Abstract
The passive influx and efflux of inorganic ions across plasma membrane vesicles purified from extracts of Avena sativa roots were investigated. Uptake was measured by incubating the vesicles in a radioisotope for various times. The “loaded” vesicles were separated from the external solution by gel filtration. Efflux was measured by dialyzing the preloaded vesicles.
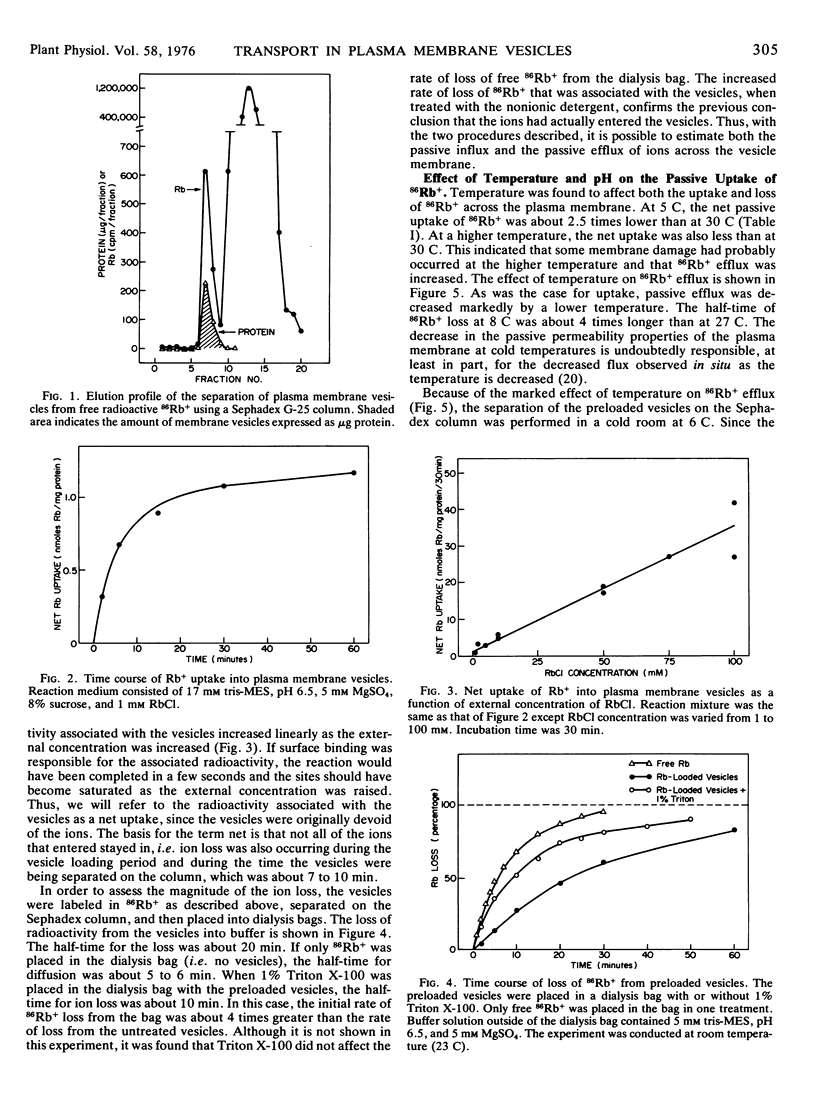
Ion transport was differentiated from superficial ion binding by (a) the time course of association of radioisotope with the vesicles; (b) the rate of loss of radioisotope from the vesicles; (c) the linear increase in isotope associated with the vesicles as the external concentration was increased; (d) the enhanced loss of radioisotope from the vesicles induced by Triton X-100; and (e) the low amount of isotope associated with the vesicles at low temperatures.
