To the Editor:
Alterations in sphingolipids have been associated with chronic lung diseases (1). A role for altered sphingolipid metabolism in asthma pathogenesis is suggested by single-nucleotide polymorphisms in the most replicated gene locus associated with childhood asthma, 17q21, which includes the gene for ORM-like protein isoform 3, a regulator of sphingolipid synthesis (2). Also, decreased sphingolipid synthesis is associated with airway hyperreactivity (3).
Childhood asthma includes multiple disease phenotypes, the disentangling of which would lead to better understanding of the underlying etiologies and potential treatments. Exercise-induced wheezing (EIW) in school-aged children has been identified as an asthma phenotype that includes both atopic and nonatopic asthma (4). Because many school-aged children with asthma will not continue to have asthma symptoms, identifying risk factors that predict persistence of wheezing has been challenging.
We hypothesized that serum sphingolipids would be altered with asthma symptoms, EIW, and asthma persistence among a case–control study of children with and without asthma, the New York City Neighborhood Asthma and Allergy Study, described previously (4). Briefly, during home visits, spirometry, fractional exhaled nitric oxide testing, and waist circumference measurements were conducted and serum was collected in 7- to 8-year-old children. Three years after enrollment, parents of all the willing subjects with asthma were queried about ongoing asthma symptoms.
Children were classified as cases on the basis of symptoms or asthma medication use in the past year (4). Children classified as a case at baseline (age 7–8 yr) were considered to have asthma persistence if they again met the case definition at age 10 to 11 years. EIW was defined as an affirmative response to questions about (1) wheezing after running or active play, and/or (2) child’s chest sounding wheezy during or after exercise in the past 12 months. Children with specific IgE greater than or equal to 0.35 IU/ml against any of the inhalant allergens tested were considered seroatopic. Sphingolipids were analyzed by mass spectrometry (5).
Multivariable analyses to examine asthma outcomes (dependent variables) were conducted using logistic regression. Variables conventionally considered as potential confounders were included in the multivariable models, including sex, race, Hispanic ethnicity, maternal asthma, environmental tobacco smoke, and material hardship.
Serum was available for 243 children at age 7 to 8 years, of whom 152 were classified as having asthma. Of those 152 subjects with asthma at age 7 to 8 years, 147 participated in the follow-up at age 10 to 11 years. Of those 147 children meeting the case definition of asthma at age 7 to 8 years, 102 (69%) also met that definition 3 years later and were considered to have asthma persistence between ages 7 to 8 and 10 to 11 years. Clinically, children with asthma had mostly mild disease with relatively normal lung function; however, they had significantly lower FEV1/FVC, higher total IgE, and were more likely to be seroatopic than the children without asthma. Children included in these analyses did not differ significantly from those lost to follow-up.
Seventeen sphingolipid species were measurable in serum. Dihydroceramides C16 and C18:1 and long-chain bases sphinganine and sphingosine were below the limit of detection in most samples and were excluded from analyses.
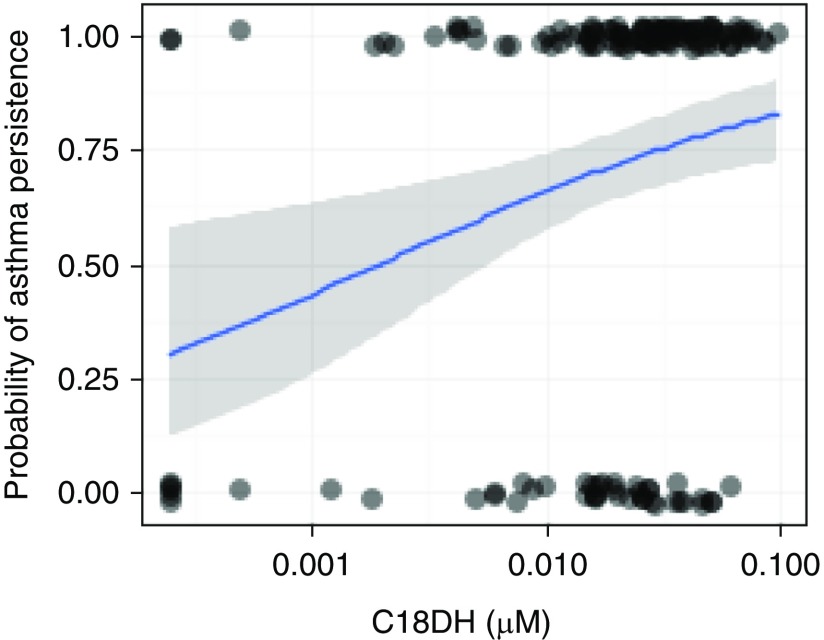
In multivariable analyses, there were no statistically significant differences in sphingolipid concentrations among the children with and without asthma (Table 1). Among the children with asthma, concentrations of dihydroceramide C18 (DHC18); ceramides C18, C20, C24, and deoxy C24:1; and sphingomyelin SM24:1 were higher among those with EIW than among those without EIW. Asthma persistence between ages 7 to 8 years and 10 to 11 years was associated with increased serum DHC18 and C20:0 (Figure 1).
