Abstract
An enzymic preparation from peas (Pisum satisum), able to form neutral and polar glycosides, is described. The best sugar donor is UDP-glucose and the acceptors are present in the enzymic system.
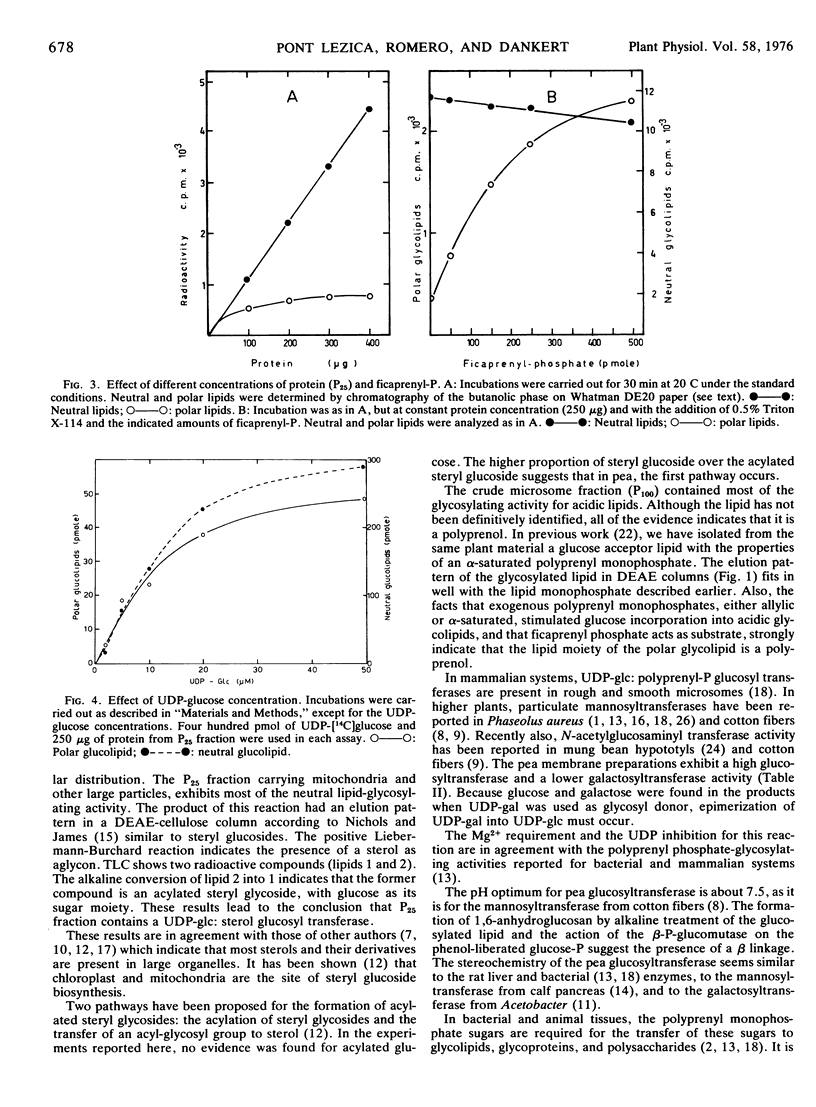
The neutral glycolipids have been characterized as steryl and acylated steryl glucosides. The polar glucolipid had been identified as polyprenyl monophosphate glucose. The glucose is linked to the phosphate in β configuration. Polar glucolipids are also formed from UDP-glucose and exogenous prenylic acceptors, either α-saturated, as dolichyl monophosphate, or allylic, as ficaprenyl monophosphate.
