Abstract
Most medical specialties including the field of gastroenterology are mainly aimed at treating diseases rather than preventing them. Genomic medicine studies the health/disease process based on the interaction of the human genes with the environment. The gastrointestinal (GI) system is an ideal model to analyze the interaction between our genes, emotions and the gut microbiota. Based on the current knowledge, this mini-review aims to provide an integrated synopsis of this interaction to achieve a better understanding of the GI disorders related to bad eating habits and stress-related disease. Since human beings are the result of an evolutionary process, many biological processes such as instincts, emotions and behavior are interconnected to guarantee survival. Nourishment is a physiological need triggered by the instinct of survival to satisfy the body’s energy demands. The brain-gut axis comprises a tightly connected neural-neuroendocrine circuitry between the hunger-satiety center, the dopaminergic reward system involved in the pleasure of eating and the gut microbiota that regulates which food we eat and emotions. However, genetic variations and the consumption of high-sugar and high-fat diets have overridden this energy/pleasure neurocircuitry to the point of addiction of several foodstuffs. Consequently, a gut dysbiosis generates inflammation and a negative emotional state may lead to chronic diseases. Balancing this altered processes to regain health may involve personalized-medicine and genome-based strategies. Thus, an integrated approach based on the understanding of the gene-emotions-gut microbiota interaction is the next frontier that awaits the gastroenterologist to prevent and treat GI disorders associated with obesity and negative emotions.
Keywords: Genes, Emotions, Brain reward system, Gut microbiota, Gastrointestinal disease, Personalized medicine, Genome-based nutrition, Nutrigenetics, Food decision-making, Obesity
Core tip: Even though instincts, emotions, and behavior are evolutionary mechanisms for humans to adapt, dysfunctional genes, chronic negative emotions and gut dysbiosis are high risk factors for different diseases. A deep study of the gene-environmental interactions and the gut-bacteria consortium is a key factor that could help to understand how negative emotions are translated into disease. Physicians do not always consider that emotional factors aggravate disease progression and severity. Therefore, personalized-medicine and genomic-based nutrition strategies may aid in the prevention and reduction in the prevalence of gastrointestinal disorders associated with obesity and negative emotions.
INTRODUCTION
One of the main functions of the human gastrointestinal (GI) tract is to sustain the natural interaction between the environment and the body’s interior. From an evolutionary standpoint, a series of anatomical changes has occurred over time. For example, wider and larger teeth allowed humans to eat a greater amount of plants and fruits[1]. Also, a longer small intestine helped to digest food and absorb nutrients, while removing non-digestible molecules, toxic matter and harmful agents from the body. During this evolutionary and historical process, humans were able to survive based on this capacity to eat foliage and roots, soil, and all kinds of animals. For instance, maguey worms, ant eggs, grasshoppers and snails once served as complementary survival foods for the Mesoamericans of Mexico. Later, they became part of the staple foodstuffs in several regions of the country, and paradoxically, they are considered exotic dishes in fancy restaurants today[2].
Globally, the prevalence of GI pathologies varies according to geographical location, which in turn is linked to genetic, environmental, and sociocultural, interactions. Thus, differences in the incidence and prevalence of GI pathologies may exist between urban and rural populations. However, regardless of these variables, the most common ailments are those related to bad eating habits and those associated with psychological or emotional factors[3].
As a result of the aforementioned issues, obesity has increased remarkably worldwide, along with its concomitant GI symptoms and associated co-morbidities, including type-2 diabetes and liver diseases such as non-alcoholic steatohepatitis[4]. Obesity ranks as the number one disease in both the United States and Mexico[5,6], while the economic devastation associated with type-2 diabetes and cirrhosis represents a serious problem for health services[7]. Eating less and more exercise has been the simplest proposal for the management of obesity. However, to date, all strategies to combat obesity have failed due to lack of a therapeutic target, or the patient’s lack of knowledge and poor attitude[8]. On the other hand, up to 60% of GI diseases are associated with stress[9]. A globalized world comes with high rates of stress and people with GI conditions struggle even more with anxiety, stress, and pain due to extensive lifestyle changes that have an impact on their quality of life. This unhealthy scenario leads us to ask why do patients overeat? Alternatively, why after losing weight by a harsh nutritional-medical treatment or even more often after bariatric surgery, patients relapse gaining more weight or recovering the lost weight? The answer may be related to the imbalance between the food we eat, genes and emotions.
Interestingly, the oldest records that allude to the food-body-emotion interaction is in Ayurvedic Medicine and in the theory of the balance between the natural elements documented by Chinese medicine. Both are considered precursors of the concepts defined by Hippocrates in which the mind, body and spirit are represented by the Four Humours theory: sanguine, phlegmatic, choleric and melancholic[10]. Based on this background, we may consider that the common denominator of these theories is the balance between the human body and the environment, i.e., what we eat, what we feel and our behavior (emotions) according to the person’s personality (genetics) or character. This balance leads to well-being, health, and happiness, while an imbalance leads to illness.
Modern or scientific medicine, as defined by the concepts derived from Descartes’ scientific method, has achieved significant advances in the understanding of how our body functions, first at the macroscopic and microscopic level, then followed by biochemical-physiological aspects, and most recently at the molecular level[11]. In the last century, modern medicine has focused more on disease than on health, leading to a fragmentation of our scientific knowledge[12]. Gastroenterologists may only address the sick digestive organ, whereas the nutritionist may recommend revisions to the kinds and amounts of the food we eat, but often neither of them consider the food-body-emotion interaction.
In the same sense, the concept of intestinal flora has advanced towards the study of the composition of the intestinal microbiota, which depends precisely on our eating habits. However, genomic medicine raises the question about how the genetic (inside)-environment (outside) interaction occurs. Currently, nutrigenetics and nutrigenomics are providing knowledge on how food interacts with our genes. With this new knowledge, doctors or health professionals have a new set of molecular tools to study GI disorders and establish genome-based treatment strategies. However, the interaction between eating and emotions has been less understood, causing knowledge again to be atomized throughout other disciplines such as neurology, psychology, psychiatry and even religion, or whatever it may be that leads to a greater degree of spirituality[13].
Returning to the Hippocratic’s concept, in which the balance between body, mind, and spirit is necessary for health, genomic medicine currently may explain at the molecular level how this may occur. Thus, the objective of this mini-review aims to provide an integrated synopsis of the interaction between genes, gut microbiota and emotions to achieve a better understanding of the GI disorders related to bad eating habits and stress-related diseases.
EMOTIONS, INSTINCTS AND BEHAVIOR
Emotions may be defined as mental and physical states that are generated in response to internal or external stimuli. This stimulation can arise from thought (thinking), or through the visual, auditory, somatosensory, gustatory, and olfactory senses. In the ancient times, one clear example of a stimulus that arises from thought was melancholy, described as an animic state that was present when a person yearned for their homeland and their activities or for loved ones that were no longer with them. Today, this emotion has been denoted as stress, anxiety, and depression, which arises because of various circumstances.
Both thoughts and senses can be activated by an internal or external stimulus, and the basis of this response is instinct, as an essential part of survival[14,15]. Through time, evolution establishes genetically an adaptability, given by the experience, to the surrounding environment. Eventually, through this adaptability of the human to its environment, a behavior arises, which is based on learning (cognition) and genetic adaptations[16,17]. An easy example to understand how genetic-environmental interactions modulate behavior is through the behavioral traits of different breeds of dogs, whose behavior or character is a mixture of the genetic aspects of the race and training (learning).
