Abstract
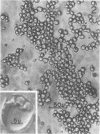
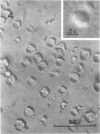
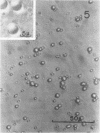
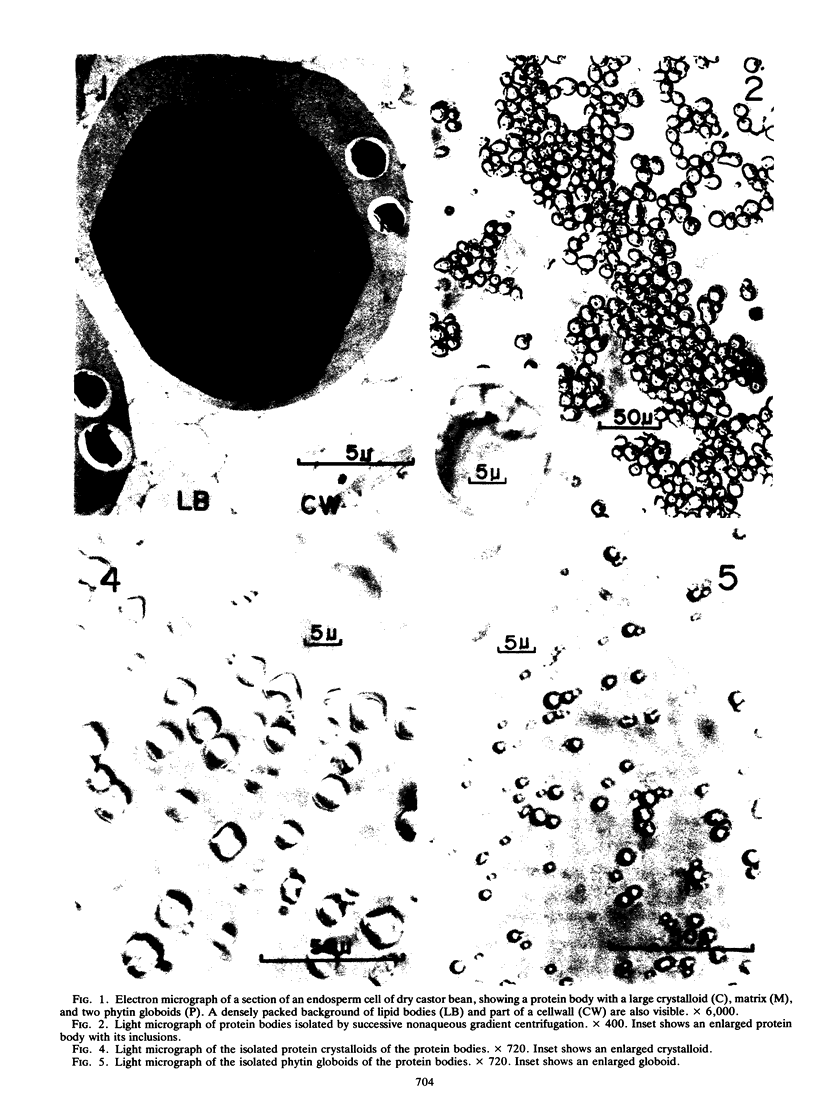
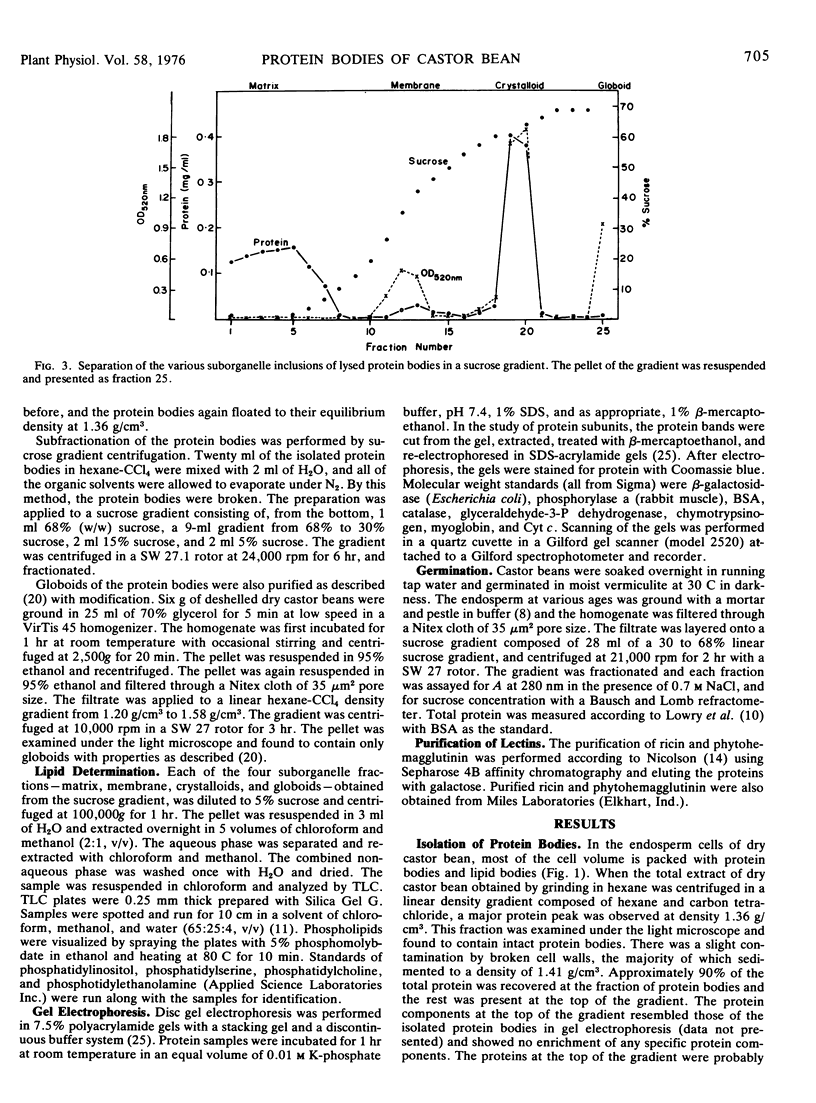
Protein bodies from the storage endosperm of dry castor bean (Ricinus communis L.) were isolated by successive nonaqueous linear density gradient centrifugation. The isolated protein bodies were lysed by the addition of water, and the various structural components of the organelles were separated by sucrose gradient centrifugation. The matrix protein remained at the top of the gradient while the membrane, the crystalloids, and the globoids migrated to densities 1.15 g/cm3, 1.30 g/cm3, and > 1.46 g/cm3, respectively. The protein of the protein bodies was distributed evenly between the crystalloids and the matrix, and little protein was present in the globoids or the membrane.
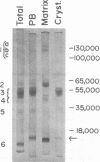
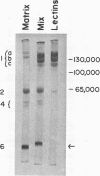
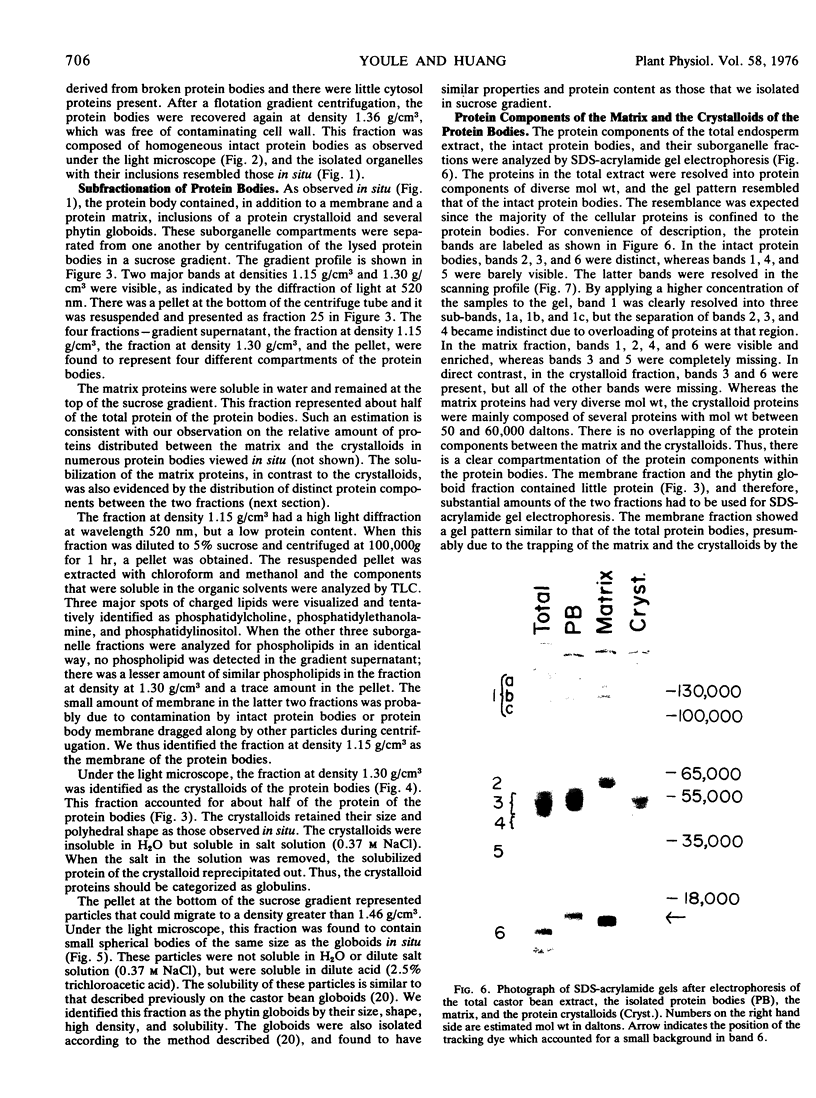
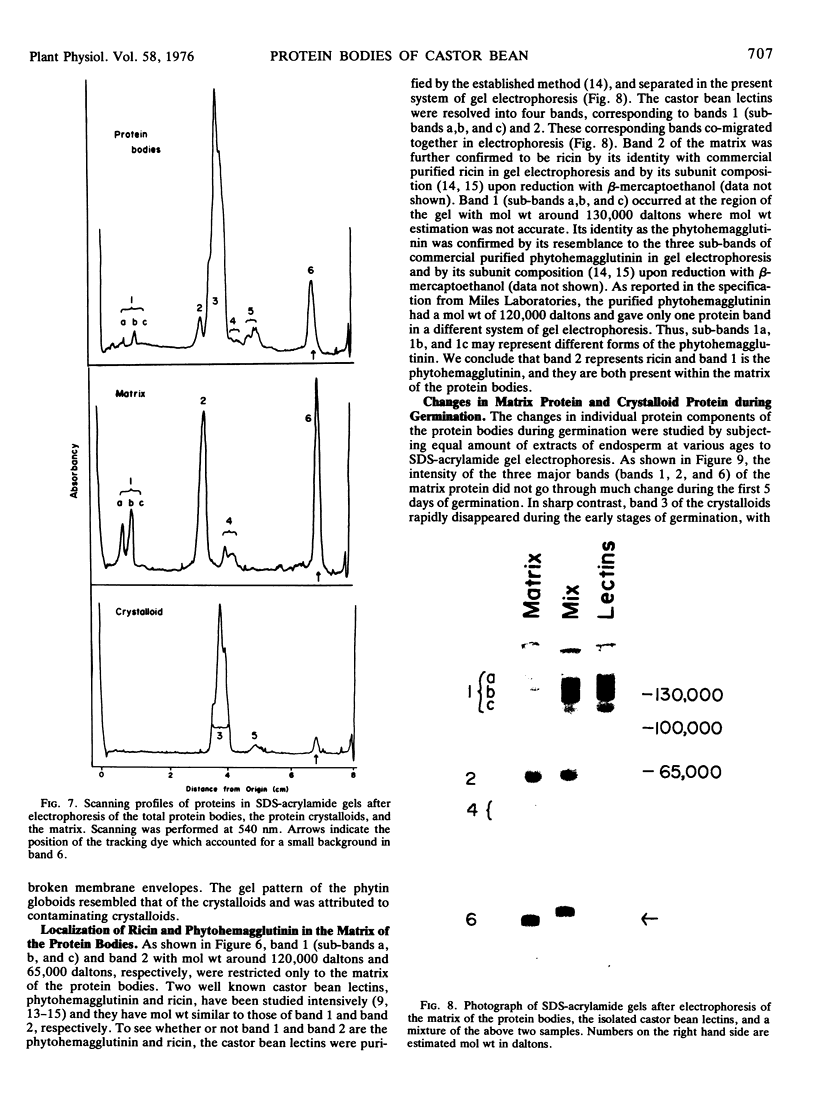
