Abstract
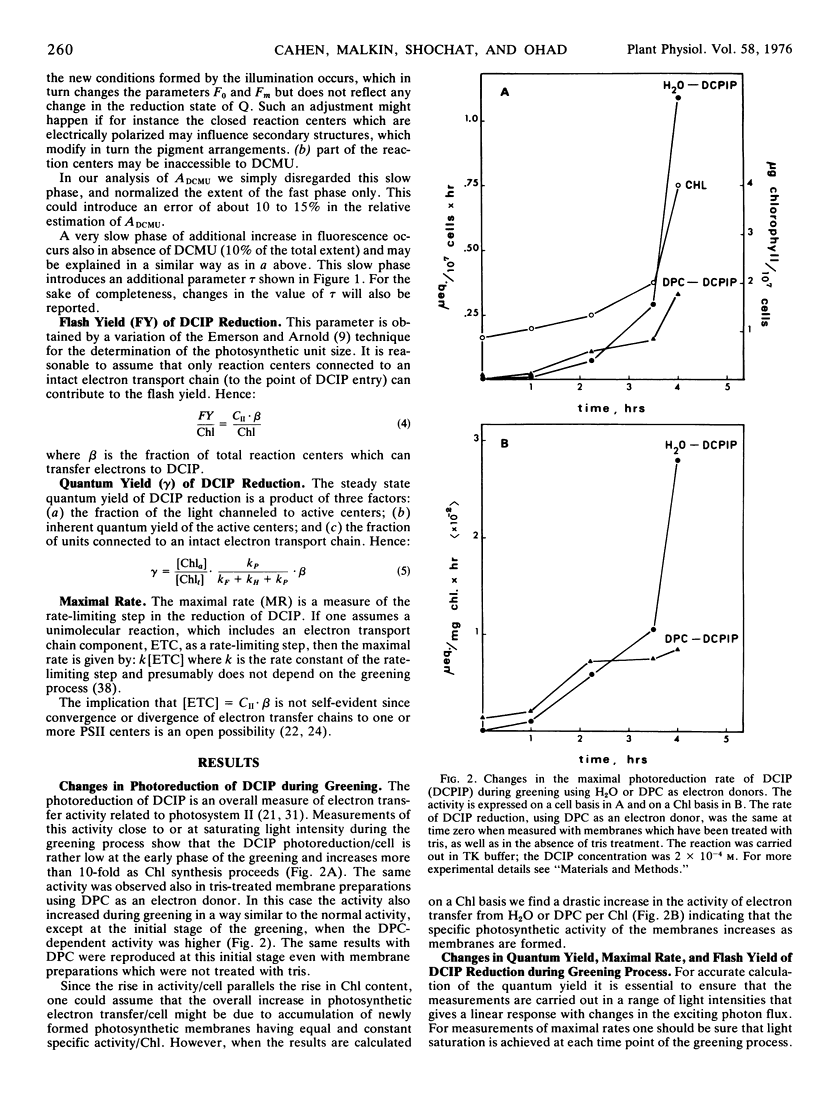
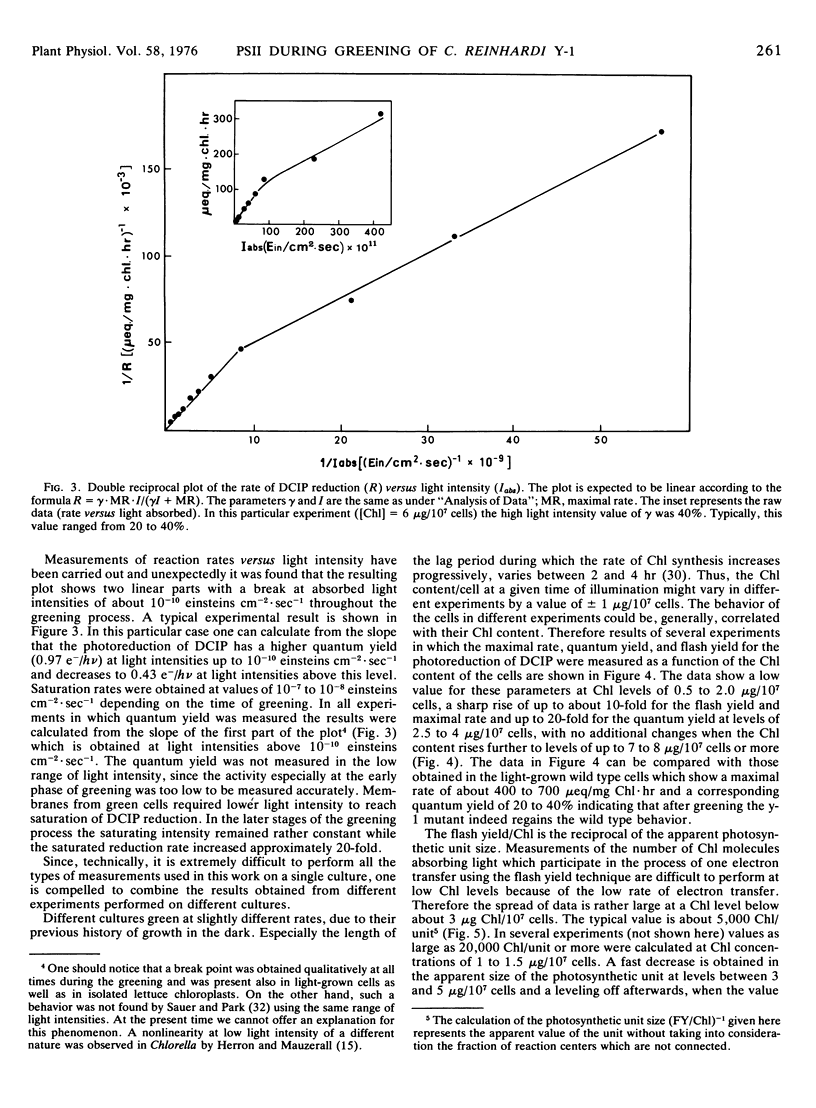
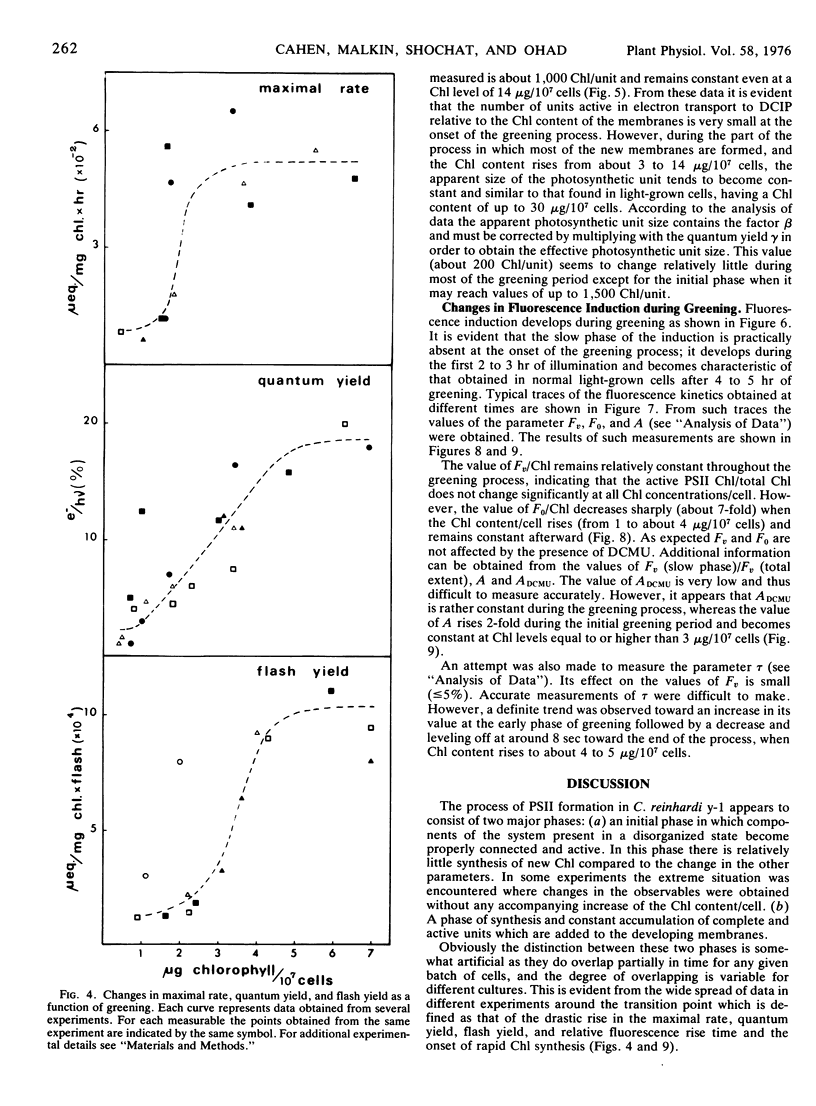
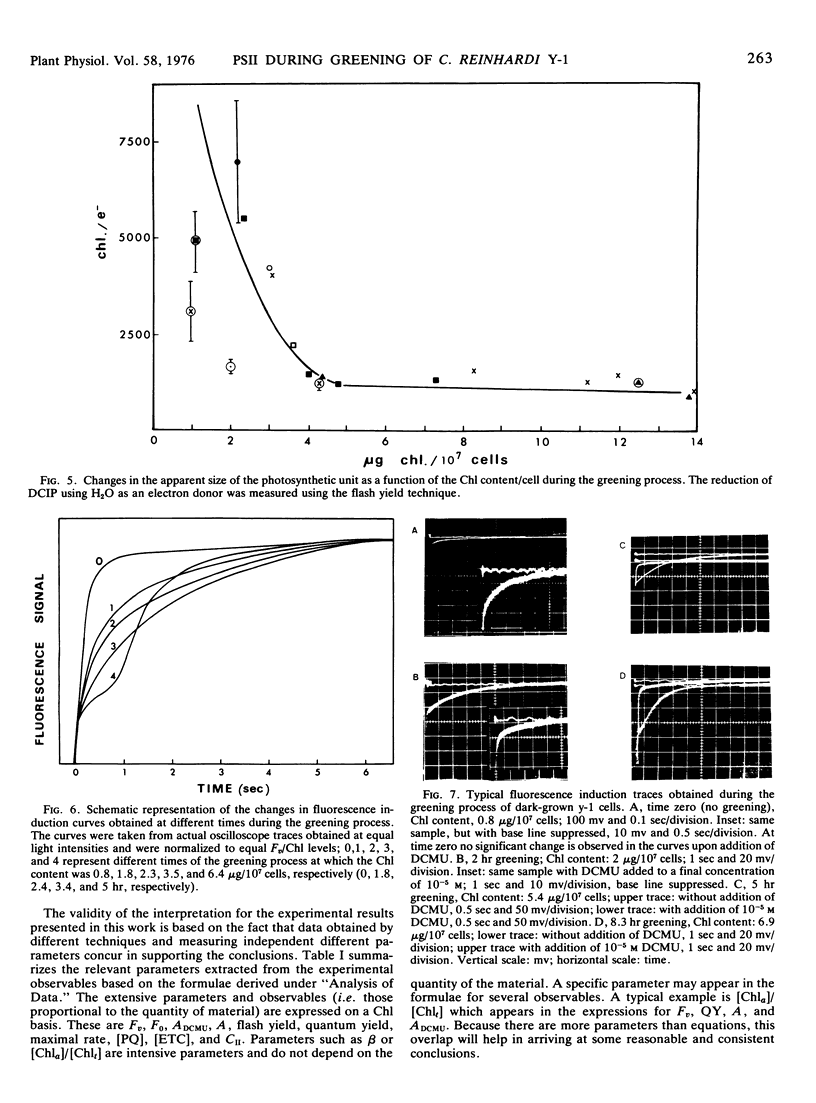
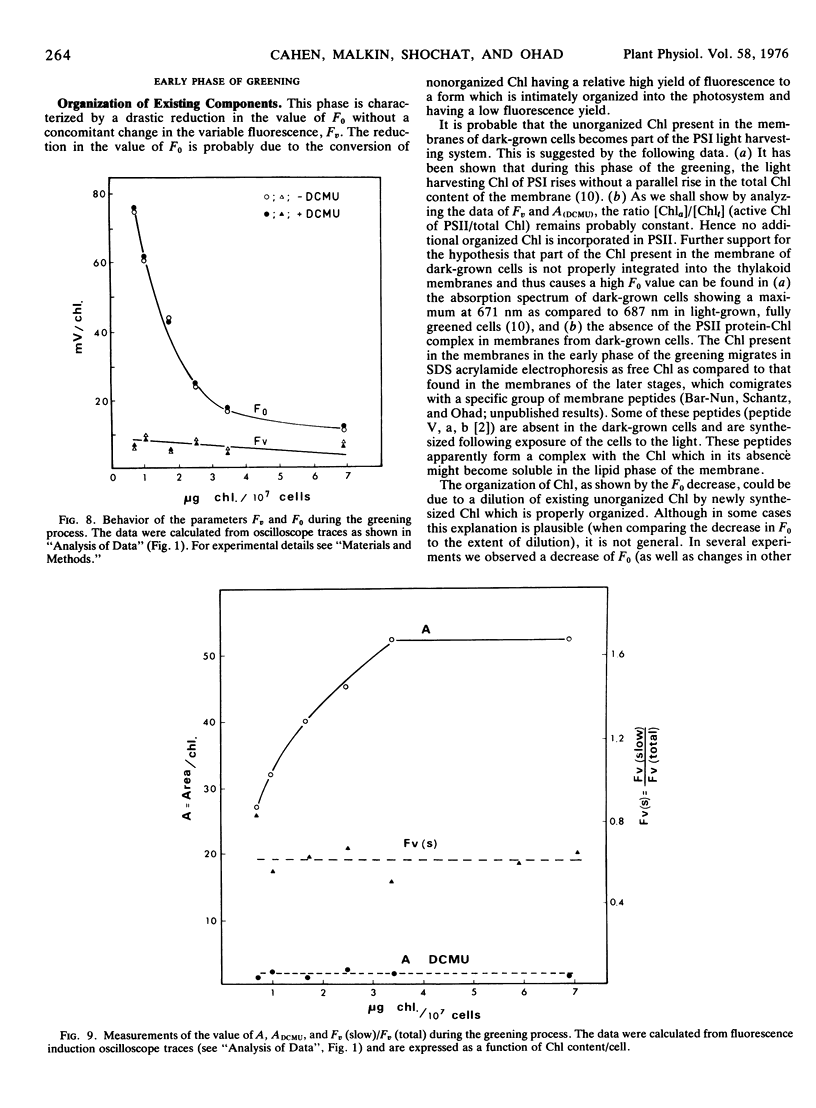
The relative content of organized pigment, active centers, and acceptor pools of photosystem II and their interconnection during the development of the photosynthetic membranes of Chlamydomonas reinhardi y-1 have been measured using the fluorescence induction technique. The degree of connectivity and efficiency of the developing system has been assessed also from measurements of maximal rates, quantum yield, and flash yield of 2,6-dichlorophenolindophenol photoreduction using H2O as the electron donor. The results obtained indicate that the process of membrane development in this organism consists of two phases: an initial phase of reorganization and connection between pre-existing components, and a second phase of actual accumulation of newly formed, complete, and active units. The ratio of active centers to Chl remains practically constant throughout the process while the degree of connectivity between the active center and the plastoquinone pool was doubled during the early phase of the greening. In addition the degree of connectivity between the plastoquinone pool and the rest of the electron transport chain increases as demonstrated by a 