Abstract
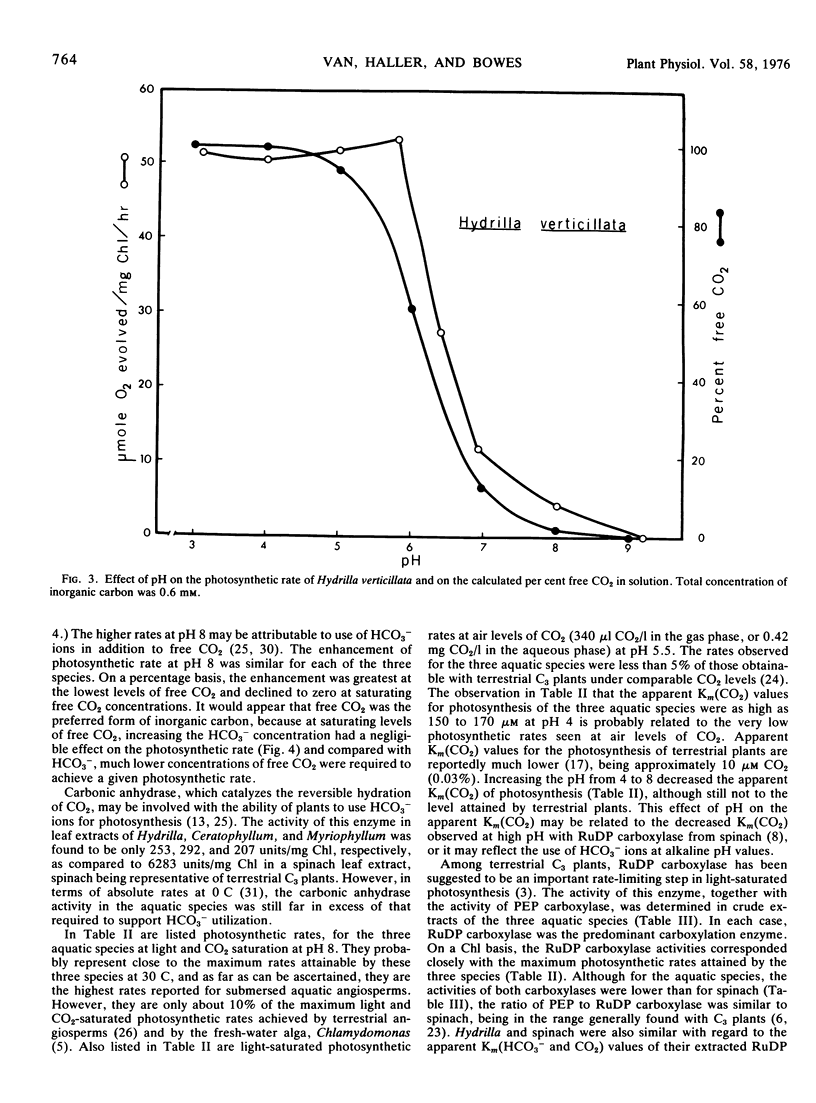
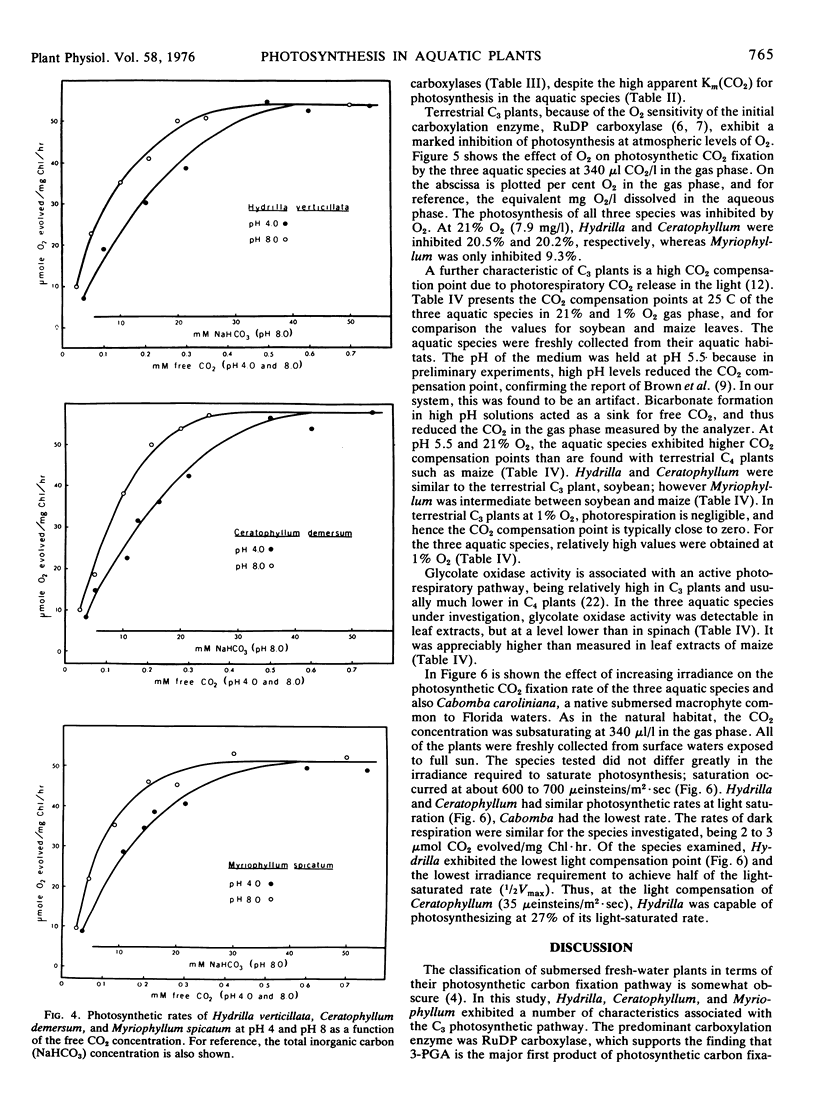
Light- and CO2-saturated photosynthetic rates of the submersed aquatic plants Hydrilla verticillata, Ceratophyllum demersum, and Myriophyllum spicatum were 50 to 60 μmol O2/mg Chl·hr at 30 C. At air levels of CO2, the rates were less than 5% of those achieved by terrestrial C3 plants. The low photosynthetic rates correlated with low activities of the carboxylation enzymes. In each species, ribulose 1,5-diphosphate carboxylase was the predominant carboxylation enzyme. The apparent Km(CO2) values for photosynthesis were 150 to 170 μm at pH 4, and 75 to 95 μm at pH 8. The Km(CO2) of Hydrilla ribulose 1,5-diphosphate