From Darwin to contemporary authors, emotions have been given different definitions and classifications to explain the health/disease process. However, it is worth rethinking the concept of instinct. Instincts are a set of physiological and mental reactions that lead to the preservation of life. These instincts arise from an internal or external stimulus; subsequently, the body responds by entering in a state of alert followed by a movement. In fact, emotion in Latin means “motion”. Darwin states that there are different facial expressions related to that movement[18]. These physical changes are fast, specific, and self-limiting; thus, the body may return to the original state after the stimulus disappears or it may chronically persist if the emotion is not resolved, for example, a feeling of resentment.
Once the state of alert is initiated, blood flows into specific body areas depending on the situation. For example, blood flows to the legs in case of “fear”, towards the chest and arms in case of “fight”, and to the genitalia when a possible mate is detected or to the stomach when the appetite or hunger arises[16]. Additionally, in regard to the blood flow, Alexander Lowen suggests sorting emotions into positive or negative[19]. Positive emotions are all those that provide well-being and pleasure, while negative emotions generate the opposite. The former favors blood flow whereas the latter generate vasoconstriction, releasing adrenalin and cortisol, which activates stress. Based on Lowen’s concept, one or a set of negative emotions over an extended period could lead to chronic illness. Therefore, in the medical context, a clear and integrated approach could help us to understand the role of instinct, emotions, and behavior in the health/disease process, and to establish therapeutic targets.
FUNCTIONAL GASTROINTESTINAL DISORDERS AND EMOTIONS
Functional gastrointestinal disorders (FGIDs) are a broad spectrum of chronic abnormalities, some of which arise from dysfunctional brain-gut interactions that can lead to dysmotility and hypersensitivity[20-22]. Several factors such as genetic susceptibility, gut physiology, microbiota composition, and psychological factors have been associated with FGIDs[23-25]. Episodes of anxiety and depression are experienced more frequently in individuals with FGIDs than in healthy subjects[26,27]. They also have been related to physiological changes in colonic motility, abdominal pain, mucosal blood flow and hyperreactivity among patients with intestinal bowel syndrome (IBS)[22]. Furthermore, negative emotions, stressful life events and personality traits like neuroticism have been associated with IBS, colitis, Crohn’s disease (CD) and dyspepsia[28]. At the same time, impaired attention and emotion regulation elicit symptoms of anxiety, hypervigilance, and hypersensitivity[20,21].
Among patients with FGIDs, quality of life is affected in two ways: first, anxiety and depression seem to predict the presence, severity, and frequency of symptoms[29,30]; and second, GI disorders may exacerbate the presence of negative emotions[31]. In fact, overall GI functions such as hunger, appetite, satiety, digestion, absorption and evacuation are affected by negative emotions[32]. However, the pathophysiological process of how emotions relate to GI disorders is not clearly understood. It has been proposed that homeostatic signals between the GI system and emotions are integrated by the gut-brain axis. This axis comprises the interaction between the endocrine, the immune and the enteric nervous systems[33], which in turn, interact with the autonomic and central nervous systems. For example, chronic stress promotes the release of pro-inflammatory cytokines and C-reactive protein. This protein stimulates the hypothalamic-pituitary-adrenal (HPA) axis by liberating corticotrophin-releasing hormone from the hypothalamus, which stimulates the activation of the sympathetic nervous system and the secretion of adrenocorticotropic hormone, which finally stimulates the release of cortisol from the adrenal cortex to limit stress[34]. In fact, patients with FGIDs and exacerbated anxiety and depression have high cortisol levels[35]. Due to HPA axis dysregulation the mesolimbic brain reward system (BRS) is altered, resulting in cognitive and emotional disturbance. As a result, FGIDs patients, predominantly IBS patients, are characterized by high rates of hypersensitivity related to GI symptoms such as pain[20].
EMOTIONS AND MICROBIOTA
The gut hosts almost 100 trillion microorganisms that share symbiotic properties with humans. Intestinal microbiota regulates part of the host’s metabolic and energy balance, modulate intestinal motility, and regulate immune system maturation. Also, it confers protection against pathogens and toxins, regulates cytokines secretion from adipose tissue, insulin signaling and finally, modulates host emotions and cognition[36,37]. The gut microbiota is considered our second genome, because it constitutes 90% of the total number of cells that interact with our bodies[38].
As shown in Figure 1A, the gut microbiota can help regulate emotions and cognition because it maintains a two-way communication with the brain[39] using the nervous, endocrine and immune systems[40]. Brain-gut communication is driven by the vagal nerve, which connects to nearly 100 million neurons in the enteric nervous system together with afferent (vagal and spinal) and efferent adrenergic neurons (sympathetic and parasympathetic)[41]. Moreover, certain gut bacteria synthesize neurotransmitters[42] and close to 20 neuropeptides produced in the enteroendocrine cells (central and peripheral neurons) serve as second messengers in the brain, thus regulating mood and cognition[43]. Some of these include substance P, calcitonin, corticotropin releasing factor, pancreatic polypeptide, vasoactive intestinal polypeptide, GLP-1 and somatostatin, neuropeptide Y, and peptide YY, among others[42]. These last two neuropeptides play a major role in body energy homeostasis[44]. The endocrine system regulates the release of gut bacteria neurotransmitters[43] and ghrelin, influencing the levels of neurotransmitters such as dopamine[45] whereas the brain controls the neuroendocrine factors. Finally, adhesion molecules maintain the integrity of the intestinal mucosa, which serves as a physical and chemical barrier against pathogenic bacteria[46]. Also, antigen recognition of pathogen-associated molecular patterns are recognized by the Toll-like receptors, modulating the activation of the immune response against nocive bacteria[47].
Figure 1.
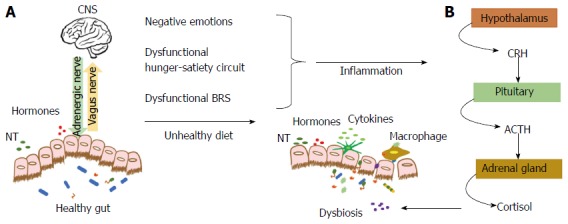
Gut-brain axis and dysbiosis. A: The gut microbiota maintains a two-way communication with the CNS using hormones, neuropeptides, NT, cytokines and the afferent (vagus nerve) and efferent signals (adrenergic nerve); B: Alterations in the energy balance circuit and BRS that lead to negative emotions chronically activate the HPA axis elevating cortisol levels. Cortisol results in dysbiosis, allowing pathogens to permeate the gut barrier and activate inflammation. Unhealthy dietary patterns also lead to dysbiosis, inflammation and negative emotions. CNS: Central nervous system; BRS: Brain reward system; CRH: Corticotrophin-releasing hormone; ACTH: Adrenocorticotropic hormone; HPA: Hypothalamus-pituitary-adrenal.
As shown in Figure 1B, alterations of the BRS and negative emotions, together with other unhealthy lifestyle factors produce a dysbiosis, which is an imbalance between beneficial and non-beneficial bacteria[46]. As mentioned before, activation of the HPA axis[40] releases free systemic stress hormones such as adrenaline, noradrenaline, and cortisol that promote bacterial growth of pathogens such as E. coli (E. coli0157), Yersinia enterocolitic, and Pseudomonas aerugins that further promote the synthesis of pro-inflammatory cytokines[43]. This scenario facilitates the loss of intestinal mucosa integrity. Lipopolysaccharides, pathogenic bacteria and toxins can permeate into the systemic circulation producing a metabolic endotoxemia[48,49]. This state generates pro-inflammatory conditions, insulin resistance and metabolic abnormalities related to chronic diseases[50].
