable to hypothesize that A. baumannii transcription machinery would successfully recognize the bla promoter and express the transgene in a manner similar to that in E. coli. Sequencing of the assembled construct confirmed that the pac gene had replaced the bla open reading frame (ORF) in frame with the promoter. As that method did not work, we next tried to leave the promoter region of the pac gene intact and instead add the A. baumannii ori sequence to the pBacPuroR_NeoR plasmid. The promoter sequence differed from the bla promoter that was present in pABBR_MCS so it was reasonable that a change in the promoter sequence would improve gene expression; however, this approach was also unsuccessful.
Thus, we were unable to develop a functional puromycin selectable marker in A. baumannii despite the functional activity displayed by E. coli transformants. This difficulty could be due to the use of genetic elements that have not been optimized for expression in A. baumannii such as the promoter elements and codon sequence. While we were unable to express a functional pac gene in Acinetobacter, successful expression may be possible with a different promoter or codon optimized sequence. Additionally, the robustness of the antibiotic resistance conferred by the Sh ble and tetA genes used in the plasmids could be improved with similar promoter and codon optimization considerations.
We also observed that A. baumannii ATCC 17978 and HUMC1 were susceptible to drugs that are not used clinically, including puromycin and zeocin. This is most likely due to lack of exposure to these antimicrobial agents so selective pressure has not promoted mutants with resistance to these drugs. Recent publications have shown that other nonclinically relevant antimicrobials, such as tellurite, can be used for in vitro selection schemes (30, 37). Further effort to characterize selection systems, for drug resistance strains in particular, for basic science purposes continues to be of value.
A national surveillance study of U.S. intensive care units found that 50% of clinical isolates of A. baumannii were carbapenem-resistant, XDR strains (38). Further research is needed to better understand the basic physiology and host-pathogen interactions of the most difficult-to-treat and most lethal drug-resistant strains. Molecular tools such as selectable markers are needed to facilitate basic genetic studies and engender further research of these intractable strains. Our results enable transformation of antibiotic-resistant strains of A. baumannii by identifying alternative selectable markers and establishing effective constructs that are potentially useful in spite of an XDR phenotype.
MATERIALS AND METHODS
Bacterial strains.
E. coli DH5α, A. baumannii HUMC1 (15, 16, 33, 34), and A. baumannii ATCC 17978 were cultured using aseptic technique. Single colonies were first streaked out on tryptic soy agar (TSA) from frozen glycerol stocks. Single colonies were picked and used to inoculate overnight broth cultures in tryptic soy broth (TSB).
Resazurin MIC assays.
The colometric resazurin assay was conducted as previously described (39, 40). Antibiotics were acquired from Sigma-Aldrich (St. Louis, MO) or ThermoFisher (Waltham, MA).
Overnight cultures of the bacteria (A. baumannii HUMC1 or ATCC 17978) grown in TSB were diluted 1:100 into Mueller-Hinton II (MH2) broth and subcultured in a shaking incubator at 200 rpm and 37°C until the optical density at 600 nm (OD600) reached 0.5. Bacteria were diluted to a working concentration of 1 × 106 CFU/ml. The bacterial density was confirmed by plating serial dilutions on TSA and counting CFU.
MIC assays were conducted in standard, sterile, round-bottom (U-shaped), 96-well plates. Drug dilutions were done by serial twofold dilutions across plate columns. Wells of bacteria and media alone were included as positive and negative controls, respectively. One hundred microliters of 1 × 106 CFU/ml bacterial culture was added to each one of the requisite wells. The plates were incubated for 24 h at 37°C. Twenty microliters of 0.1% resazurin dye was added to each well, and metabolism of the dye was measured after 1 h.
Plasmids.
Details for the plasmids used in this study are listed in Table 2.
pMo130-TelR was a gift from Kim Lee Chua (Addgene plasmid no. 50799) (30). pBacPuroR-NeoR was a gift from Ben Lehner (Addgene plasmid no. 34921) (41). pMSG360zeo was a gift from Michael Glickman (Addgene plasmid no. 27154) (42).
Primers.
Primers were purchased from Integrated DNA Technologies, Inc. (IDT) (Coralville, IA). Primer sequences are listed in Table 3.
TABLE 3 .