carboxylase was 45 μm at pH 8. Optimum temperatures for the photosynthesis of Hydrilla, Myriophyllum, and Ceratophyllum were 36.5, 35.0, and 28.5 C, respectively. The apparent ability of each species to use HCO3− ions for photosynthesis was similar, but at saturating free CO2 levels, there was no indication of HCO3− use. Increasing the pH from 3.1 to 9.2 affected the photosynthetic rate indirectly, by decreasing the free CO2. With saturating free CO2 (0.5 mm), the maximum photosynthetic rates were similar at pH 4 and 8. Carbonic anhydrase activity, although much lower than in terrestrial C3 plants, was still in excess of that required to support HCO3− utilization.
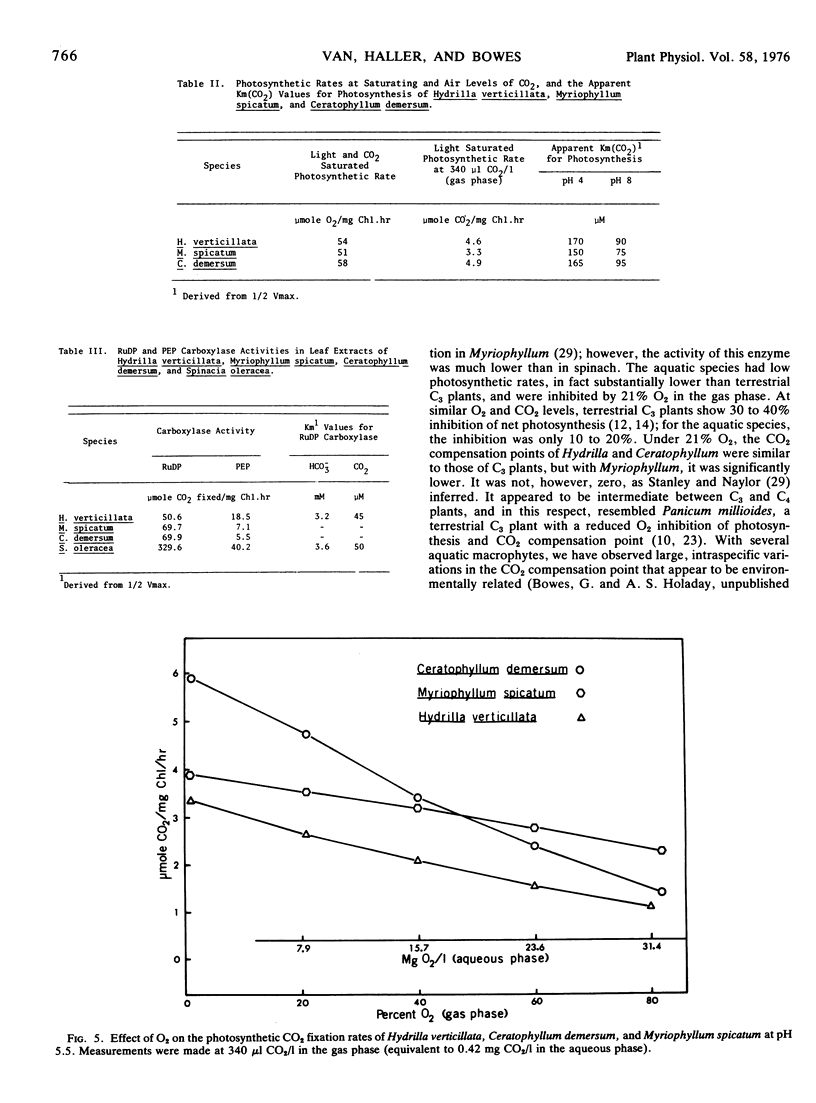