Furthermore, a Western diet containing a high-sugar and high-fat composition contributes to dysbiosis and to a lower production of short-chain fatty acids from fiber fermentation, which have an anti-inflammatory role[51]. A high-fat diet promotes inflammation by increasing the expression of TNF-α and IL-6 inflammation-related cytokines, and macrophage infiltration[52]. Moreover, epidemiological studies have shown that central obesity and BMI are predictors of depression, anxiety and low quality of life[53,54]. Dysfunctions in adipose tissue are implicated in the development of stress and depression[55]. Adipocytes mediate a neuro-inflammatory profile affecting emotion and cognitive brain regulatory centers[56]. Also, inflammation is related to neurobiochemical alterations such as impaired serotonin synthesis, depletion of melatonin and tryptophan and neuronal damage in the hippocampus due to altered glutamatergic pathways[57]. Moreover, the prevalence of psychopathologies increases proportionally with the number of obesity-related metabolic diseases and metabolic syndrome components such as abdominal obesity, hypertriglyceridemia, and reduced high-density lipoprotein levels[58,59].
In this vein, changes in gut microbiota composition and diversity are related to immune-mediated diseases such as colitis, inflammatory bowel disease (IBD) and CD. For example, when compared to healthy subjects, patients with colitis have a reduced abundance of Akkermansia (phylum Veruccomicrobia), which promotes mucin degradation and protection against toxins[60]. Bacteria composition and diversity is also affected in IBD patients, who show a reduced number of Firmicutes and a higher number of Proteobacteria and Tenericutes; increases in E. coli populations is also associated with IBD[61]. Patients with CD tend to have a decreased number of the beneficial bacteria Firmicutes and increased number of Bacteroidetes when compared with healthy controls, together with reductions in bacteria gene protein expression related to nutrient and energy metabolism, intracellular traffic and defense[62].
HUNGER-SATIETY CIRCUIT: THE ENERGY BALANCE SYSTEM
Hunger and appetite stimulate feeding, although they may not be directly associated with each other. Hunger, an essential part of the instinct of survival, is the physical sensation that triggers eating to refuel the body (calorie input)[44], whereas appetite is the desire for food. Satiety is the end result, the state of feeling full to the point of satisfaction after food consumption.
The hypothalamic hunger-satiety neurocircuitry system, in coordination with external cues, regulates body fat stores by balancing energy intake and energy expenditure over time[44,63]. Systemic hunger-satiety signals include ghrelin, peptide YY, leptin, and insulin. Ghrelin and peptide YY are gut hormones that communicate to the brain the absence/presence of food in the GI tract. Leptin is an anorectic hormone secreted by white adipose tissue[64], and promotes satiety and increasing energy expenditure[65]. Insulin is a hormone secreted by the pancreas that participates in glucose homeostasis[66] and has a similar action as leptin[44].
Figure 2 shows the hunger-satiety circuit. It is essentially comprised of two sets of neuronal subpopulations situated in the arcuate nucleus. The agouti-related protein (AgRP) neurons co-expressing orexigenic AgRP and neuropeptide Y (NPY) stimulate eating and lower energy expenditure. Additionally, adjacent to the AgRP neurons are the anorective cells that co-express pro-opiomelanocortin (POMC) and cocaine and amphetamine regulated transcript (CART). These neurons release α-melanocyte stimulating hormone (α-MSH), which suppresses hunger and increases energy expenditure by binding to the melanocortin-4-receptor (MC4R)[67].
Figure 2.
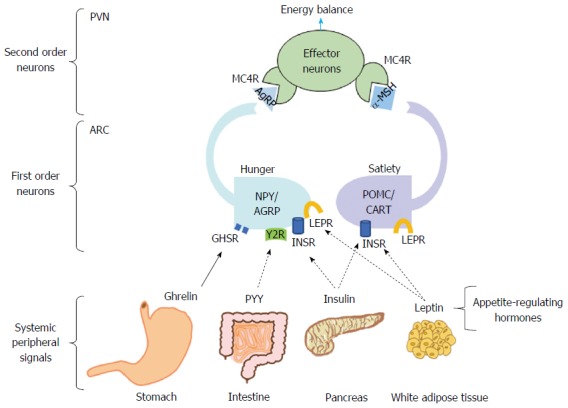
Hunger-satiety circuit and energy balance. Energy balance depends on the appropriate compensatory signals from the hypothalamic nucleus. Food intake is promoted by the binding of ghrelin to the GHSR in the NPY/AgRP neurons, blocking anorexigenic signaling. Once food consumption occurs, PYY-Y2R binding has an antagonistic effect on the orexigenic pathway. Leptin and insulin bind to their specific receptors in POMC/CART neurons that in turn induces α-MSH to bind to its MC4R, promoting satiety. Furthermore, leptin and insulin in NYP/AGRP neurons inhibit AgRP signaling. GHSR: Growth hormone secretagogue receptor; NPY/AgRP: Neuropeptide Y/Agouti-related receptor protein; PYY: Peptide YY; Y2R: Y2 receptor; POMC/CART: Pro-opiomelanocortin/cocaine and amphetamine regulated transcript; α-MSH: α-melanocyte-stimulating hormone; PVN: Paraventricular nucleus; ARC: Arcuate nucleus.
In the fasting state, feeding is stimulated by the binding of ghrelin to the growth hormone secretagogue receptor[68] in the AgRP/NPY neurons. AgRP is then liberated, which antagonizes the binding of α-MSH to the MC4R. In contrast, to end the act of eating, satiety is induced by the binding of the peptide YY, insulin, and leptin to their respective receptors. These actions block the orexigenic effect of the NPY/AgRP neurons and activate the satiety effect of α-MSH[68].
People with excess weight have altered energy balances. Furthermore, they tend more often to make unhealthy food choices compared to those with a healthy weight. It has been documented that the intake of energy-dense food produces a high neuronal reward and stimulation of the dopaminergic BRS pathways, bypassing the physiological regulation of hunger and satiety, especially in subjects that are driven to intake food in response to emotional stimuli[69]. These emotional eaters will repeat this behavior to re-experience this pleasure, which eventually leads to overeating behaviors[70,71]. People with either emotional or cognition alterations can thus develop addictive behaviors, such as low self-control, and low self-efficacy[72,73]. For these people, the small, short-term reward from indulgent foods is more powerful than the long-term benefit of eating healthier food choices.
BRAIN REWARD SYSTEM: THE PLEASURE CENTER
The BRS controls emotions, behavior, learning and memory, which impact food patterns[74-76]. Various stimuli such as positive emotions, sex, food, and even drugs activate BRS neurotransmitters resulting in feelings of comfort. Not surprisingly, the BRS is more stimulated by highly energy-dense food than to low-calorie food[77]. The BRS is localized in the mesolimbic region and is comprised of the ventral tegmental area (VTA), nucleus accumbens (NAC), amygdala, prefrontal cortex and hippocampus. The BRS is regulated by dopamine, serotonin, Υ-aminobutyric acid and glutamate as well as endogenous opioids[76].
Dopamine is the most important neurotransmitter in the BRS. Dopaminergic neurons project from VTA to the amygdala, NAC, pre-frontal cortex, and hippocampus[78]. Figure 3 illustrates the VTA-NAC dopaminergic pathway of the BRS. After stimulation, dopamine synthesis and release from the VTA neurons activates excitatory dopamine one receptors (DRD1) in the NAC. Dopamine transmission concludes by degradation of dopamine by catechol-O-methyl transferase (COMT) by incorporating methyl groups donated by S-adenosyl methionine. When the stimuli have concluded, the dopamine receptor D2/ankyrin repeat domain and content kinase 1 (DRD2/ANKK1) or protein kinase 2 exerts its inhibitory function. Dopamine transporter 1/Solute Carrier Family 6 (DAT1/SLC6A3) uptakes dopamine from the synaptic cleft[79].
Figure 3.