Primers used for this study
| Plasmid or process and primer | Target | Template | Sequencea |
|---|---|---|---|
| pMSG360Zeo_AB | |||
| ZeoF_pMSG_F | Linear pMSG360 | pMSG360 | CGTTCTTCTTCGTCATAACTTAATG |
| ZeoR_pMSG_R | Linear pMSG360 | pMSG360 | GAAACGCCTTAAACCGGAAAATTTTC |
| Zeo_OriF | Acinetobacter ori | pABBR_MCS | tttccggtttaaggcgtttcGGATTTTAACATTTTGCGTTG |
| Zeo_OriR | Acinetobacter ori | pABBR_MCS | agttatgacgaagaagaacgGATCGTAGAAATATCTATGATTATCTTG |
| pCR-Blunt II-TOPO_AB | |||
| ZeoF_TOPO | Linear pCR-Blunt II-TOPO | pCR-Blunt II-TOPO | tcatagatatttctacgatcTTAAGGGCGAATTCTGCAG |
| ZeoR_TOPO | Linear pCR-Blunt II-TOPO | pCR-Blunt II-TOPO | aacgcaaaatgttaaaatccTCTATAGTGTCACCTAAATAGC |
| TOPOZeo_OriF | Acinetobacter ori | pABBR_MCS | GGATTTTAACATTTTGCGTTG |
| TOPOZeo_OriR | Acinetobacter ori | pABBR_MCS | GATCGTAGAAATATCTATGATTATCTTG |
| pMU125_ZeoR | |||
| ZeoR_F | Zeocin resistance cassette | pCR-Blunt II-TOPO | agcgagtcagtgagcgaggaCGTTGGCTACCCGTGATATT |
| ZeoR_R | Zeocin resistance cassette | pCR-Blunt II-TOPO | ccgcatcaggcgctcttccgGATTAGCAGAGCGAGGTATGTAG |
| pABBR_GFP | |||
| pABBR_GFP_F | eGFP | pMU125 | agcgagtcagtgagcgaggaCCCTTTCGTCTTCAAGAATTCTC |
| pABBR_GFP_R | eGFP | pMU125 | ccgcatcaggcgctcttccgTGAAGGCTCTCAAGGGCATC |
| pABBR_PuroR | |||
| PuroF1 | Linear pBacPuroR-NeoR | pBacPuroR-NeoR | GCGTCAGCGGGTGTTGGC |
| PuroR1 | Linear pBacPuroR-NeoR | pBacPuroR-NeoR | CAGTCATAGCCGAATAGCCTCTCC |
| Puro_OriF1 | Acinetobacter ori | pABBR_MCS | aggctattcggctatgactgGGATTTTAACATTTTGCGTTG |
| Puro_OriR1 | Acinetobacter ori | pABBR_MCS | ccgccaacacccgctgacgcGATCGTAGAAATATCTATGATTATCTTG |
| pBacPuroR_AB | |||
| PuroF2 | Linear pBacPuroR-NeoR | pBacPuroR-NeoR | gaggtgccgccggcttccatTCAGGCACCGGGCTTGCGGGTCA |
| PuroR2 | Linear pBacPuroR-NeoR | pBacPuroR-NeoR | aacgcagtcaggcaccgtgtATGACCGAGTACAAGCCCACGGTGC |
| Puro_OriF2 | Acinetobacter ori | pABBR_MCS | ACACGGTGCCTGACTGCG |
| Puro_OriR2 | Acinetobacter ori | pABBR_MCS | ATGGAAGCCGGCGGCACC |
| Confirmation PCR | |||
| Zeo_Confir_F1 | Zeocin resistance | pCR-Blunt II-TOPO | CGACGTGACCCTGTTCATC |
| Zeo_Confir_R1 | Zeocin resistance | pCR-Blunt II-TOPO | TCGCCGATCTCGGTCAT |
| Zeo_Confir_F2 | Kanamycin resistance | pCR-Blunt II-TOPO | CTTGTCGATCAGGATGATCTGG |
| Zeo_Confir_R2 | Kanamycin resistance | pCR-Blunt II-TOPO | CTCTTCAGCAATATCACGGGTAG |
| Puro_Confir_F1 | Puromycin resistance | pBacPuroR-NeoR | GTCACCGAGCTGCAAGAA |
| Puro_Confir_R1 | Puromycin resistance | pBacPuroR-NeoR | GGCCTTCCATCTGTTGCT |
| Puro_Confir_F2 | Amp resistance | pBacPuroR-NeoR | GCTATGTGGCGCGGTATTAT |
| Puro_Confir_R2 | Amp resistance | pBacPuroR-NeoR | CTCCGATCGTTGTCAGAAGTAAG |
| TetR_ConfirF | Tetracycline resistance | HUMC1 genomic DNA | TAAATCGCCGTGACGATCAG |
| TetR_ConfirR | Tetracycline resistance | pAT04 | GCGAGAAGCAGGCCATTAT |
Uppercase nucleotides represent exact matches to those in the template sequence. Lowercase nucleotides represent nucleotides in the 5′ adapter sequence needed for the Gibson assembly reaction but do not match the nucleotides in the template sequence.