10- to 20-fold rise in the quantum yield and a 10-fold rise in the maximal rate and the flash yield. The ratio of light harvesting Chl to active centers remains apparently constant during the second phase of the greening as indicated by light saturation experiments and by the constancy of the apparent photosynthetic unit size. Electron donation from H2O seems to develop slower than the activity of the rest of the complex as demonstrated by measurements of 2,6-dichlorophenolindophenol photoreduction using 1,5-diphenylcarbazide as the electron donor. The value of all the above parameters which remain constant during the second phase of the greening are comparable to those obtained with membranes of light-grown cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Nun S., Wallach D., Ohad I. Biogenesis of chloroplast membranes. X. Changes in the photosynthetic specific activity and the relationship between the light harvesting system and photosynthetic electron transfer chain during greening of Chlamydomonas reinhardi y-1 cells. Biochim Biophys Acta. 1972 Apr 20;267(1):138–148. doi: 10.1016/0005-2728(72)90145-4. [DOI] [PubMed] [Google Scholar]
- Beck D. P., Levine R. P. Synthesis of chloroplast membrane polypeptides during synchronous growth of Chlamydomonas reinhardtii. J Cell Biol. 1974 Dec;63(3):759–772. doi: 10.1083/jcb.63.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton R. K. Characteristics of prompt and delayed fluorescence from spinach chloroplasts. Biophys J. 1969 Jan;9(1):60–76. doi: 10.1016/S0006-3495(69)86369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubertret G., Joliot P. Structure and organization of system II photosynthetic units during the greening of a dark-grown Chlorella mutant. Biochim Biophys Acta. 1974 Sep 20;357(3):399–411. doi: 10.1016/0005-2728(74)90030-9. [DOI] [PubMed] [Google Scholar]
- Eytan G., Jennings R. C., Forti G., Ohad I. Biogenesis of chloroplast membranes. Changes in photosystem I activity and membrane organization during degreening and greening of a Chlamydomonas reinhardi mutant, Y-1. J Biol Chem. 1974 Feb 10;249(3):738–744. [PubMed] [Google Scholar]
- Eytan G., Ohad I. Biogenesis of chloroplast membranes. 8. Modulation of chloroplast lamellae composition and function induced by discontinuous illumination and inhibition of ribonucleic acid and protein synthesis during greening of Chlamydomonas reinhardi y-1 mutant cells. J Biol Chem. 1972 Jan 10;247(1):122–129. [PubMed] [Google Scholar]
- Eytan G., Ohad I. Biogenesis of chloroplast membranes. VI. Cooperation between cytoplasmic and chloroplast ribosomes in the synthesis of photosynthetic lamellar proteins during the greening process in a mutant of Chlamydomonas reinhardi y-1. J Biol Chem. 1970 Sep 10;245(17):4297–4307. [PubMed] [Google Scholar]