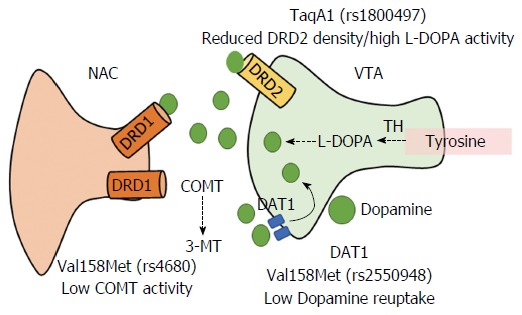
Brain reward dopaminergic signaling between ventral tegmental area and nucleus accumbens neurons. Dopamine in the VTA area activates excitatory DRD1 in the NAC. Several genetic polymorphisms in the regulatory proteins DRD2, DAT1, and COMT modify the reward response to dopamine. VTA: Ventral tegmental area; NAC: Nucleus accumbens; DRD1: Dopamine receptor 1; DRD2: Dopamine receptor 2; DAT1: Dopamine transporter 1; COMT: Catechol-O-methyl-transferase; TH: Tyrosine hydroxylase.
However, structural variations in the genetic basis of the hunger-satiety circuit and the BRS may modify the biological response to stimuli, thus explaining some of the differences in emotions, cognition and behavior among individuals.
GENETIC FACTORS
The unveiling of the sequences of the human genome has led to the discovery of heritable single nucleotide polymorphisms (SNPs) that in some cases can have little to no observable effects, but often can significantly affect protein structure (where it leads to amino acid sequence changes) and gene expression. Table 1 summarizes several SNPs seen in the energy-balance system and BRS genes involved in less satiety and obesity as well as emotional and cognitive disturbances[80-83] that may be lead to a dysfunctional gut-brain axis. For example, the -2548 G>A polymorphism in the leptin gene decreases leptin concentrations[84,85], whereas the 688 A>G polymorphism in the leptin receptor (LEPR)[86] reduces the receptor’s affinity for leptin[87]. The -188 kb T>C polymorphism of the MC4R gene[88,89] has been associated with higher energy consumption of fat and proteins, low postprandial satiety and less feeling of nausea in response to overfeeding[88-90].
Table 1.
Gene polymorphisms of the central energy balance and brain reward system
| Gene | Locus | SNP (reference sequence) | Risk allele | Clinical implications | Ref. |
| Central nervous system genes related to hunger and satiety | |||||
| LEP | 7q31.3 | -2548 G>A, (rs7799039) | G | Deficient anorectic signal | Mammès et al[84] Hoffstedt et al[85] |
| LEPR | 1p31 | 668 A>G, Gln223Arg | G | Obesity, low satiety | Boumaiza et al[80] Dougkas et al[81]Mizuta et al[83] |
| (rs1137101) | |||||
| MC4R | 18q22 | -188 kb T>C, (rs17782313) | C | High energy intake | Loos et al[88] |
| Low satiety | Acosta et al[90] | ||||
| Brain reward system genes related to emotional disturbances | |||||
| DRD2/ANKK1 | 11q23.2 | 2137 G>A, Glu713Lys | A | Addictions, impulsivity, | Blum et al[94] |
| (rs1800497) | emotional disturbance | ||||
| DAT 1/SLC6A3 | 5p15.3 | G>A, (rs2550948) | A | Impulsivity, | Genro et al[96] |
| G>A, (rs2652511) | A | increased food intake | Fontana et al[97] | ||
| G>A, (rs1048953) | A | ||||
| COMT | 22q11.21 | 472 G>A, Val158Met | A (Met) | Impulsivity, cognitive function, anxiety, depression | Egan et al[101] |
| (rs4680) | Gao et al[102] | ||||
| BDNF | 11p13 | 196 G>A, Val66Met | A (Met) | Learning, depression | Bonaccorso et al[104] |
| (rs6265) | |||||
DRD2/ANKK1: Dopamine receptor 2/ankyrin repeat domain and containing kinase 1 or protein kinase 2 gene; DAT 1/SLC6A3: Dopamine transporter 1/solute carrier family 6 member 3 gene; COMT: Catechol-O-methyltransferase gene; BDNF: Brain-derived neurotrophic factor gene; LEP: Leptin gene; LEPR: Leptin receptor gene; MC4R: Melanocortin-4-receptor gene.
Furthermore, BRS signaling genes such as DRD2/ANKK1, COMT, DAT 1/SLC6A3 and BDNF (Brain-derived neurotrophic factor) have been related to Reward Deficiency Syndrome (RDS), which consists of a dopamine-based neuronal sensorial deprivation that affects emotions, cognition, and promotes addictive behaviors[91]. Distinct SNPs have been associated with alterations in the core mechanisms of the dopaminergic VTA-NAC pathway. For example, the DRD2/ANKK1 TaqA1 (rs1800497) polymorphism affects receptor density while increasing L-DOPA activity[92-94]. Several SNPs in the DAT1 transporter protein gene alter the recapture of dopamine[95], which have been associated with impulsivity and increased food intake[96,97]. A reduced COMT activity in the Val158Met polymorphism affects the degradation of dopamine and other catecholamines (epinephrine, norepinephrine) causing higher dopamine synaptic levels[98,99]. Stein et al[100] have proposed that Met/Met carriers be identified as “worriers” whereas the Val/Val carriers were known as “warriors” in response to stress resistance. Furthermore, it is thought that obesity behaviors could be related to the Met/Met genotype[101-102]. Additionally, the Val66Met polymorphism reduces BDNF expression and activity[103], which has been related to obsessive-compulsive disorder, eating disorders, hyperactivity and, attention deficit hyperactive disorder[104]. In fact, Met carriers are more susceptible to depression and anxiety after being exposed to stressful events[105].
GENES-EMOTIONS-GUT MICROBIOTA INTERACTION: REGAINING HEALTH AND HAPPINESS
People with addictions have difficulty in halting the experience of the pleasure reward system, regardless of whether this excessiveness is causing serious damage to the body. Alcohol, tobacco, and a wide variety of drugs are pleasurable stimuli that induce well-being, which of course when consumed in large quantities and over a prolonged period of time, lead to chronic diseases such as alcoholic cirrhosis, lung cancer, or drug abuse. In ancient times, stimulants such as alcohol, peyote, coca leaves and ayahuasca were used by a limited number of people, mainly native priests or shamans, in different religious rites to achieve an altered state of consciousness, while most people did not have free access to them. However, once these were tasted and enjoyed by the rest of the society, the risk of overconsumption leading to addiction became eminent. Nonetheless, these substances are not vital for life, in a biological sense, whereas food is essential for survival. How is it then that the innate need of eating, a pleasure promoted by hunger and appetite ends up as an addiction affecting the GI tract? As explained before, an altered genes-emotions-gut microbiota interaction may be involved. However, social factors should also be considered.
Naturally, a healthy body will respond to the hunger-satiety/reward cycle. However, several decades ago, the global obesity epidemic was not a public health problem. On the contrary, famines and poverty limited access to food and industrially processed food was uncommon, and malnutrition and GI infectious diseases were the mainstream health issues worldwide. Currently, macroeconomic changes resulting from globalization challenge today’s societies with a wide variety of foods and flavors, food abundance and 24-h accessibility that promotes overeating. Unfortunately, despite this relative food “wealth”, poor people still suffer from hunger, eating cheap, energy-dense foods that cause malnutrition (excess weight), particularly in developing countries.
On the other hand, why people overeat and overload the hunger-satiety/reward system may also be attributable to the genetics of taste preferences. Human taste genes have evolved to distinguish “good” and “bad” tastes, and these genes are highly polymorphic. Additionally, differencing safe food from poisoned (bitter) or damaged food (sour), and to detect sweet or fat tastes in natural/endemic foodstuffs related to energy molecules is a matter of survival[106]. Furthermore, the BRS depends highly on dopamine receptors to elicit the hedonic phase of many human actions, including feeding. What we choose to eat is not based on what is “good” nutritionwise. Instead, our food decision-making depends on the liking of the “good” taste. People who have non-taster alleles for sensing sweets and fats, and have an altered BRS prefer energy-dense foods. Therefore, in an obesogenic environment, these individuals may be at risk for addiction to certain food flavors.