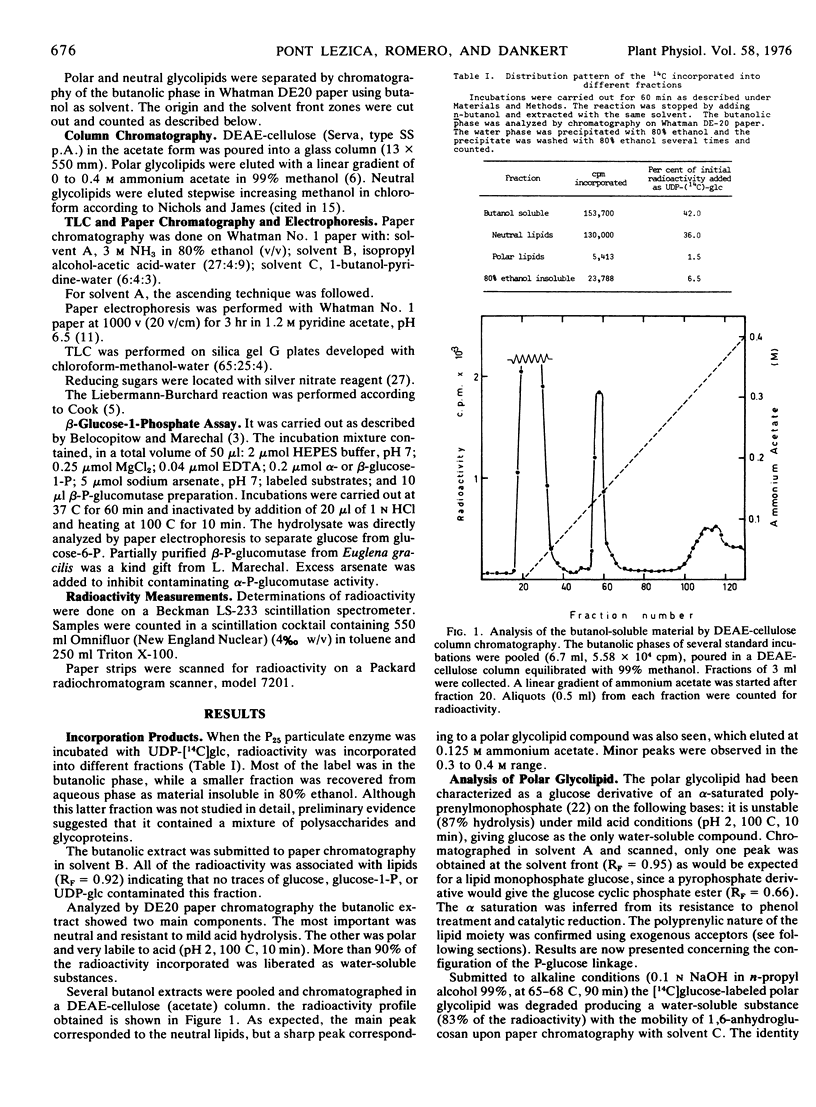
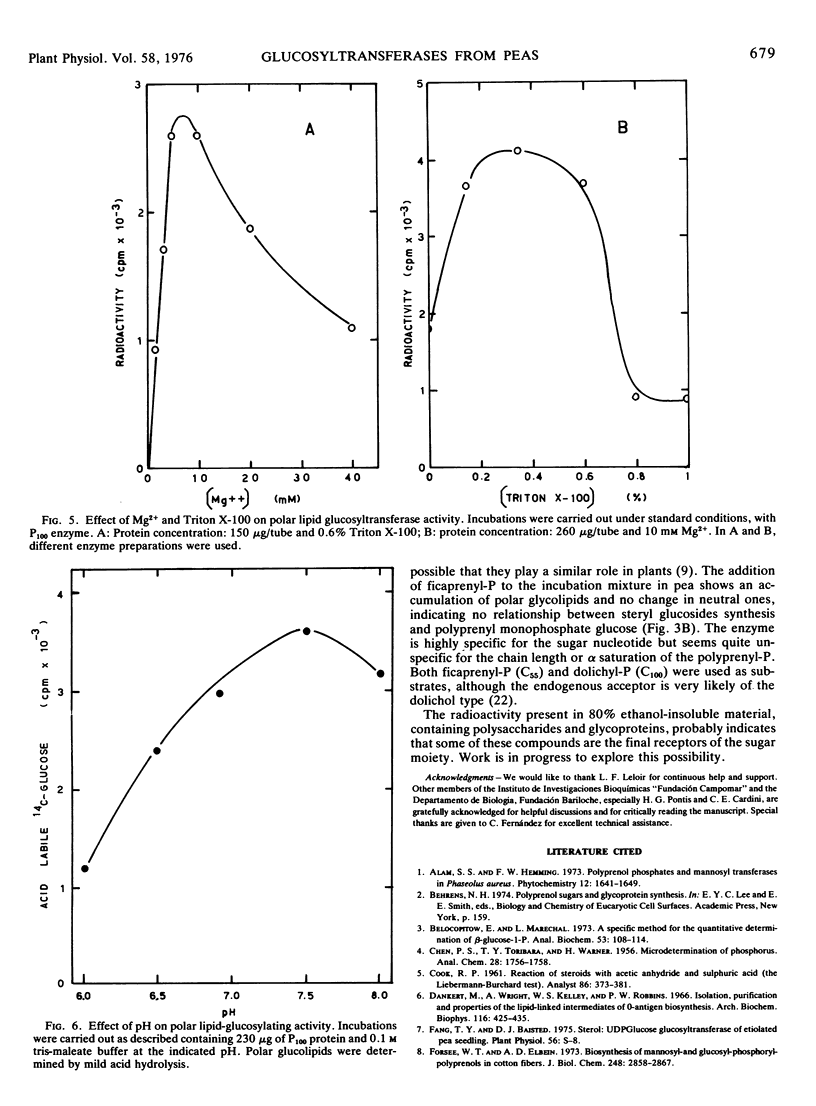
The two glucosylating activities have been partially separated by differential centrifugation: the fraction that precipitates at 25,000g has most of the neutral glucolipid-synthesizing activity, and the fraction that sediments at 100,000g is rich in polar glucolipid glucosyl transferase activity. This latter activity was strongly dependent on Mg2+ concentration, the optimum being around 5 to 10 mm. UDP inhibits the reaction and 0.2 to 0.5% (v/v) Triton X-100 has a stimulatory effect. The optimum pH is 7.5.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dankert M., Wright A., Kelley W. S., Robbins P. W. Isolation, purification, and properties of the lipid-linked intermediates of O-antigen biosynthesis. Arch Biochem Biophys. 1966 Sep 26;116(1):425–435. doi: 10.1016/0003-9861(66)90049-x. [DOI] [PubMed] [Google Scholar]
- Forsee W. T., Elbein A. D. Glycoprotein biosynthesis in plants. Demonstration of lipid-linked oligosaccharides of mannose and N-acetylglucosamine. J Biol Chem. 1975 Dec 25;250(24):9283–9293. [PubMed] [Google Scholar]
- García R. C., Recondo E., Dankert M. Polysaccharide biosynthesis in Acetobacter xylinum. Enzymatic synthesis of lipid diphosphate and monophospate sugars. Eur J Biochem. 1974 Mar 15;43(1):93–105. doi: 10.1111/j.1432-1033.1974.tb03389.x. [DOI] [PubMed] [Google Scholar]
- Herscovics A., Warren C. D., Jeanloz R. W. Anomeric configuration of the dolichyl D-mannosyl phosphate formed in calf pancreas microsomes. J Biol Chem. 1975 Oct 25;250(20):8079–8084. [PubMed] [Google Scholar]
- Kauss H. A plant mannosyl-lipid acting in reversible transfer of mannose. FEBS Lett. 1969 Sep;5(1):81–84. doi: 10.1016/0014-5793(69)80298-x. [DOI] [PubMed] [Google Scholar]
