Abstract
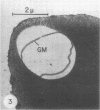
Protein bodies in the endosperm of castor bean seeds (Ricinus communis L.) contain phytin globoids and protein crystalloids embedded in an amorphous proteinaceous matrix. The protein bodies are apparently surrounded by a single membrane. The protein bodies were isolated by grinding and centrifuging in glycerol. Such isolated protein bodies were almost identical (after cytological fixation) to those observed in situ, except that the globoids were lost. However, membrane-like structures appear to have surrounded the globoids. Histochemical analysis of the isolated protein bodies showed that carbohydrates (glycoproteins) are localized only in the matrix region.
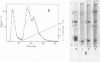
Addition of water to protein bodies in glycerol caused dissolution of the matrix, and release of the globoids and crystalloids. When the crystalloids were centrifuged on sucrose density gradients, they were recovered at an equilibrium density of 1.29 to 1.30 g/ml. The crystalloids were only slightly soluble in most aqueous buffers but were very soluble in sodium dodecyl sulfate, urea, or NaOH solutions.
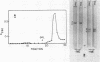
Polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate and chromatography on ion exchange celluloses show that the protein bodies are composed of one major and several minor anodic proteins. The major protein, along with a few of the minor proteins, is localized in the crystalloids.
The major protein (molecular weight 65,000) was converted by mercaptoethanol into subunits with molecular weights of 32,000 and 15,800. It is proposed that the protein is made up of two of the smaller subunits and one of the larger, linked by disulfide bridges. None of the crystalloid proteins appear to be glycosylated.
The water-soluble matrix fraction is composed mainly of two proteins, with molecular weights of 12,500 and 10,300 on the gels. Neither is a glycoprotein, and neither can be reduced with mercaptoethanol to give subunits. The soluble fraction also contains other lesser components among which are several glycoprotein lectins. One of these is ricin D, which is the main glycoprotein in the protein bodies.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daussant J., Neucere N. J., Yatsu L. Y. Immunochemical Studies on Arachis hypogaea Proteins With Particular Reference to the Reserve Proteins. I. Characterization, Distribution, and Properties of alpha-Arachin and alpha-Conarachin. Plant Physiol. 1969 Apr;44(4):471–479. doi: 10.1104/pp.44.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M. C., Chrispeels M. J. Isolation and Characterization of Glucosamine-containing Storage Glycoproteins from the Cotyledons of Phaseolus aureus. Plant Physiol. 1973 Aug;52(2):98–104. doi: 10.1104/pp.52.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Horner H. T., Jr, Arnott H. J. A histochemical and ultrastructural study of yucca seed proteins. Am J Bot. 1965 Nov-Dec;52(10):1027–1038. [PubMed] [Google Scholar]
- Lugnier A., Dirheimer G. Differences between ricin and phytohemagglutinins from Ricinus communis seeds. FEBS Lett. 1973 Sep 1;35(1):117–120. doi: 10.1016/0014-5793(73)80590-3. [DOI] [PubMed] [Google Scholar]
- Lui N. S., Altschul A. M. Isolation of globoids from cottonseed aleurone grain. Arch Biochem Biophys. 1967 Sep;121(3):678–684. doi: 10.1016/0003-9861(67)90053-7. [DOI] [PubMed] [Google Scholar]
- Moore H. G., Jr Penetrating vascular injuries. N C Med J. 1966 Jul;27(7):321–325. [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J., Etzler M. E. Characterization of two plant lectins from Ricinus communis and their quantitative interaction with a murine lymphoma. Biochemistry. 1974 Jan 1;13(1):196–204. doi: 10.1021/bi00698a029. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Refsnes K., Pihl A. Mechanism of action of the toxic lectins abrin and ricin. Nature. 1974 Jun 14;249(458):627–631. doi: 10.1038/249627a0. [DOI] [PubMed] [Google Scholar]
- Ory R. L., Henningsen K. W. Enzymes associated with protein bodies isolated from ungerminated barley seeds. Plant Physiol. 1969 Nov;44(11):1488–1498. doi: 10.1104/pp.44.11.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ory R. L., Yatsu L. Y., Kircher H. W. Association of lipase activity with the spherosomes of Ricinus communis. Arch Biochem Biophys. 1968 Feb;123(2):255–264. doi: 10.1016/0003-9861(68)90132-x. [DOI] [PubMed] [Google Scholar]
- Paleg L., Hyde B. Physiological Effects of Gibberellic Acid. VII. Electron Microscopy of Barley Aleurone Cells. Plant Physiol. 1964 Jul;39(4):673–680. doi: 10.1104/pp.39.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero J., Sun S. M., McLeester R. C., Bliss F. A., Hall T. C. Heritable Variation in a Polypeptide Subunit of the Major Storage Protein of the Bean, Phaseolus vulgaris L. Plant Physiol. 1975 Dec;56(6):776–779. doi: 10.1104/pp.56.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- St Angelo A. J., Yatsu L. Y., Altschul A. M. Isolation of edestin from aleurone grains of Cannabis sativa. Arch Biochem Biophys. 1968 Mar 20;124(1):199–205. doi: 10.1016/0003-9861(68)90320-2. [DOI] [PubMed] [Google Scholar]
- Thanh V. H., Okubo K., Shibasaki K. Isolation and Characterization of the Multiple 7S Globulins of Soybean Proteins. Plant Physiol. 1975 Jul;56(1):19–22. doi: 10.1104/pp.56.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombs M. P. Protein bodies of the soybean. Plant Physiol. 1967 Jun;42(6):797–813. doi: 10.1104/pp.42.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yatsu L. Y., Jacks T. J. Association of lysosomal activity with aleurone grains in plant seeds. Arch Biochem Biophys. 1968 Mar 20;124(1):466–471. doi: 10.1016/0003-9861(68)90354-8. [DOI] [PubMed] [Google Scholar]