From a metabolic perspective, other genes involved in carbohydrate and lipid metabolism also present risk alleles. Consequently, individuals with these risk alleles consuming an obesogenic diet may develop dyslipidemia, metabolic syndrome and chronic diseases. As the shown in Figure 4, this natural physiological need, as modulated by the brain’s energy balance/reward system, makes us seek pleasure. However, by eating the wrong food and feeling negative emotions, this same system may eventually lead to obesity and changes in the gut microbiota starting a vicious cycle. As mentioned before, negative emotions lead to taking refuge in excessively pleasurable stimuli, altering the intestinal microbiota and generating a chronic inflammatory state. The bottom line is that it seems that we no longer enjoy drinking a fine wine, smoking a good tobacco or having a delicious meal in moderation to fulfill the joy of celebration. Instead, this excessiveness has led to addiction, obesity and chronic diseases.
Figure 4.
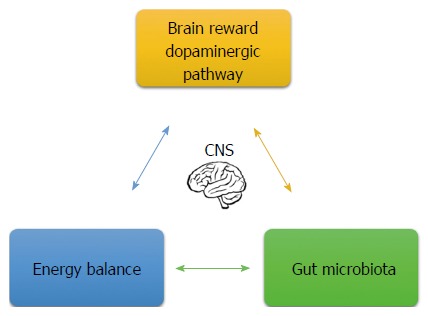
Signals involved in the health/disease process. Brain dopaminergic pathway and energy balance regulate emotional eating behaviors. Negative emotion contributes to alterations in gut microbiota composition. At the same time, gut microbiota environment and its metabolites communicate to the central to the brain and regulate the brain’s reward system and energy balance. CNS: Central nervous system.
So what is required to regain a healthy genes-emotions-gut microbiota interaction? Based on an integrative genomic medical and nutritional approach, we can state:
Eat ecologically
Globalization, climate changes, and acculturation are only a few of the major threats that have disrupted our relationship with Mother Nature. Human populations that have shifted their regional and traditional ways of life, including their food culture, towards a Westernized lifestyle typical of the “developed world” have lost their ancestral gene-food-culture interconnection, thus leading to higher rates of illness and death due to non-transmissible diseases.
For example, most populations in Latin America countries, such as Mexico are an admixture of Amerindian, Caucasian and African lineages with a rich and traditional food culture. Recent studies in the Mexican population have revealed a higher prevalence of risk alleles of sweet (TAS1R2)[107], fat (CD36)[108,109] and bitter (TAS2R38)[110] taste receptors, lipid-transporting proteins (APO e2 and e4, FABP2)[111], lactose intolerance (LCT-13910 C>T)[112] and neurotransmitter transporters (DRD2 Taq1)[113]. These alleles have been associated with metabolic abnormalities, obesity and alcohol abuse disorders, aggravated by the consumption of a hepatopathogenic and obesogenic diet[114]. Implementation of a genome-based nutritional strategy has been our recommendation to combat these bad eating habits to restore health. The rationale of this strategy is to select foods that balance our inheritance of polymorphic nutrient-interacting genes with the regional ecosystem while preserving the traditional food culture[115].
Enjoy life and be healthy
As the Good Book cites: “The only worthwhile thing for a human being is to eat, drink, and enjoy life’s goodness that he finds in what he accomplishes. This, I observed, is also from the hand of God himself” (Ecclesiastes 2:24). In regards to this point, the BRS allow us to feel pleasure as the natural response to the intrinsic rewards of human life. In other words, we should enjoy eating, drinking and working but without stress, depression, and anxiety. In contrast, extrinsic rewards such as money, are conditioned rewards[116] that may cause in many people who live only for money to have mental and body illnesses more than happiness.
CONCLUSION
In summary, nourishment is a natural and physiological need to obtain energy and seek food from the environment. The brain-gut axis comprises a neural-neuroendocrine circuit between the brain’s hunger-satiety and dopaminergic reward systems in conjunction with the gut microbiota, which regulates our emotions and food-decision making. However, genetic variations and the consumption of high-sugar and high-fat diets have overridden this energy/pleasure circuitry to the point of addiction to several foodstuffs as well as obesity and other associated chronic co-morbidities. Balancing this altered physiological process to regain health may involve personalized-medicine and genome-based strategies. Thus, an integrated approach based on the understanding of the genes, emotions and gut microbiota interactions is the next frontier that awaits the gastroenterologist to prevent and treat GI disorders associated with obesity and negative emotions.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Mexico
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Authors declare no conflict of interest for this article.
Peer-review started: February 6, 2017
First decision: March 3, 2017
Article in press: April 12, 2017
P- Reviewer: Chandra D, Satake H S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
References
- 1.Teaford MF, Ungar PS. Diet and the evolution of the earliest human ancestors. Proc Natl Acad Sci USA. 2000;97:13506–13511. doi: 10.1073/pnas.260368897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ojeda-Granados C, Panduro A, Ramos-López O, Román S. Construyendo una dieta correcta con base genoma latino. Rev Endocrinol Nutr. 2013;21:84–92. [Google Scholar]
- 3.Pratt LA, Brody DJ. Depression and obesity in the U.S. adult household population, 2005-2010. NCHS Data Brief. 2014;167:1–8. [PubMed] [Google Scholar]
- 4.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 6.Barquera S, Campos-Nonato I, Hernández-Barrera L, Pedroza A, Rivera-Dommarco JA. [Prevalence of obesity in Mexican adults 2000-2012] Salud Publica Mex. 2013;55 Suppl 2:S151–S160. [PubMed] [Google Scholar]
- 7.McPhail SM. Multimorbidity in chronic disease: impact on health care resources and costs. Risk Manag Healthc Policy. 2016;9:143–156. doi: 10.2147/RMHP.S97248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivera-Iñiguez I, Panduro A. ¿Porqué fracasan los tratamientos médico-nutricionales en el manejo de la obesidad en México? Rev Mex Endocrinol Metab Nutr. 2014;1:193–202. [Google Scholar]
- 9.Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut. 2000;47:861–869. doi: 10.1136/gut.47.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berrios GE. Melancholia and depression during the 19th century: a conceptual history. Br J Psychiatry. 1988;153:298–304. doi: 10.1192/bjp.153.3.298. [DOI] [PubMed] [Google Scholar]
- 11.Mehta N. Mind-body Dualism: A critique from a Health Perspective. Mens Sana Monogr. 2011;9:202–209. doi: 10.4103/0973-1229.77436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fani Marvasti F, Stafford RS. From sick care to health care--reengineering prevention into the U.S. system. N Engl J Med. 2012;367:889–891. doi: 10.1056/NEJMp1206230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roman S, Panduro A. Genomic medicine in gastroenterology: A new approach or a new specialty? World J Gastroenterol. 2015;21:8227–8237. doi: 10.3748/wjg.v21.i27.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watt DF. Consciousness, emotional self-regulation and the brain: Review article. J Conscious Stud. 2004;11:77–82. [Google Scholar]