Table 1.
Odds Ratio for Asthma Outcomes with Each Interquartile Range Increase in Serum Sphingolipid
| Cases vs. Control Subjects (n = 243) | EIW vs. Non-EIW (Cases and Control Subjects)* (n = 243) | EIW vs. Non-EIW (Cases)† (n = 152) | Asthma Persistence vs. Remission (n = 147) | |
|---|---|---|---|---|
| Dihydroceramides (d18:0) | ||||
| DHC18 | 1.2 (0.82–1.7) | 1.7‡ (1.1–2.5) | 1.9‡ (1.1–3.0) | 2.3§ (1.3–4.4) |
| DHC24:1 | 0.88 (0.61–1.3) | 1.3 (0.88–2.0) | 1.7 (0.97–2.9) | 1.3 (0.70–2.4) |
| DHC24 | 0.96 (0.68–1.4) | 1.3 (0.87–1.8) | 1.4 (0.87–2.2) | 1.0 (0.60–1.7) |
| Ceramides (d18:1) | ||||
| C16 | 1.2 (0.78–1.8) | 1.6‡ (1.05–2.4) | 1.7 (0.99–2.8) | 1.5 (0.80–2.7) |
| C18 | 1.1 (0.76–1.5) | 1.5 (1.0–2.2) | 1.7‡ (1.1–2.9) | 1.3 (0.78–2.2) |
| C20 | 0.86 (0.59–1.2) | 1.5 (0.99–2.2) | 1.9‡ (1.2–3.1) | 1.8‡ (1.0–3.1) |
| C22 | 0.87 (0.59–1.3) | 1.2 (0.77–1.8) | 1.3 (0.78–2.3) | 1.5 (0.84–2.8) |
| C24 | 0.92 (0.66–1.3) | 1.4 (0.95–2.0) | 1.8‡ (1.1–3.0) | 1.1 (0.62–1.9) |
| C24:1 | 0.81 (0.52–1.3) | 1.1 (0.71–1.8) | 1.4 (0.78–2.5) | 1.6 (0.81–3.1) |
| Long-chain bases (d18:1) | ||||
| S1p | 1.4 (0.89–2.4) | 1.2 (0.78–2.2) | 1.1 (0.61–1.9) | 1.5 (0.72–2.9) |
| Sa1p | 1.3 (0.80–2.0) | 1.2 (0.78–2.0) | 1.1 (0.69–1.9) | 1.7 (0.92–3.3) |
| Sphingomyelins (d18:1) | ||||
| SM C16 | 0.99 (0.68–1.4) | 1.2 (0.80–1.9) | 1.2 (0.73–1.9) | 1.7 (0.96–3.0) |
| SM C18 | 1.1 (0.75–1.7) | 1.3 (0.84–2.1) | 1.3 (0.77–2.2) | 1.6 (0.86–2.9) |
| SM C18:1 | 1.2 (0.81–1.7) | 1.5 (0.94–2.3) | 1.4 (0.85–2.4) | 1.3 (0.74–2.3) |
| SM C24:1 | 1.0 (0.66–1.5) | 1.6 (1.0–2.5) | 1.7‡ (1.0–3.0) | 1.5 (0.83–2.8) |
| Alanine-derived ceramides (m18:1) | ||||
| DeoxyC24:1 | 0.93 (0.68–1.3) | 1.3 (0.94–1.9) | 1.7‡ (1.04–2.7) | 1.0 (0.62–1.8) |
Definition of abbreviation: EIW = exercise-induced wheezing.
Adjusted for sex, race, Hispanic ethnicity, seroatopy, season of serum collection, assay batch, and number of freeze/thaws of serum before analyses. To standardize across sphingolipids, odds ratios were calculated for an interquartile range increase in sphingolipid.
Analyses were conducted comparing the children with EIW to those without EIW. The group without EIW included cases with asthma and control subjects without asthma.
Analyses were conducted comparing the children with EIW to those with asthma without EIW. The group without EIW only included subjects with asthma and did not include control subjects without asthma.
P < 0.05.
P < 0.01.
Figure 1.
Association between dihydroceramide C18 (C18DH) and asthma persistence. The blue line represents logistic regression estimated odds ratio, with the 95% confidence interval in gray. The dots along the top and bottom of the figure represent individual children. Those in line with 0 on the y-axis did not have persistence, whereas those in line with 1 had asthma that persisted. P = 0.005 for unadjusted model (depicted), and P = 0.008 for the adjusted model described in Table 1.