Transformation. (i) Acinetobacter baumannii.
A. baumannii cells were made electrocompetent according to published protocols (36). Briefly, 500 µl of an overnight culture was used to inoculate 50 ml of TSB medium, and the subculture was incubated until it reached an OD600 of 0.5. The cells were pelleted by centrifugation (10 min at 10,000 × g) and washed five times with 1 ml of 10% glycerol. The cells were separated into 100-µl aliquots and stored at −80°C for later use as we have previously described (33).
Plasmid DNA (25 ng) was mixed with electrocompetent cells, and the mixture was incubated on ice for 10 min. The mixture was transferred to a 1-mm cuvette and electroporated at 25 µF, 100 Ω, and 2.5 kV. Following electroporation, 900 µl of superoptimal broth with catabolite repression (SOC) was added to the cuvette, and the cells were transferred to a 2-ml microcentrifuge tube and then incubated in a shaking incubator at 200 rpm and 37°C for 1 h. The cells were then plated on TSA supplemented with 100 µg/ml tetracycline, 250 µg/ml zeocin, or 250 µg/ml puromycin.
(ii) Escherichia coli.
Chemically competent or electrocompetent E. coli DH5α cells were used for the transformations. E. coli DH5α competent cells were made using the Mix & Go E. coli transformation kit per the manufacturer’s suggested protocol (catalog no. T3001; Zymo Research). Briefly, the DNA was incubated with competent cells on ice for 1 h prior to plating on TSA supplemented with 10 µg/ml tetracycline, 25 µg/ml zeocin, 125 µg/ml puromycin, 50 µg/ml kanamycin, or 100 µg/ml ampicillin. The concentration of antibiotics used for selection of E. coli was chosen according to the manufacturer’s directions. Electrocompetent E. coli DH5α cells were prepared using the same methods as described above for A. baumannii.
Construct assembly.
The constructs were assembled using the Gibson assembly method (Fig. 2) (20). Overlap sequences for the vector and insert were determined using the NEBuilder assembly tool (New England BioLabs). Vector backbones were prepared by PCR amplification of plasmid DNA or by restriction enzyme digestion. Assembly of the parts to create the final constructs was accomplished using the NEBuilder HiFi assembly master mix per the manufacturer’s protocol. Briefly, the corresponding linearized vector (100 ng) and insert were added in a 1:2 molar ratio of vector to insert. The linear fragments were incubated with 10 µl of enzyme master mix at 50°C for 15 min. Two microliters of the assembly product was then used for bacterial transformation.
Preparation of the vector backbone and insert sequence for each plasmid were done as follows. For pBacPuroR_AB, the pBacPuroR-NeoR vector backbone was linearized by PCR, and the A. baumannii ori insert sequence was amplified by PCR from pABBR_MCS. For pABBR_PuroR, the pABBR vector backbone was linearized by PCR, and the puromycin resistance cassette insert sequence was amplified by PCR from pBacPuroR-NeoR. For pCR-Blunt II_AB, the pCR-Blunt II_AB vector backbone was linearized by PCR, and the A. baumannii ori insert sequence was amplified by PCR from pABBR_MCS. For pMSG360zeo_AB, the pMSG360zeo vector backbone was linearized by PCR, and A. baumannii ori insert sequence was amplified by PCR from pABBR_MCS. For pABBR_GFP, the linearized vector backbone was prepared by digestion with SapI, and the gfp insert sequence was amplified by PCR from pMU125. For pMU125_ZeoR, the linearized vector backbone was prepared by digestion with SapI, and the zeocin resistance cassette insert was amplified by PCR from pCR-Blunt II-TOPO.