- 15.Jablonka E, Ginsburg S, Dor D. The co-evolution of language and emotions. Philos Trans R Soc Lond B Biol Sci. 2012;367:2152–2159. doi: 10.1098/rstb.2012.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekman P, Levenson RW, Friesen WV. Autonomic nervous system activity distinguishes among emotions. Science. 1983;221:1208–1210. doi: 10.1126/science.6612338. [DOI] [PubMed] [Google Scholar]
- 17.Foxall GR. Cognitive requirements of competing neuro-behavioral decision systems: some implications of temporal horizon for managerial behavior in organizations. Front Hum Neurosci. 2014;8:184. doi: 10.3389/fnhum.2014.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darwin C, Ekman P, Prodger P. The Expression of the Emotions in Man and Animals. 3rd ed: Oxford University Press; 1998: 33 [Google Scholar]
- 19.Lowen A. The Spirituality of the Body: Bioenergetics for Grace and Harmony: Macmillan, 1990 [Google Scholar]
- 20.Drossman DA. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.032. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 21.Mazaheri M. Difficulties in Emotion Regulation and Mindfulness in Psychological and Somatic Symptoms of Functional Gastrointestinal Disorders. Iran J Psychiatry Behav Sci. 2015;9:e954. doi: 10.17795/ijpbs-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wouters MM, Vicario M, Santos J. The role of mast cells in functional GI disorders. Gut. 2016;65:155–168. doi: 10.1136/gutjnl-2015-309151. [DOI] [PubMed] [Google Scholar]
- 23.Zhu L, Huang D, Shi L, Liang L, Xu T, Chang M, Chen W, Wu D, Zhang F, Fang X. Intestinal symptoms and psychological factors jointly affect quality of life of patients with irritable bowel syndrome with diarrhea. Health Qual Life Outcomes. 2015;13:49. doi: 10.1186/s12955-015-0243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu S, Ren J, Hong Z, Li X, Yao M, Yan D, Ren H, Wu X, Wang G, Gu G, et al. An evil backstage manipulator: psychological factors correlated with health-related quality of life in Chinese patients with Crohn’s disease. ScientificWorldJournal. 2013;2013:464698. doi: 10.1155/2013/464698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Palma G, Collins SM, Bercik P. The microbiota-gut-brain axis in functional gastrointestinal disorders. Gut Microbes. 2014;5:419–429. doi: 10.4161/gmic.29417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jerndal P, Ringström G, Agerforz P, Karpefors M, Akkermans LM, Bayati A, Simrén M. Gastrointestinal-specific anxiety: an important factor for severity of GI symptoms and quality of life in IBS. Neurogastroenterol Motil. 2010;22:646–e179. doi: 10.1111/j.1365-2982.2010.01493.x. [DOI] [PubMed] [Google Scholar]
- 27.Tayama J, Nakaya N, Hamaguchi T, Tomiie T, Shinozaki M, Saigo T, Shirabe S, Fukudo S. Effects of personality traits on the manifestations of irritable bowel syndrome. Biopsychosoc Med. 2012;6:20. doi: 10.1186/1751-0759-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lackner JM, Gudleski GD, Thakur ER, Stewart TJ, Iacobucci GJ, Spiegel BM. The impact of physical complaints, social environment, and psychological functioning on IBS patients’ health perceptions: looking beyond GI symptom severity. Am J Gastroenterol. 2014;109:224–233. doi: 10.1038/ajg.2013.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yi ZH, Yang ZB, Kang L, Feng L, Yang L. [Clinical features, quality of life and psychological health of patients with irritable bowel syndrome and functional dyspepsia] Sichuan Daxue Xuebao Yixueban. 2014;45:493–496. [PubMed] [Google Scholar]
- 30.Pinto-Sanchez MI, Ford AC, Avila CA, Verdu EF, Collins SM, Morgan D, Moayyedi P, Bercik P. Anxiety and Depression Increase in a Stepwise Manner in Parallel With Multiple FGIDs and Symptom Severity and Frequency. Am J Gastroenterol. 2015;110:1038–1048. doi: 10.1038/ajg.2015.128. [DOI] [PubMed] [Google Scholar]
- 31.Lackner JM, Gudleski GD, Ma CX, Dewanwala A, Naliboff B. Fear of GI symptoms has an important impact on quality of life in patients with moderate-to-severe IBS. Am J Gastroenterol. 2014;109:1815–1823. doi: 10.1038/ajg.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 33.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 34.Pritchard SE, Garsed KC, Hoad CL, Lingaya M, Banwait R, Thongborisute W, Roberts E, Costigan C, Marciani L, Gowland PA, et al. Effect of experimental stress on the small bowel and colon in healthy humans. Neurogastroenterol Motil. 2015;27:542–549. doi: 10.1111/nmo.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ehlert U, Nater UM, Böhmelt A. High and low unstimulated salivary cortisol levels correspond to different symptoms of functional gastrointestinal disorders. J Psychosom Res. 2005;59:7–10. doi: 10.1016/j.jpsychores.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Cani PD. Crosstalk between the gut microbiota and the endocannabinoid system: impact on the gut barrier function and the adipose tissue. Clin Microbiol Infect. 2012;18 Suppl 4:50–53. doi: 10.1111/j.1469-0691.2012.03866.x. [DOI] [PubMed] [Google Scholar]
- 37.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 38.Grice EA, Segre JA. The human microbiome: our second genome. Annu Rev Genomics Hum Genet. 2012;13:151–170. doi: 10.1146/annurev-genom-090711-163814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neufeld KA, Kang N, Bienenstock J, Foster JA. Effects of intestinal microbiota on anxiety-like behavior. Commun Integr Biol. 2011;4:492–494. doi: 10.4161/cib.4.4.15702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selkrig J, Wong P, Zhang X, Pettersson S. Metabolic tinkering by the gut microbiome: Implications for brain development and function. Gut Microbes. 2014;5:369–380. doi: 10.4161/gmic.28681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2:94. doi: 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43:164–174. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 43.Holzer P, Farzi A. Neuropeptides and the microbiota-gut-brain axis. Adv Exp Med Biol. 2014;817:195–219. doi: 10.1007/978-1-4939-0897-4_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barsh GS, Schwartz MW. Genetic approaches to studying energy balance: perception and integration. Nat Rev Genet. 2002;3:589–600. doi: 10.1038/nrg862. [DOI] [PubMed] [Google Scholar]
- 45.Sustkova-Fiserova M, Jerabek P, Havlickova T, Kacer P, Krsiak M. Ghrelin receptor antagonism of morphine-induced accumbens dopamine release and behavioral stimulation in rats. Psychopharmacology (Berl) 2014;231:2899–2908. doi: 10.1007/s00213-014-3466-9. [DOI] [PubMed] [Google Scholar]
- 46.Galley JD, Nelson MC, Yu Z, Dowd SE, Walter J, Kumar PS, Lyte M, Bailey MT. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014;14:189. doi: 10.1186/1471-2180-14-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalliomäki MA, Walker WA. Physiologic and pathologic interactions of bacteria with gastrointestinal epithelium. Gastroenterol Clin North Am. 2005;34:383–399, vii. doi: 10.1016/j.gtc.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 48.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 49.de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:G440–G448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abraham C, Medzhitov R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology. 2011;140:1729–1737. doi: 10.1053/j.gastro.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim CH, Park J, Kim M. Gut microbiota-derived short-chain Fatty acids, T cells, and inflammation. Immune Netw. 2014;14:277–288. doi: 10.4110/in.2014.14.6.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daniel H, Gholami AM, Berry D, Desmarchelier C, Hahne H, Loh G, Mondot S, Lepage P, Rothballer M, Walker A, et al. High-fat diet alters gut microbiota physiology in mice. ISME J. 2014;8:295–308. doi: 10.1038/ismej.2013.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pokrajac-Bulian A, Kukić M, Bašić-Marković N. Quality of life as a mediator in the association between body mass index and negative emotionality in overweight and obese non-clinical sample. Eat Weight Disord. 2015;20:473–481. doi: 10.1007/s40519-015-0208-x. [DOI] [PubMed] [Google Scholar]
