Abstract
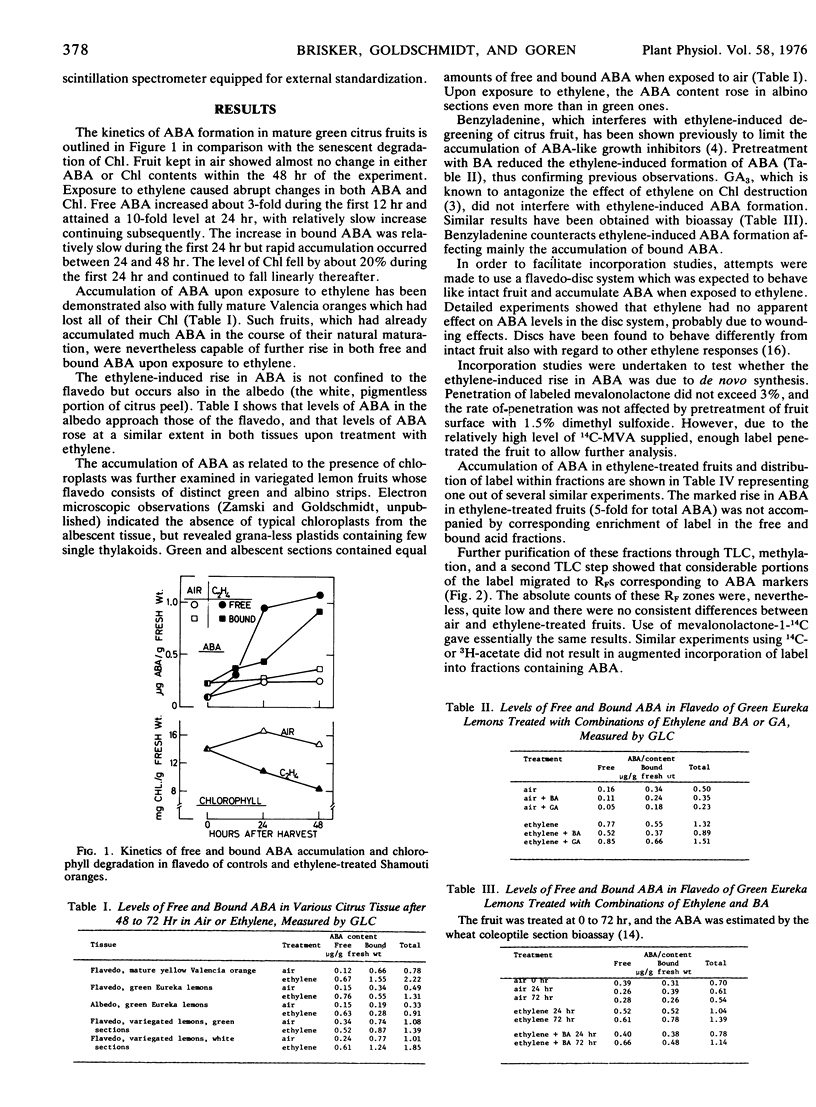
When mature green harvested Shamouti oranges (Citrus sinensis L. Osbeck) were exposed to 35 μl/liter of ethylene, a 3-fold increase in free abscisic acid (ABA) of the flavedo could be detected after 12 hours and a 10-fold increase after 24 hours, while chlorophyll destruction did not exceed 20%. The increase in free ABA continued up to 24 hours and leveled off. Bound ABA accumulated strongly after 24 hours suggesting that excess of free ABA was being converted into the bound form. Similar increases in ABA upon exposure to ethylene occurred also in fully mature orange fruits which had already lost all of their chlorophyll, in white and green portions of the flavedo of variegated lemons, and in the colorless albedo of Eureka lemons.
The synthetic cytokinin benzyladenine which retards chlorophyll breakdown delayed the ethylene-induced accumulation of free and bound ABA. GA3 had no effect on endogenous ABA levels.