ACKNOWLEDGMENT
This work was supported by NIAID R01 AI117211.
REFERENCES
- 1.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 2.Pendleton JN, Gorman SP, Gilmore BF. 2013. Clinical relevance of the ESKAPE pathogens. Expert Rev Anti Infect Ther 11:297–308. doi: 10.1586/eri.13.12. [DOI] [PubMed] [Google Scholar]
- 3.Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. 2011. Carbapenems: past, present, and future. Antimicrob Agents Chemother 55:4943–4960. doi: 10.1128/AAC.00296-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eliopoulos GM, Maragakis LL, Perl TM. 2008. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis 46:1254–1263. doi: 10.1086/529198. [DOI] [PubMed] [Google Scholar]
- 5.Song JY, Cheong HJ, Choi WS, Heo JY, Noh JY, Kim WJ. 2011. Clinical and microbiological characterization of carbapenem-resistant Acinetobacter baumannii bloodstream infections. J Med Microbiol 60:605–611. doi: 10.1099/jmm.0.029439-0. [DOI] [PubMed] [Google Scholar]
- 6.Spellberg B, Rex JH. 2013. The value of single-pathogen antibacterial agents. Nat Rev Drug Discov 12:963. doi: 10.1038/nrd3957-c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leite GC, Oliveira MS, Perdigão-Neto LV, Rocha CKD, Guimarães T, Rizek C, Levin AS, Costa SF. 2016. Antimicrobial combinations against pan-resistant Acinetobacter baumannii isolates with different resistance mechanisms. PLoS One 11:e0151270. doi: 10.1371/journal.pone.0151270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim W-Y, Moon J-Y, Huh JW, Choi S-H, Lim C-M, Koh Y, Chong YP, Hong S-B. 2016. Comparable efficacy of tigecycline versus colistin therapy for multidrug-resistant and extensively drug-resistant Acinetobacter baumannii pneumonia in critically ill patients. PLoS One 11:e0150642. doi: 10.1371/journal.pone.0150642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tal-Jasper R, Katz DE, Amrami N, Ravid D, Avivi D, Zaidenstein R, Lazarovitch T, Dadon M, Kaye KS, Marchaim D. 2016. Clinical and epidemiological significance of carbapenem resistance in Acinetobacter baumannii infections. Antimicrob Agents Chemother 60:3127–3131. doi: 10.1128/AAC.02656-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luciani F, Sisson SA, Jiang H, Francis AR, Tanaka MM. 2009. The epidemiological fitness cost of drug resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 106:14711–14715. doi: 10.1073/pnas.0902437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deris JB, Kim M, Zhang Z, Okano H, Hermsen R, Groisman A, Hwa T. 2013. The innate growth bistability and fitness landscapes of antibiotic-resistant bacteria. Science 342:1237435. doi: 10.1126/science.1237435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gagneux S, Long CD, Small PM, Van T, Schoolnik GK, Bohannan BJM. 2006. The competitive cost of antibiotic resistance in Mycobacterium tuberculosis. Science 312:1944–1946. doi: 10.1126/science.1124410. [DOI] [PubMed] [Google Scholar]
- 13.Roux D, Danilchanka O, Guillard T, Cattoir V, Aschard H, Fu Y, Angoulvant F, Messika J, Ricard J-D, Mekalanos JJ, Lory S, Pier GB, Skurnik D. 2015. Fitness cost of antibiotic susceptibility during bacterial infection. Sci Transl Med 7:297ra114. doi: 10.1126/scitranslmed.aab1621. [DOI] [PubMed] [Google Scholar]
- 14.Baker S, Duy PT, Nga TVT, Dung TTN, Phat VV, Chau TT, Turner AK, Farrar J, Boni MF. 2013. Fitness benefits in fluoroquinolone-resistant Salmonella Typhi in the absence of antimicrobial pressure. Elife 2:e01229. doi: 10.7554/eLife.01229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin L, Tan B, Pantapalangkoor P, Ho T, Baquir B, Tomaras A, Montgomery JI, Reilly U, Barbacci EG, Hujer K, Bonomo RA, Fernandez L, Hancock REW, Adams MD, French SW, Buslon VS, Spellberg B. 2012. Inhibition of LpxC protects mice from resistant Acinetobacter baumannii by modulating inflammation and enhancing phagocytosis. mBio 3:e00312-12. doi: 