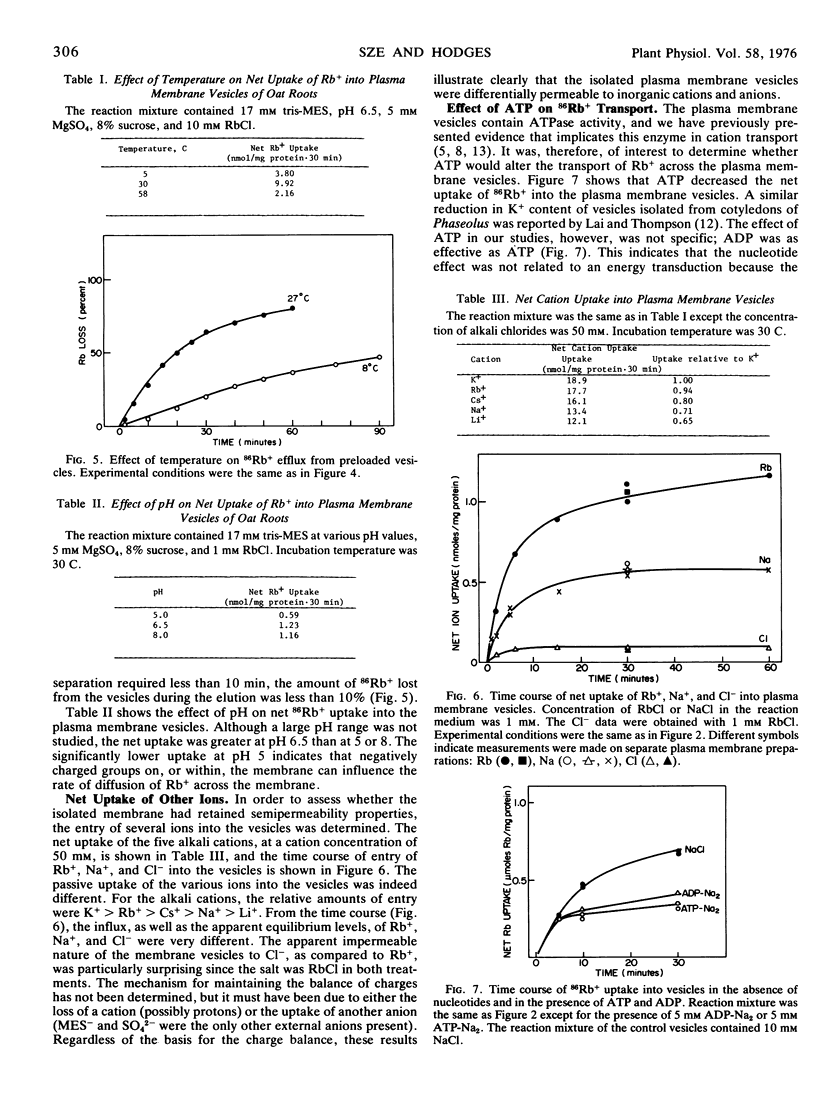
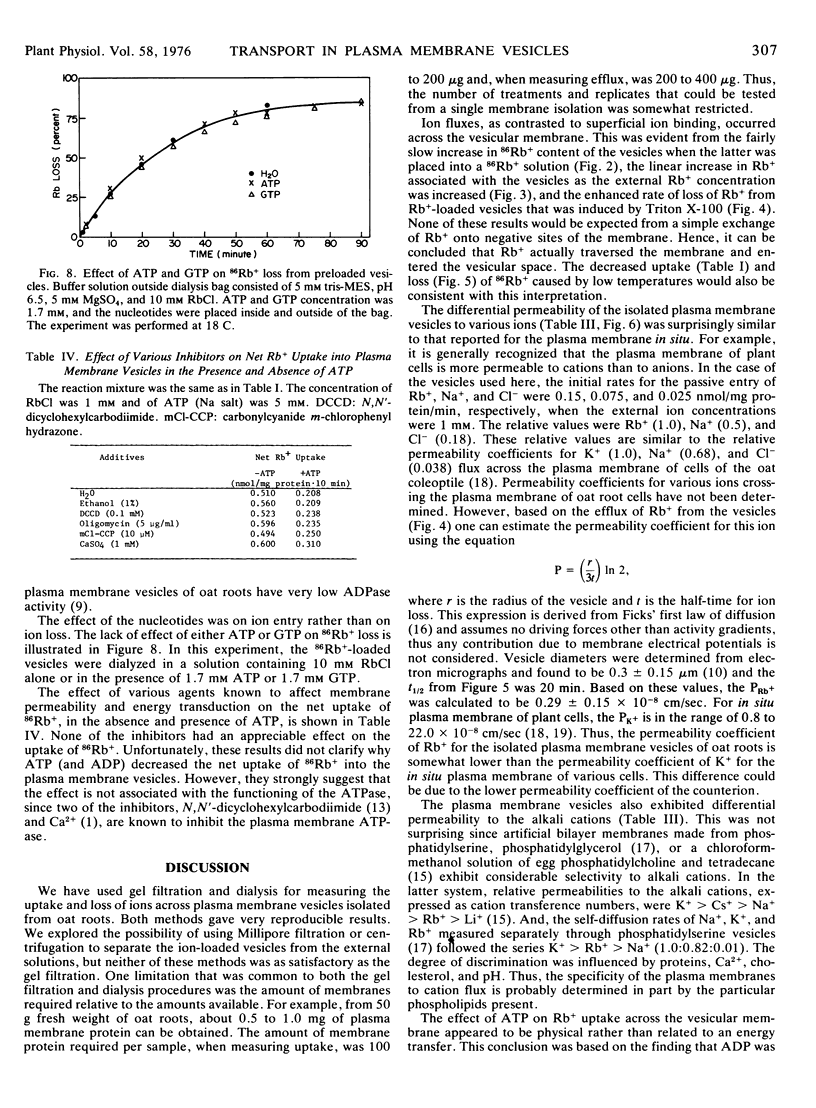
The plasma membrane vesicles were differentially permeable to the alkali cations with the order of decreasing permeation being K+ > Rb+ > Cs+ > Na+ > Li+. The relative transport of Rb+, Na+, and Cl− across the plasma membrane vesicles was about 1.0:0.50:0.18. The permeability coefficient (P) for Rb+ was estimated to be 0.29 ± 0.15 × 10−8 cm/sec.
ATP (and ADP) decreased the passive uptake of Rb+ into the vesicles, however, this effect did not appear to be related to the ATPase of the plasma membrane.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodemann H., Passow H. Factors controlling the resealing of the membrane of human erythrocyte ghosts after hypotonic hemolysis. J Membr Biol. 1972;8(1):1–26. doi: 10.1007/BF01868092. [DOI] [PubMed] [Google Scholar]
- Fisher J. D., Hansen D., Hodges T. K. Correlation between ion fluxes and ion-stimulated adenosine triphosphatase activity of plant roots. Plant Physiol. 1970 Dec;46(6):812–814. doi: 10.1104/pp.46.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin S. M., Tong S. W. Reconstitution of active transport catalyzed by the purified sodium and potassium ion-stimulated adenosine triphosphatase from canine renal medulla. J Biol Chem. 1974 Sep 25;249(18):5907–5915. [PubMed] [Google Scholar]