- Forbush B., Kok B. Reaction between primary and secondary electron acceptors of photosystem II of photosynthesis. Biochim Biophys Acta. 1968 Aug 20;162(2):243–253. doi: 10.1016/0005-2728(68)90106-0. [DOI] [PubMed] [Google Scholar]
- Goldberg I., Ohad I. Biogenesis of chloroplast membranes. IV. Lipid and pigment changes during synthesis of chloroplast membranes in a mutant of Chlamydomonas reinhardi y-1. J Cell Biol. 1970 Mar;44(3):563–571. doi: 10.1083/jcb.44.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron H. A., Mauzerall D. The development of photosynthesis in a greening mutant of chlorella and an analysis of the light saturation curve. Plant Physiol. 1972 Jul;50(1):141–148. doi: 10.1104/pp.50.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoober J. K., Siekevitz P., Palade G. E. Formation of chloroplast membranes in Chlamydomonas reinhardi y-1. Effects of inhibitors of protein synthesis. J Biol Chem. 1969 May 25;244(10):2621–2631. [PubMed] [Google Scholar]
- Hoober J. K. Sites of synthesis of chloroplast membrane polypeptides in Chlamydomonas reinhardi y-1. J Biol Chem. 1970 Sep 10;245(17):4327–4334. [PubMed] [Google Scholar]
- Kok B., Malkin S., Owens O., Forbush B. Observations on the reducing side of the O2-evolving photoact. Brookhaven Symp Biol. 1966;19:446–459. [PubMed] [Google Scholar]