The proteins of the protein bodies were resolved into protein components of diverse molecular weights in sodium dodecyl sulfate-acrylamide gel electrophoresis. The protein components of the organelle matrix were distinct from those of the crystalloids. Whereas the matrix proteins had very diverse molecular weights, the crystalloid proteins were mainly composed of several proteins with molecular weights between 50,000 and 60,000 daltons. Also, the matrix proteins were soluble in water while the crystalloid proteins were insoluble in water but soluble in salt solution, thus representing albumins and globulins, respectively. Two of the matrix proteins with molecular weights approximately 120,000 and 65,000 daltons were identified as the phytohemagglutimin and the toxic protein ricin, respectively.
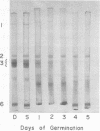
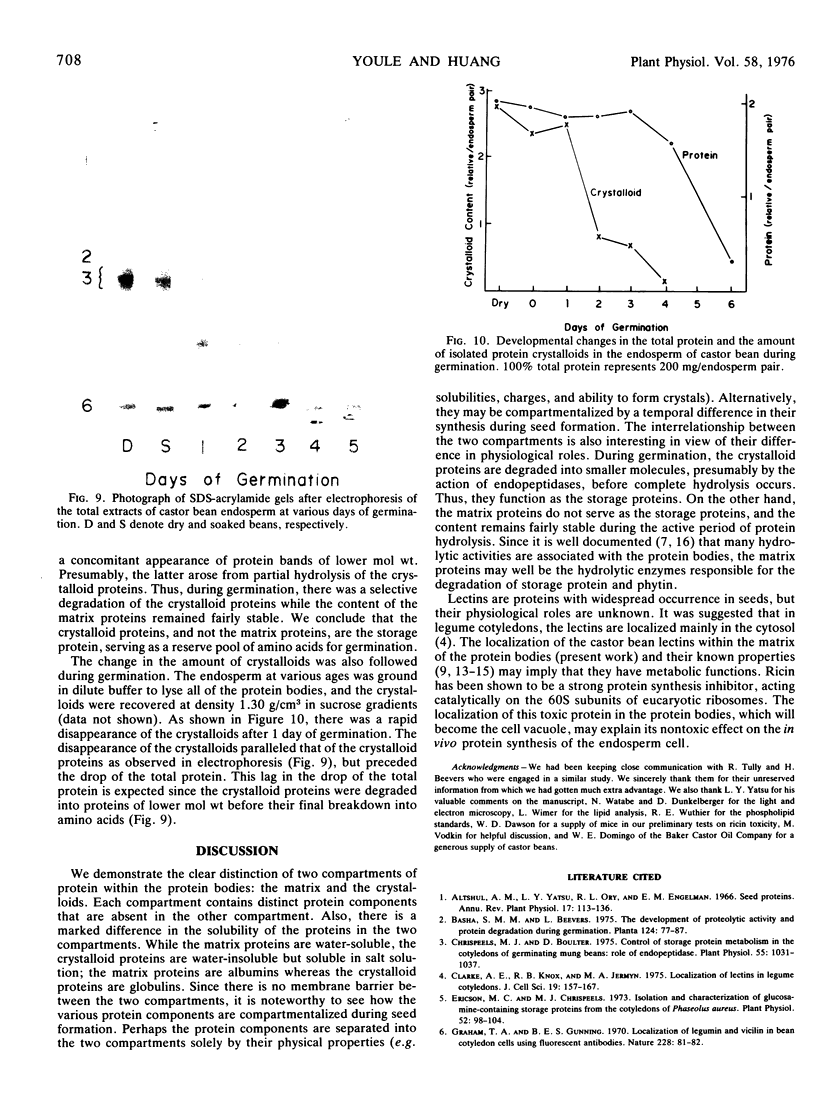
During germination, the crystalloid proteins served as the storage protein and went through rapid degradation with smaller polypeptides formed as intermediates. In contrast, the proteins of the matrix under-went a much slower degradation during the same period and did not appear to be storage protein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chrispeels M. J., Boulter D. Control of storage protein metabolism in the cotyledons of germinating mung beans: role of endopeptidase. Plant Physiol. 