- Hilden S., Hokin L. E. Active potassium transport coupled to active sodium transport in vesicles reconstituted from purified sodium and potassium ion-activated adenosine triphosphatase from the rectal gland of Squalus acanthias. J Biol Chem. 1975 Aug 25;250(16):6296–6303. [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T., Bracker C. E., Keenan T. W. Purification of an ion-stimulated adenosine triphosphatase from plant roots: association with plasma membranes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3307–3311. doi: 10.1073/pnas.69.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Transport studies in bacterial membrane vesicles. Science. 1974 Dec 6;186(4167):882–892. doi: 10.1126/science.186.4167.882. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lai Y. F., Thompson J. E. Effects of Germination on NA-K-stimulated Adenosine 5'-Triphosphatase and ATP-dependent Ion Transport of Isolated Membranes from Cotyledons. Plant Physiol. 1972 Oct;50(4):452–457. doi: 10.1104/pp.50.4.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hodges T. K. Characterization of Plasma Membrane-associated Adenosine Triphosphase Activity of Oat Roots. Plant Physiol. 1973 Jul;52(1):6–12. doi: 10.1104/pp.52.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papahadjopoulos D. Na + -K + discrimination by "pure" phospholipid membranes. Biochim Biophys Acta. 1971 Jul 6;241(1):254–259. doi: 10.1016/0005-2736(71)90323-3. [DOI] [PubMed] [Google Scholar]
- Pierce W. S., Higinbotham N. Compartments and Fluxes of K, NA, and CL in Avena Coleoptile Cells. Plant Physiol. 1970 Nov;46(5):666–673. doi: 10.1104/pp.46.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]