10.1128/mBio.00312-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruhn KW, Pantapalangkoor P, Nielsen T, Tan B, Junus J, Hujer KM, Wright MS, Bonomo RA, Adams MD, Chen W, Spellberg B. 2014. Host fate is rapidly determined by innate effector-microbial interactions during Acinetobacter baumannii bacteremia. J Infect Dis 211:1296–1305. doi: 10.1093/infdis/jiu593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs AC, Thompson MG, Black CC, Kessler JL, Clark LP, McQueary CN, Gancz HY, Corey BW, Moon JK, Si Y, Owen MT, Hallock JD, Kwak YI, Summers A, Li CZ, Rasko DA, Penwell WF, Honnold CL, Wise MC, Waterman PE, Lesho EP, Stewart RL, Actis LA, Palys TJ, Craft DW, Zurawski DV. 2014. AB5075, a highly virulent isolate of Acinetobacter baumannii, as a model strain for the evaluation of pathogenesis and antimicrobial treatments. mBio 5:e01076-14. doi: 10.1128/mBio.01076-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. 2013. Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lajoie MJ, Kosuri S, Mosberg JA, Gregg CJ, Zhang D, Church GM. 2013. Probing the limits of genetic recoding in essential genes. Science 342:361–363. doi: 10.1126/science.1241460. [DOI] [PubMed] [Google Scholar]
- 20.Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, Smith HO. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 21.Wetmore KM, Price MN, Waters RJ, Lamson JS, He J, Hoover CA, Blow MJ, Bristow J, Butland G, Arkin AP, Deutschbauer A. 2015. Rapid quantification of mutant fitness in diverse bacteria by sequencing randomly bar-coded transposons. mBio 6:e00306-15. doi: 10.1128/mBio.00306-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cameron DE, Bashor CJ, Collins JJ. 2014. A brief history of synthetic biology. Nat Rev Microbiol 12:381–390. doi: 10.1038/nrmicro3239. [DOI] [PubMed] [Google Scholar]
- 23.van Opijnen T, Bodi KL, Camilli A. 2009. Tn-seq: high-throughput parallel sequencing for fitness and genetic interaction studies in microorganisms. Nat Methods 6:767–772. doi: 10.1038/nmeth.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Opijnen T, Camilli A. 2013. Transposon insertion sequencing: a new tool for systems-level analysis of microorganisms. Nat Rev Microbiol 11:435–442. doi: 10.1038/nrmicro3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Berardinis V, Vallenet D, Castelli V, Besnard M, Pinet A, Cruaud C, Samair S, Lechaplais C, Gyapay G, Richez C, Durot M, Kreimeyer A, Le Fèvre F, Schächter V, Pezo V, Döring V, Scarpelli C, Médigue C, Cohen GN, Marlière P, Salanoubat M, Weissenbach J. 2008. A complete collection of single-gene deletion mutants of Acinetobacter baylyi ADP1. Mol Syst Biol 4:174. doi: 10.1038/msb.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallagher LA, Ramage E, Weiss EJ, Radey M, Hayden HS, Held KG, Huse HK, Zurawski DV, Brittnacher MJ, Manoil C. 2015. Resources for genetic and genomic analysis of emerging pathogen Acinetobacter baumannii. J Bacteriol 197:2027–2035. doi: 10.1128/JB.00131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biswas I. 2015. Genetic tools for manipulating Acinetobacter baumannii genome: an overview. J Med Microbiol 64:657–669. doi: 10.1099/jmm.0.000081. [DOI] [PubMed] [Google Scholar]
- 29.Wang N, Ozer EA, Mandel MJ, Hauser AR. 2014. Genome-wide identification of Acinetobacter baumannii genes necessary for persistence in the lung. mBio 5:e01163-14. doi: 10.1128/mBio.01163-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amin IM, Richmond GE, Sen P, Koh TH, Piddock LJ, Chua KL. 2013. A method for generating marker-less gene deletions in multidrug-resistant Acinetobacter baumannii. BMC Microbiol 13:158. doi: 10.1186/1471-2180-13-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aranda J, Poza M, Pardo BG, Rumbo S, Rumbo C, Parreira JR, Rodríguez-Velo P, Bou G. 2010. A rapid and simple method for constructing stable mutants of Acinetobacter baumannii. BMC