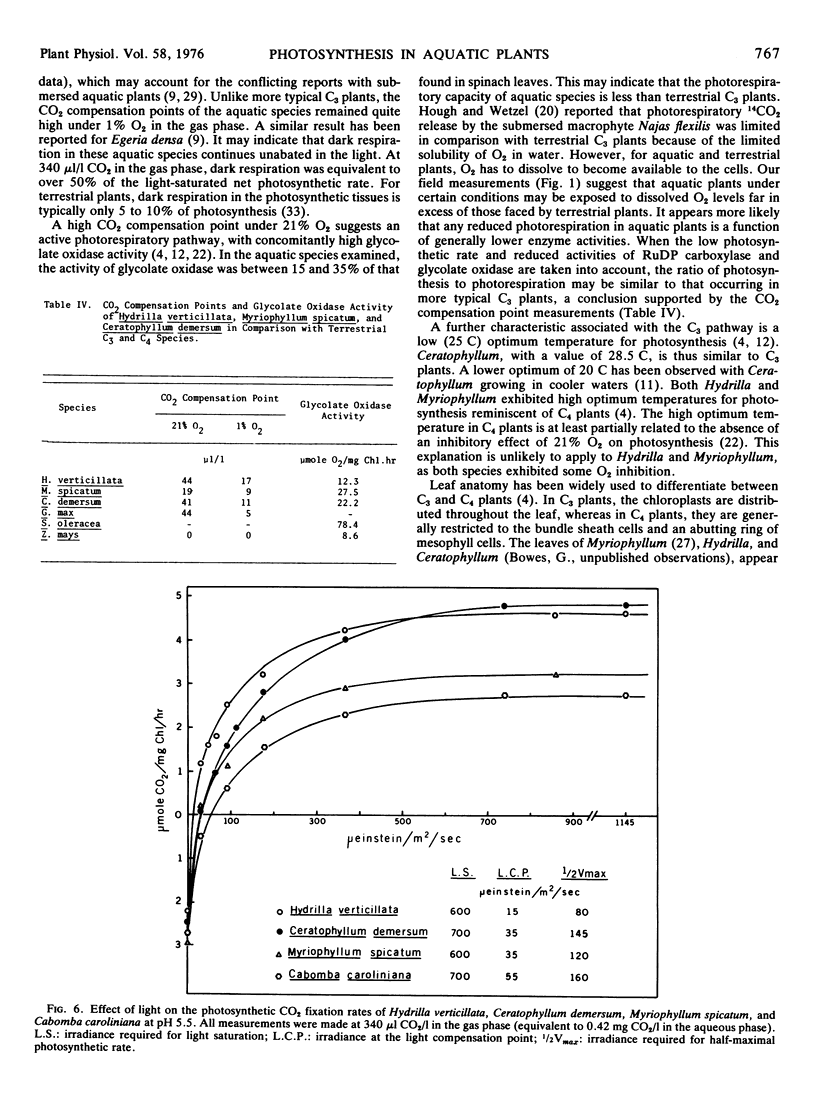
Hydrilla and Ceratophyllum had CO2 compensation points of 44 and 41 μl/l, respectively, whereas the value for Myriophyllum was 19. Relatively high CO2 compensation points under 1% O2 indicated that some “dark” respiration occurred in the light. The inhibition of photosynthesis by O2 was less than with terrestrial C3 plants. Glycolate oxidase activity was 12.3 to 27.5 μmol O2/mg Chl·hr, as compared to 78.4 for spinach. Light saturation of photosynthesis occurred at 600 to 700 μeinsteins/m2·sec in each species grown under full sunlight. Hydrilla had the lowest light compensation point, and required the least irradiance to achieve the half-maximal photosynthetic rate.