- Malkin S. Energy transfer in the photosynthetic unit. I. The concept of independent units for photosystem II analyzed by flash yields for dichlorophenolindophenol reduction. Biophys Chem. 1974 Dec;2(4):327–337. doi: 10.1016/0301-4622(74)80059-1. [DOI] [PubMed] [Google Scholar]
- Malkin S., Kok B. Fluorescence induction studies in isolated chloroplasts. I. Number of components involved in the reaction and quantum yields. Biochim Biophys Acta. 1966 Nov 8;126(3):413–432. doi: 10.1016/0926-6585(66)90001-x. [DOI] [PubMed] [Google Scholar]
- Malkin S., Siderer Y. The effect of salt concentration on the fluorescence parameters of isolated chloroplasts. Biochim Biophys Acta. 1974 Dec 19;368(3):422–431. doi: 10.1016/0005-2728(74)90187-x. [DOI] [PubMed] [Google Scholar]
- Ohad I., Siekevitz P., Palade G. E. Biogenesis of chloroplast membranes. I. Plastid dedifferentiation in a dark-grown algal mutant (Chlamydomonas reinhardi). J Cell Biol. 1967 Dec;35(3):521–552. doi: 10.1083/jcb.35.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad I., Siekevitz P., Palade G. E. Biogenesis of chloroplast membranes. II. Plastid differentiation during greening of a dark-grown algal mutant (Chlamydomonas reinhardi). J Cell Biol. 1967 Dec;35(3):553–584. doi: 10.1083/jcb.35.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer K., Park R. B. The Hill reaction of chloroplasts. Action spectra and quantum requirements. Biochemistry. 1965 Dec;4(12):2791–2798. doi: 10.1021/bi00888a032. [DOI] [PubMed] [Google Scholar]