To our knowledge, this is the first study to evaluate the association between serum sphingolipid profiles and asthma in children. We identified a distinct serum dihydroceramide and ceramide profile associated with EIW and found that elevated concentrations of DHC18 and C20:0 at age 7 to 8 years predicted asthma persistence to age 10 to 11 years. These associations were not altered by seroatopy, suggesting a mechanism independent of allergic sensitization. This is consistent with the observation that nonallergic childhood asthma was the major phenotype found associated with single-nucleotide polymorphisms that affect expression of ORM-like protein isoform 3 (6). Although it is not known whether serum sphingolipid profiles reflect those tissues of the respiratory tract, these results strongly support that altered systemic sphingolipid metabolism is present with EIW and that increased serum DHC18 and C20 may predict asthma persistence. We acknowledge the possibility of spurious findings, given the number of sphingolipids tested in these analyses. As such, these findings must be interpreted with caution. Still, we believe that these findings are compelling for replication in future studies.
All serum sphingolipids that distinguished EIW and asthma persistence were higher than levels measured among control subjects. Notably, these serum signatures are opposite from what we found in murine models with impaired de novo sphingolipid synthesis demonstrating airway hyperreactivity. In this mouse model, genetic or pharmacologic inhibition of serine palmitoyltransferase, the rate-limiting step of de novo sphingolipid synthesis, results in airway hyperreactivity and is associated with decreased dihydroceramide and ceramide levels in the lungs (3). In the current study, sphingolipid synthesis cannot be directly assessed, as sphinganine (a specific product of de novo sphingolipid synthesis) was not measurable. The maintenance of ceramide and dihydroceramide levels is complex and subject to compensatory regulation. De novo sphingolipid synthesis may have only minor contributions to the total serum sphingolipid pool, with other regulatory feedback mechanisms involving ceramide synthases and sphingosine kinase playing a role. It is therefore possible that dihydroceramides and ceramides in serum originate from recycling pathways that are up-regulated when serine palmitoyltransferase is inhibited (7).
Overall, the alterations of distinct sphingolipid species in the serum of children with an asthma phenotype not defined by allergic sensitization suggest that sphingolipids are associated with childhood asthma. This metabolic pathway should be subject to further exploration for improved identification and characterization of asthma phenotypes and as a potential target for therapy.
Acknowledgments
Acknowledgment
The authors thank the New York City Neighborhood Asthma and Allergy Study field staff and the families who have participated in the study. They also thank Christine and Pasco Alfaro for their generous support.
Footnotes
Supported by the National Institute of Environmental Health Sciences grants R01 ES 014400 and P30 ES 009089, Housing and Urban Development Healthy Homes Technical Studies grant NYHHU 0003-11, Columbia University’s Department of Pathology and Cell Biology (T.S.W. and B.I.K.), and the National Center for Advancing Translational Sciences grant KL2TR000458 (J.G.O.).
Author Contributions: M.S.P., S.W., and T.S.W. contributed to the conception and design of the study and analyses and interpretation of data and were involved in drafting, revising, and final approval of the manuscript. J.G.O. contributed to the analyses and interpretation of data and was involved in revising and final approval of the manuscript. L.M.A. contributed to the acquisition, analyses, and interpretation of data and was involved in drafting, revising, and final approval of the manuscript. B.I.K. and A.D. contributed to the acquisition of data and were involved in revising and final approval of the manuscript. R.M. and A.R. contributed to the conception and design of the study and analyses and interpretation of data and were involved in revising and final approval of the manuscript.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Petrache I, Berdyshev EV. Ceramide signaling and metabolism in pathophysiological states of the lung. Annu Rev Physiol. 2016;78:463–480. doi: 10.1146/annurev-physiol-021115-105221. [DOI] [PubMed] [Google Scholar]
- 2.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, von Mutius E, Farrall M, Lathrop M, Cookson WO GABRIEL Consortium. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Worgall TS, Veerappan A, Sung B, Kim BI, Weiner E, Bholah R, Silver RB, Jiang XC, Worgall S. Impaired sphingolipid synthesis in the respiratory tract induces airway hyperreactivity. Sci Transl Med. 2013;5:186ra67. doi: 10.1126/scitranslmed.3005765. [DOI] [PubMed] [Google Scholar]
- 4.Mainardi TR, Mellins RB, Miller RL, Acosta LM, Cornell A, Hoepner L, Quinn JW, Yan B, Chillrud SN, Olmedo OE, et al. Exercise-induced wheeze, urgent medical visits, and neighborhood asthma prevalence. Pediatrics. 2013;131:e127–e135. doi: 10.1542/peds.2012-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bui HH, Leohr JK, Kuo MS. Analysis of sphingolipids in extracted human plasma using liquid chromatography electrospray ionization tandem mass spectrometry. Anal Biochem. 2012;423:187–194. doi: 10.1016/j.ab.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 6.Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, Depner M, von Berg A, Bufe A, Rietschel E, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448:470–473. doi: 10.1038/nature06014. [DOI] [PubMed] [Google Scholar]
- 7.Oyeniran C, Sturgill JL, Hait NC, Huang WC, Avni D, Maceyka M, Newton J, Allegood JC, Montpetit A, Conrad DH, et al. Aberrant ORM (yeast)-like protein isoform 3 (ORMDL3) expression dysregulates ceramide homeostasis in cells and ceramide exacerbates allergic asthma in mice. J Allergy Clin Immunol. 2015;136:1035–1046.e6. doi: 10.1016/j.jaci.2015.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]