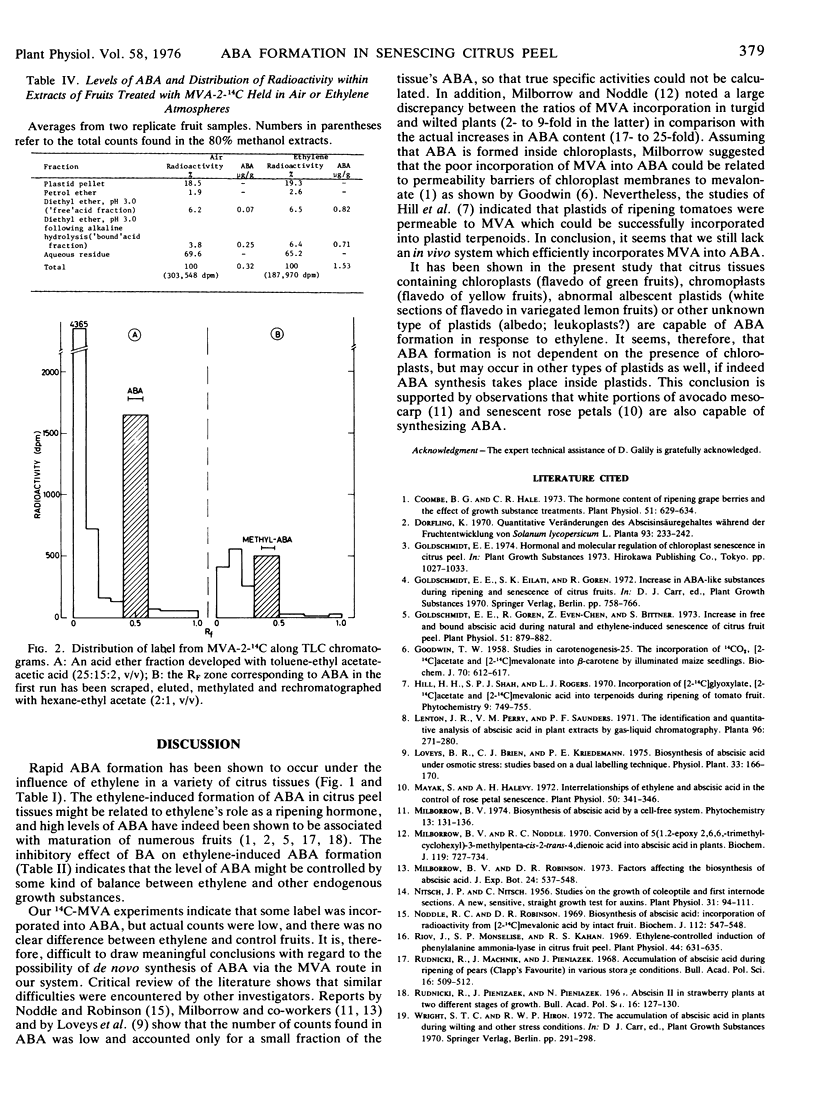
Application of 14C-2-mevalonolactone to fruits resulted in low but distinct labeling of RF zones corresponding to ABA markers, in both free and bound acid fractions. However, there were no significant differences in patterns of incorporation between ethylene-treated and control fruits.
The results indicate that ABA accumulates in citrus peel upon exposure to ethylene irrespective of the type of plastids present in the tissue. The possible role of chloroplasts in ABA formation is discussed.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coombe B. G., Hale C. R. The hormone content of ripening grape berries and the effects of growth substance treatments. Plant Physiol. 1973 Apr;51(4):629–634. doi: 10.1104/pp.51.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODWIN T. W. Studies in carotenogenesis. 25. The incorporation of 14CO2, [2-14C] acetate and [2-14C]mevalonate into beta-carotene by illuminated etiolated maize seedings. Biochem J. 1958 Dec;70(4):612–617. doi: 10.1042/bj0700612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt E. E., Goren R., Even-Chen Z., Bittner S. Increase in Free and Bound Abscisic Acid during Natural and Ethylene-induced Senescence of Citrus Fruit Peel. Plant Physiol. 1973 May;51(5):879–882. doi: 10.1104/pp.51.5.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayak S., Halevy A. H. Interrelationships of ethylene and abscisic Acid in the control of rose petal senescence. Plant Physiol. 1972 Sep;50(3):341–346. doi: 10.1104/pp.50.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milborrow B. V., Noddle R. C. Conversion of 5-(1,2-epoxy-2,6,6-trimethylcyclohexyl)-3-methylpenta-cis-2-trans-4-dienoic acid into abscisic acid in plants. Biochem J. 1970 Oct;119(4):727–734. doi: 10.1042/bj1190727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noddle R. C., Robinson D. R. Biosynthesis of abscisic acid: incorporation of radioactivity from [2-14C]mevalonic acid by intact fruit. Biochem J. 1969 May;112(4):547–548. doi: 10.1042/bj1120547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riov J., Monselise S. P., Kahan R. S. Ethylene-controlled Induction of Phenylalanine Ammonia-lyase in Citrus Fruit Peel. Plant Physiol. 1969 May;44(5):631–635. doi: 10.1104/pp.44.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki R., Pieniazek J., Pieniazek N. Abscisin II in strawberry plants at two different stages of growth. Bull Acad Pol Sci Biol. 1968;16(2):127–130. [PubMed] [Google Scholar]