- Schor S., Siekevitz P., Palade G. E. Cyclic Changes in Thylakoid Membranes of Synchronized Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1970 May;66(1):174–180. doi: 10.1073/pnas.66.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner S., Ohad I. Biogenesis of chloroplast membranes. 3. Light-dependent induction of proton pump activity in whole cells and its correlation to cytochrome f photooxidation during greening of a Chlamydomonas reinhardti mutant (y-I). Biochim Biophys Acta. 1969 May;180(1):165–177. doi: 10.1016/0005-2728(69)90203-5. [DOI] [PubMed] [Google Scholar]
- Vernon L. P., Shaw E. R. Photoreduction of 2,6-dichlorophenolindophenol by diphenylcarbazide: a photosystem 2 reaction catalyzed by tris-washed chloroplasts and subchloroplast fragments. Plant Physiol. 1969 Nov;44(11):1645–1649. doi: 10.1104/pp.44.11.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D., Bar-Nun S., Ohad I. Biogenesis of chloroplast membranes. IX. Development of photophosphorylation and proton pump activities in greening Chlamydomonas reinhardi y-I as measured with an open-cell preparation. Biochim Biophys Acta. 1972 Apr 20;267(1):125–137. doi: 10.1016/0005-2728(72)90144-2. [DOI] [PubMed] [Google Scholar]