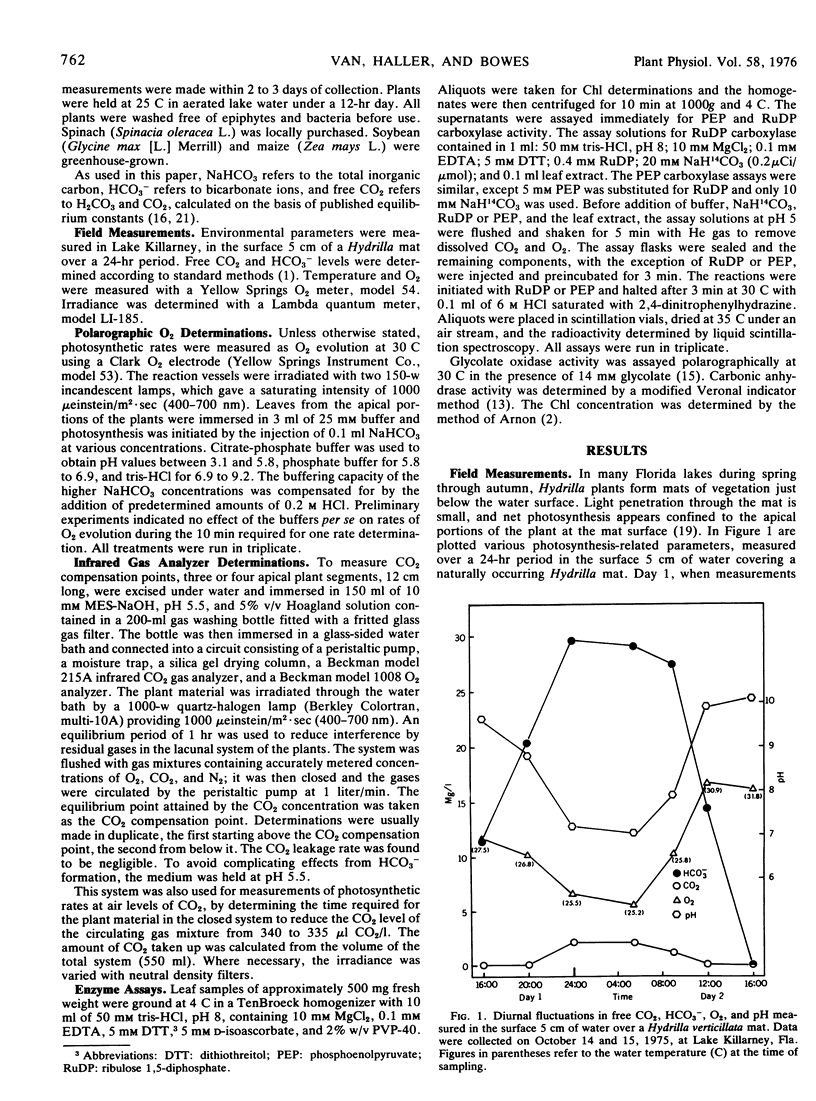
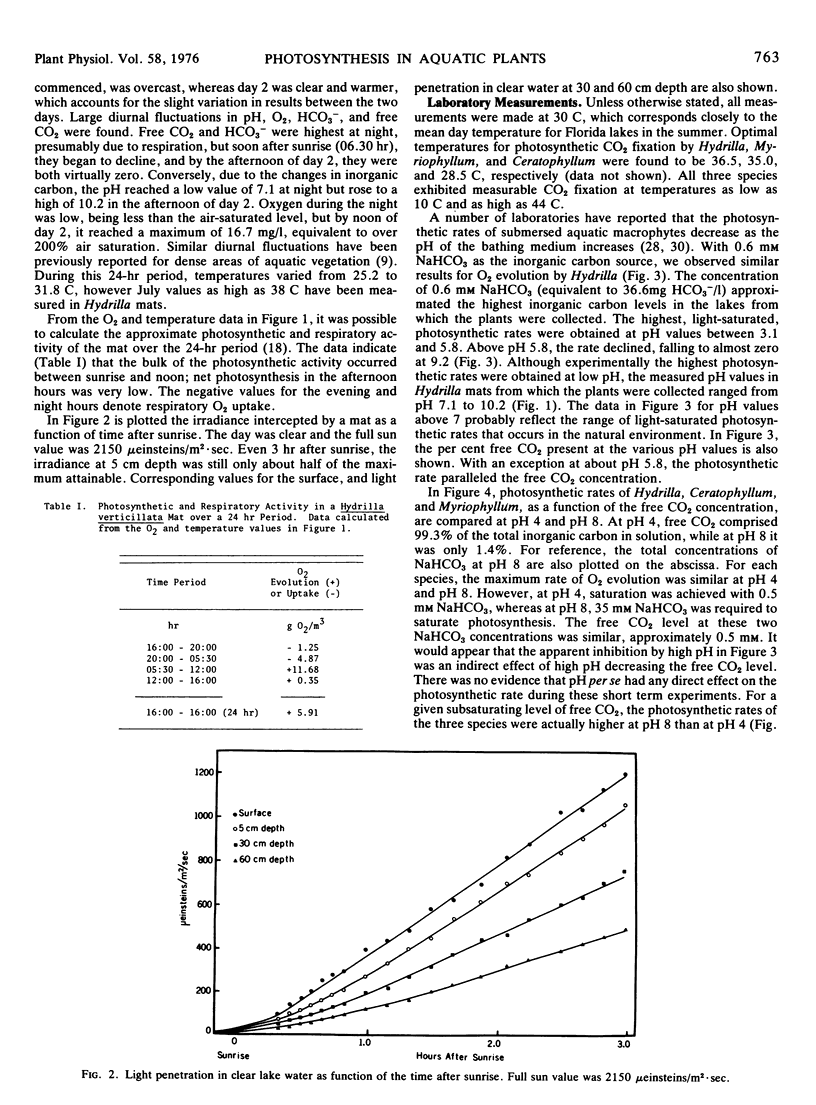
Field measurements in a Hydrilla mat indicated that in the afternoon, free CO2 dropped to zero, and O2 rose to over 200% air saturation. Most photosynthetic activity occurred in the morning when the free CO2 was highest and O2 and solar radiation lowest. The low light requirement of Hydrilla probably provides a competitive advantage under these field conditions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes G., Ogren W. L., Hageman R. H. Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem Biophys Res Commun. 1971 Nov 5;45(3):716–722. doi: 10.1016/0006-291x(71)90475-x. [DOI] [PubMed] [Google Scholar]
- Bowes G., Ogren W. L. Oxygen inhibition and other properties of soybean ribulose 1,5-diphosphate carboxylase. J Biol Chem. 1972 Apr 10;247(7):2171–2176. [PubMed] [Google Scholar]
- Bowes G. pH Dependence of the Km(CO(2)) of Ribulose 1,5-Diphosphate Carboxylase. Plant Physiol. 1975 Nov;56(5):630–633. doi: 10.1104/pp.56.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester M. L., Krotkov G., Nelson C. D. Effect of oxygen on photosynthesis, photorespiration and respiration in detached leaves. I. Soybean. Plant Physiol. 1966 Mar;41(3):422–427. doi: 10.1104/pp.41.