1975 Jun;55(6):1031–1037. doi: 10.1104/pp.55.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. E., Knox R. B., Jermyn M. A. Localization of lectins in legume cotyledons. J Cell Sci. 1975 Oct;19(1):157–167. doi: 10.1242/jcs.19.1.157. [DOI] [PubMed] [Google Scholar]
- Ericson M. C., Chrispeels M. J. Isolation and Characterization of Glucosamine-containing Storage Glycoproteins from the Cotyledons of Phaseolus aureus. Plant Physiol. 1973 Aug;52(2):98–104. doi: 10.1104/pp.52.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham T. A., Gunning B. E. Localization of legumin and vicilin in bean cotyledon cells using fluorescent antibodies. Nature. 1970 Oct 3;228(5266):81–82. doi: 10.1038/228081a0. [DOI] [PubMed] [Google Scholar]
- Harris N., Chrispeels M. J. Histochemical and biochemical observations on storage protein metabolism and protein body autolysis in cotyledons of germinating mung beans. Plant Physiol. 1975 Aug;56(2):292–299. doi: 10.1104/pp.56.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Isolation of microbodies from plant tissues. Plant Physiol. 1971 Nov;48(5):637–641. doi: 10.1104/pp.48.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lis H., Sharon N. The biochemistry of plant lectins (phytohemagglutinins). Annu Rev Biochem. 1973;42(0):541–574. doi: 10.1146/annurev.bi.42.070173.002545. [DOI] [PubMed] [Google Scholar]
- Montanaro L., Sperti S., Stirpe F. Inhibition by ricin of protein synthesis in vitro. Ribosomes as the target of the toxin. Biochem J. 1973 Nov;136(3):677–683. doi: 10.1042/bj1360677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NACHMIAS V. T., PADYKULA H. A. A histochemical study of normal and denervated red and white muscles of the rat. J Biophys Biochem Cytol. 1958 Jan 25;4(1):47–54. doi: 10.1083/jcb.4.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J., Etzler M. E. Characterization of two plant lectins from Ricinus communis and their quantitative interaction with a murine lymphoma. Biochemistry. 1974 Jan 1;13(1):196–204. doi: 10.1021/bi00698a029. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Saltvedt E., Pihl A. Isolation and comparison of galactose-binding lectins from Abrus precatorius and Ricinus communis. J Biol Chem. 1974 Feb 10;249(3):803–810. [PubMed] [Google Scholar]
- Ory R. L., Henningsen K. W. Enzymes associated with protein bodies isolated from ungerminated barley seeds. Plant Physiol. 1969 Nov;44(11):1488–1498. doi: 10.1104/pp.44.11.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ory R. L., Yatsu L. Y., Kircher H. W. Association of lipase activity with the spherosomes of Ricinus communis. Arch Biochem Biophys. 1968 Feb;123(2):255–264. doi: 10.1016/0003-9861(68)90132-x. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Angelo A. J., Yatsu L. Y., Altschul A. M. Isolation of edestin from aleurone grains of Cannabis sativa. Arch Biochem Biophys. 1968 Mar 20;124(1):199–205. doi: 10.1016/0003-9861(68)90320-2. [DOI] [PubMed] [Google Scholar]
- Tombs M. P. Protein bodies of the soybean. Plant Physiol. 1967 Jun;42(6):797–813. doi: 10.1104/pp.42.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varner J. E., Schidlovsky G. Intracellular Distribution of Proteins in Pea Cotyledons. Plant Physiol. 1963 Mar;38(2):139–144. doi: 10.1104/pp.38.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]