- 54.Nigatu YT, Bültmann U, Reijneveld SA. The prospective association between obesity and major depression in the general population: does single or recurrent episode matter? BMC Public Health. 2015;15:350. doi: 10.1186/s12889-015-1682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hryhorczuk C, Sharma S, Fulton SE. Metabolic disturbances connecting obesity and depression. Front Neurosci. 2013;7:177. doi: 10.3389/fnins.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castanon N, Luheshi G, Layé S. Role of neuroinflammation in the emotional and cognitive alterations displayed by animal models of obesity. Front Neurosci. 2015;9:229. doi: 10.3389/fnins.2015.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 58.Foley DL, Morley KI, Madden PA, Heath AC, Whitfield JB, Martin NG. Major depression and the metabolic syndrome. Twin Res Hum Genet. 2010;13:347–358. doi: 10.1375/twin.13.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wiltink J, Michal M, Wild PS, Zwiener I, Blettner M, Münzel T, Schulz A, Kirschner Y, Beutel ME. Associations between depression and different measures of obesity (BMI, WC, WHtR, WHR) BMC Psychiatry. 2013;13:223. doi: 10.1186/1471-244X-13-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vigsnæs LK, Brynskov J, Steenholdt C, Wilcks A, Licht TR. Gram-negative bacteria account for main differences between faecal microbiota from patients with ulcerative colitis and healthy controls. Benef Microbes. 2012;3:287–297. doi: 10.3920/BM2012.0018. [DOI] [PubMed] [Google Scholar]
- 61.Tong M, Li X, Wegener Parfrey L, Roth B, Ippoliti A, Wei B, Borneman J, McGovern DP, Frank DN, Li E, et al. A modular organization of the human intestinal mucosal microbiota and its association with inflammatory bowel disease. PLoS One. 2013;8:e80702. doi: 10.1371/journal.pone.0080702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Erickson AR, Cantarel BL, Lamendella R, Darzi Y, Mongodin EF, Pan C, Shah M, Halfvarson J, Tysk C, Henrissat B, et al. Integrated metagenomics/metaproteomics reveals human host-microbiota signatures of Crohn’s disease. PLoS One. 2012;7:e49138. doi: 10.1371/journal.pone.0049138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tulloch AJ, Murray S, Vaicekonyte R, Avena NM. Neural responses to macronutrients: hedonic and homeostatic mechanisms. Gastroenterology. 2015;148:1205–1218. doi: 10.1053/j.gastro.2014.12.058. [DOI] [PubMed] [Google Scholar]
- 64.Maffei M, Fei H, Lee GH, Dani C, Leroy P, Zhang Y, Proenca R, Negrel R, Ailhaud G, Friedman JM. Increased expression in adipocytes of ob RNA in mice with lesions of the hypothalamus and with mutations at the db locus. Proc Natl Acad Sci USA. 1995;92:6957–6960. doi: 10.1073/pnas.92.15.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walley AJ, Asher JE, Froguel P. The genetic contribution to non-syndromic human obesity. Nat Rev Genet. 2009;10:431–442. doi: 10.1038/nrg2594. [DOI] [PubMed] [Google Scholar]
- 66.Bagdade JD, Bierman EL, Porte D. The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J Clin Invest. 1967;46:1549–1557. doi: 10.1172/JCI105646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fox SI. Regulación del metabolismo. In: Fox SI. Fisiología humana. 10th ed. Madrid: McGraw Hill; 2008: 637-657 In: Fox SI, editor. [Google Scholar]
- 68.Bell CG, Walley AJ, Froguel P. The genetics of human obesity. Nat Rev Genet. 2005;6:221–234. doi: 10.1038/nrg1556. [DOI] [PubMed] [Google Scholar]
- 69.Pula K, Parks CD, Ross CF. Regulatory focus and food choice motives. Prevention orientation associated with mood, convenience, and familiarity. Appetite. 2014;78:15–22. doi: 10.1016/j.appet.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 70.Ganley RM. Emotion and eating in obesity: A review of the literature. Inter J Eating Disorders. 1989;8:343–361. [Google Scholar]
- 71.Singh M. Mood, food, and obesity. Front Psychol. 2014;5:925. doi: 10.3389/fpsyg.2014.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mela DJ. Determinants of food choice: relationships with obesity and weight control. Obes Res. 2001;9 Suppl 4:249S–255S. doi: 10.1038/oby.2001.127. [DOI] [PubMed] [Google Scholar]
- 73.MacKillop J. Integrating behavioral economics and behavioral genetics: delayed reward discounting as an endophenotype for addictive disorders. J Exp Anal Behav. 2013;99:14–31. doi: 10.1002/jeab.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schoenbaum G, Roesch M. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47:633–636. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gardner EL. Addiction and brain reward and antireward pathways. Adv Psychosom Med. 2011;30:22–60. doi: 10.1159/000324065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van der Laan LN, de Ridder DT, Viergever MA, Smeets PA. Activation in inhibitory brain regions during food choice correlates with temptation strength and self-regulatory success in weight-concerned women. Front Neurosci. 2014;8:308. doi: 10.3389/fnins.2014.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 79.Oganesyan GA, Romanova IV, Aristakesyan EA, Kuzik VV, Makina DM, Morina IY, Khramenkova AE, Artamokhina IV, Belova VA. The dopaminergic system of the telencephalo-diencephalic areas of the vertebrate brain in the organization of the sleep-waking cycle. Neurosci Behav Physiol. 2009;39:805–817. doi: 10.1007/s11055-009-9191-x. [DOI] [PubMed] [Google Scholar]
- 80.Boumaiza I, Omezzine A, Rejeb J, Rebhi L, Ouedrani A, Ben Rejeb N, Nabli N, Ben Abdelaziz A, Bouslama A. Relationship between leptin G2548A and leptin receptor Q223R gene polymorphisms and obesity and metabolic syndrome risk in Tunisian volunteers. Genet Test Mol Biomarkers. 2012;16:726–733. doi: 10.1089/gtmb.2011.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dougkas A, Yaqoob P, Givens DI, Reynolds CK, Minihane AM. The impact of obesity-related SNP on appetite and energy intake. Br J Nutr. 2013;110:1151–1156. doi: 10.1017/S0007114513000147. [DOI] [PubMed] [Google Scholar]
- 82.Hinuy HM, Hirata MH, Sampaio MF, Armaganijan D, Arazi SS, Salazar LA, Hirata RD. Relationship between variants of the leptin gene and obesity and metabolic biomarkers in Brazilian individuals. Arq Bras Endocrinol Metabol. 2010;54:282–288. doi: 10.1590/s0004-27302010000300006. [DOI] [PubMed] [Google Scholar]
- 83.Mizuta E, Kokubo Y, Yamanaka I, Miyamoto Y, Okayama A, Yoshimasa Y, Tomoike H, Morisaki H, Morisaki T. Leptin gene and leptin receptor gene polymorphisms are associated with sweet preference and obesity. Hypertens Res. 2008;31:1069–1077. doi: 10.1291/hypres.31.1069. [DOI] [PubMed] [Google Scholar]
- 84.Mammès O, Betoulle D, Aubert R, Herbeth B, Siest G, Fumeron F. Association of the G-2548A polymorphism in the 5’ region of the LEP gene with overweight. Ann Hum Genet. 2000;64:391–394. doi: 10.1017/s0003480000008277. [DOI] [PubMed] [Google Scholar]