- Lavintman N., Cardini C. E. Biosynthesis of a glycolipid in starch grains from sweet corn. Biochim Biophys Acta. 1970 Mar 24;201(3):508–510. doi: 10.1016/0304-4165(70)90175-3. [DOI] [PubMed] [Google Scholar]
- Lennarz W. J., Scher M. G. Metabolism and function of polyisoprenol sugar intermediates in membrane-associated reactions. Biochim Biophys Acta. 1972 Aug 4;265(3):417–441. doi: 10.1016/0304-4157(72)90015-9. [DOI] [PubMed] [Google Scholar]
- Lezica R. P., Brett C. T., Martinez P. R., Dankert M. A. A glucose acceptor in plants with the properties of an alpha-saturated polyprenyl-monophosphate. Biochem Biophys Res Commun. 1975 Oct 6;66(3):980–987. doi: 10.1016/0006-291x(75)90736-6. [DOI] [PubMed] [Google Scholar]
- POPJAK G., CORNFORTH J. W., CORNFORTH R. H., RYHAGE R., GOODMAN D. S. Studies on the biosynthesis of cholesterol. XVI. Chemical synthesis of 1-H2-3-2-C-14- and 1-D2-2-C-14-trans-trans-farnesyl pyrophosphate and their utilization in squalene biosynthesis. J Biol Chem. 1962 Jan;237:56–61. [PubMed] [Google Scholar]
- Roberts R. M., Pollard W. E. The Incorporation of d-Glucosamine into Glycolipids and Glycoproteins of Membrane Preparations from Phaseolus aureus Hypocotyls. Plant Physiol. 1975 Mar;55(3):431–436. doi: 10.1104/pp.55.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone K. J., Wellburn A. R., Hemming F. W., Pennock J. F. The characterization of ficaprenol-10, -11 and 12 from the leaves of Ficus elastica (decorative rubber plant). Biochem J. 1967 Jan;102(1):325–330. doi: 10.1042/bj1020325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm D. L., Hassid W. Z. The Role of a d-Mannosyl-Lipid as an Intermediate in the Synthesis of Polysaccharide in Phaseolus aureus Seedlings. Plant Physiol. 1972 Oct;50(4):473–476. doi: 10.1104/pp.50.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]