Microbiol 10:279. doi: 10.1186/1471-2180-10-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subashchandrabose S, Smith S, DeOrnellas V, Crepin S, Kole M, Zahdeh C, Mobley HLT. 2015. Acinetobacter baumannii genes required for bacterial survival during bloodstream infection. mSphere 1:e00013-15. doi: 10.1128/mSphere.00013-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen TB, Bruhn KW, Pantapalangkoor P, Junus JL, Spellberg B. 2015. Cryopreservation of virulent Acinetobacter baumannii to reduce variability of in vivo studies. BMC Microbiol 15:252. doi: 10.1186/s12866-015-0580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo G, Lin L, Ibrahim AS, Baquir B, Pantapalangkoor P, Bonomo RA, Doi Y, Adams MD, Russo TA, Spellberg B. 2012. Active and passive immunization protects against lethal, extreme drug resistant-Acinetobacter baumannii infection. PLoS One 7:e29446. doi: 10.1371/journal.pone.0029446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hombach M, Courvalin P, Böttger EC. 2014. Validation of antibiotic susceptibility testing guidelines in a routine clinical microbiology laboratory exemplifies general key challenges in setting clinical breakpoints. Antimicrob Agents Chemother 58:3921–3926. doi: 10.1128/AAC.02489-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobs AC, Thompson MG, Gebhardt M, Corey BW, Yildirim S, Shuman HA, Zurawski DV. 2014. Genetic manipulation of Acinetobacter baumannii. Curr Protoc Microbiol 35:6G.2.1–6G.2.11. doi: 10.1002/9780471729259.mc06g02s35. [DOI] [PubMed] [Google Scholar]
- 37.Trebosc V, Gartenmann S, Royet K, Manfredi P, Tötzl M, Schellhorn B, Pieren M, Tigges M, Lociuro S, Sennhenn PC, Gitzinger M, Bumann D, Kemmer C. 2016. A novel genome-editing platform for drug-resistant Acinetobacter baumannii reveals an AdeR-unrelated tigecycline resistance mechanism. Antimicrob Agents Chemother 60:7263–7271. doi: 10.1128/AAC.01275-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keen EF III, Murray CK, Robinson BJ, Hospenthal DR, Co EM, Aldous WK. 2010. Changes in the incidences of multidrug-resistant and extensively drug-resistant organisms isolated in a military medical center. Infect Control Hosp Epidemiol 31:728–732. doi: 10.1086/653617. [DOI] [PubMed] [Google Scholar]
- 39.Palomino JC, Martin A, Camacho M, Guerra H, Swings J, Portaels F. 2002. Resazurin microtiter assay plate: simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 46:2720–2722. doi: 10.1128/AAC.46.8.2720-2722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin A, Camacho M, Portaels F, Palomino JC. 2003. Resazurin microtiter assay plate testing of Mycobacterium tuberculosis susceptibilities to second-line drugs: rapid, simple, and inexpensive method. Antimicrob Agents Chemother 47:3616–3619. doi: 10.1128/AAC.47.11.3616-3619.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Semple JI, Biondini L, Lehner B. 2012. Generating transgenic nematodes by bombardment and antibiotic selection. Nat Methods 9:118–119. doi: 10.1038/nmeth.1864. [DOI] [PubMed] [Google Scholar]
- 42.Barkan D, Rao V, Sukenick GD, Glickman MS. 2010. Redundant function of cmaA2 and mmaA2 in Mycobacterium tuberculosis cis cyclopropanation of oxygenated mycolates. J Bacteriol 192:3661–3668. doi: 10.1128/JB.00312-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tucker AT, Nowicki EM, Boll JM, Knauf GA, Burdis NC, Trent MS, Davies BW. 2014. Defining gene-phenotype relationships in Acinetobacter baumannii through one-step chromosomal gene inactivation. mBio 5:e01313-14. doi: 10.1128/mBio.01313-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dorsey CW, Tomaras AP, Actis LA. 2002. Genetic and phenotypic analysis of Acinetobacter baumannii insertion derivatives generated with a transposome system. Appl Environ Microbiol 68:6353–6360. doi: 10.1128/AEM.68.12.6353-6360.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