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick S. E., Gruber P. J., Tolbert N. E. The occurrence of glycolate dehydrogenase and glycolate oxidase in green plants: an evolutionary survey. Plant Physiol. 1973 Oct;52(4):318–323. doi: 10.1104/pp.52.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS B. H., EDSALL J. T. RATE OF HYDRATION OF CARBON DIOXIDE AND DEHYDRATION OF CARBONIC ACID AT 25 DEGREES. J Biol Chem. 1963 Oct;238:3502–3507. [PubMed] [Google Scholar]
- Hough R. A., Wetzel R. G. A C-assay for photorespiration in aquatic plants. Plant Physiol. 1972 Jun;49(6):987–990. doi: 10.1104/pp.49.6.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestler D. P., Mayne B. C., Ray T. B., Goldstein L. D., Brown R. H., Black C. C. Biochemical components of the photosynthetic CO2 compensation point of higher plants. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1439–1446. doi: 10.1016/0006-291x(75)90520-3. [DOI] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. Carbon dioxide assimilation by leaves, isolated chloroplasts, and ribulose bisphosphate carboxylase from spinach. Plant Physiol. 1975 Jun;55(6):1087–1092. doi: 10.1104/pp.55.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley R. A., Naylor A. W. Photosynthesis in Eurasian Watermilfoil (Myriophyllum spicatum L.). Plant Physiol. 1972 Jul;50(1):149–151. doi: 10.1104/pp.50.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]