- 85.Hoffstedt J, Eriksson P, Mottagui-Tabar S, Arner P. A polymorphism in the leptin promoter region (-2548 G/A) influences gene expression and adipose tissue secretion of leptin. Horm Metab Res. 2002;34:355–359. doi: 10.1055/s-2002-33466. [DOI] [PubMed] [Google Scholar]
- 86.Farooqi IS, O’Rahilly S. Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nat Clin Pract Endocrinol Metab. 2008;4:569–577. doi: 10.1038/ncpendmet0966. [DOI] [PubMed] [Google Scholar]
- 87.Yiannakouris N, Yannakoulia M, Melistas L, Chan JL, Klimis-Zacas D, Mantzoros CS. The Q223R polymorphism of the leptin receptor gene is significantly associated with obesity and predicts a small percentage of body weight and body composition variability. J Clin Endocrinol Metab. 2001;86:4434–4439. doi: 10.1210/jcem.86.9.7842. [DOI] [PubMed] [Google Scholar]
- 88.Loos RJ, Lindgren CM, Li S, Wheeler E, Zhao JH, Prokopenko I, Inouye M, Freathy RM, Attwood AP, Beckmann JS, Berndt SI; Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, Jacobs KB, Chanock SJ, Hayes RB, Bergmann S, Bennett AJ, Bingham SA, Bochud M, Brown M, Cauchi S, Connell JM, Cooper C, Smith GD, Day I, Dina C, De S, Dermitzakis ET, Doney AS, Elliott KS, Elliott P, Evans DM, Sadaf Farooqi I, Froguel P, Ghori J, Groves CJ, Gwilliam R, Hadley D, Hall AS, Hattersley AT, Hebebrand J, Heid IM; KORA, Lamina C, Gieger C, Illig T, Meitinger T, Wichmann HE, Herrera B, Hinney A, Hunt SE, Jarvelin MR, Johnson T, Jolley JD, Karpe F, Keniry A, Khaw KT, Luben RN, Mangino M, Marchini J, McArdle WL, McGinnis R, Meyre D, Munroe PB, Morris AD, Ness AR, Neville MJ, Nica AC, Ong KK, O’Rahilly S, Owen KR, Palmer CN, Papadakis K, Potter S, Pouta A, Qi L; Nurses’ Health Study, Randall JC, Rayner NW, Ring SM, Sandhu MS, Scherag A, Sims MA, Song K, Soranzo N, Speliotes EK; Diabetes Genetics Initiative, Syddall HE, Teichmann SA, Timpson NJ, Tobias JH, Uda M; SardiNIA Study, Vogel CI, Wallace C, Waterworth DM, Weedon MN; Wellcome Trust Case Control Consortium, Willer CJ; FUSION, Wraight, Yuan X, Zeggini E, Hirschhorn JN, Strachan DP, Ouwehand WH, Caulfield MJ, Samani NJ, Frayling TM, Vollenweider P, Waeber G, Mooser V, Deloukas P, McCarthy MI, Wareham NJ, Barroso I, Jacobs KB, Chanock SJ, Hayes RB, Lamina C, Gieger C, Illig T, Meitinger T, Wichmann HE, Kraft P, Hankinson SE, Hunter DJ, Hu FB, Lyon HN, Voight BF, Ridderstrale M, Groop L, Scheet P, Sanna S, Abecasis GR, Albai G, Nagaraja R, Schlessinger D, Jackson AU, Tuomilehto J, Collins FS, Boehnke M, Mohlke KL. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet. 2008;40:768–775. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xi B, Chandak GR, Shen Y, Wang Q, Zhou D. Association between common polymorphism near the MC4R gene and obesity risk: a systematic review and meta-analysis. PLoS One. 2012;7:e45731. doi: 10.1371/journal.pone.0045731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Acosta A, Camilleri M, Shin A, Carlson P, Burton D, O’Neill J, Eckert D, Zinsmeister AR. Association of melanocortin 4 receptor gene variation with satiation and gastric emptying in overweight and obese adults. Genes Nutr. 2014;9:384. doi: 10.1007/s12263-014-0384-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Cull JG, Comings DE. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J R Soc Med. 1996;89:396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pohjalainen T, Rinne JO, Någren K, Lehikoinen P, Anttila K, Syvälahti EK, Hietala J. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry. 1998;3:256–260. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- 93.Berman SM, Ozkaragoz T, Noble EP, Antolin T, Sheen C, Siddarth P, Conner BT, Ritchie T. Differential associations of sex and D2 dopamine receptor (DRD2) genotype with negative affect and other substance abuse risk markers in children of alcoholics. Alcohol. 2003;30:201–210. doi: 10.1016/j.alcohol.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 94.Blum K, Oscar-Berman M, Demetrovics Z, Barh D, Gold MS. Genetic Addiction Risk Score (GARS): molecular neurogenetic evidence for predisposition to Reward Deficiency Syndrome (RDS) Mol Neurobiol. 2014;50:765–796. doi: 10.1007/s12035-014-8726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bannon MJ, Michelhaugh SK, Wang J, Sacchetti P. The human dopamine transporter gene: gene organization, transcriptional regulation, and potential involvement in neuropsychiatric disorders. Eur Neuropsychopharmacol. 2001;11:449–455. doi: 10.1016/s0924-977x(01)00122-5. [DOI] [PubMed] [Google Scholar]
- 96.Genro JP, Polanczyk GV, Zeni C, Oliveira AS, Roman T, Rohde LA, Hutz MH. A common haplotype at the dopamine transporter gene 5’ region is associated with attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1568–1575. doi: 10.1002/ajmg.b.30863. [DOI] [PubMed] [Google Scholar]
- 97.Fontana C, Vitolo MR, Campagnolo PD, Mattevi VS, Genro JP, Almeida S. DRD4 and SLC6A3 gene polymorphisms are associated with food intake and nutritional status in children in early stages of development. J Nutr Biochem. 2015;26:1607–1612. doi: 10.1016/j.jnutbio.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 98.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 99.Solís-Ortiz S, Pérez-Luque E, Gutiérrez-Muñoz M. Modulation of the COMT Val(158)Met polymorphism on resting-state EEG power. Front Hum Neurosci. 2015;9:136. doi: 10.3389/fnhum.2015.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stein DJ, Newman TK, Savitz J, Ramesar R. Warriors versus worriers: the role of COMT gene variants. CNS Spectr. 2006;11:745–748. doi: 10.1017/s1092852900014863. [DOI] [PubMed] [Google Scholar]
- 101.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 102.Gao X, Gong P, Liu J, Hu J, Li Y, Yu H, Gong X, Xiang Y, Jiang C, Zhou X. COMT Val158Met polymorphism influences the susceptibility to framing in decision-making: OFC-amygdala functional connectivity as a mediator. Hum Brain Mapp. 2016;37:1880–1892. doi: 10.1002/hbm.23142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rosas-Vargas H, Martínez-Ezquerro JD, Bienvenu T. Brain-derived neurotrophic factor, food intake regulation, and obesity. Arch Med Res. 2011;42:482–494. doi: 10.1016/j.arcmed.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 104.Bonaccorso S, Sodhi M, Li J, Bobo WV, Chen Y, Tumuklu M, Theleritis C, Jayathilake K, Meltzer HY. The brain-derived neurotrophic factor (BDNF) Val66Met polymorphism is associated with increased body mass index and insulin resistance measures in bipolar disorder and schizophrenia. Bipolar Disord. 2015;17:528–535. doi: 10.1111/bdi.12294. [DOI] [PubMed] [Google Scholar]
- 105.Koh JY, Lim JS, Byun HR, Yoo MH. Abnormalities in the zinc-metalloprotease-BDNF axis may contribute to megalencephaly and cortical hyperconnectivity in young autism spectrum disorder patients. Mol Brain. 2014;7:64. doi: 10.1186/s13041-014-0064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li D, Zhang J. Diet shapes the evolution of the vertebrate bitter taste receptor gene repertoire. Mol Biol Evol. 2014;31:303–309. doi: 10.1093/molbev/mst219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ramos-Lopez O, Panduro A, Martinez-Lopez E, Roman S. Sweet Taste Receptor TAS1R2 Polymorphism (Val191Val) Is Associated with a Higher Carbohydrate Intake and Hypertriglyceridemia among the Population of West Mexico. Nutrients. 2016;8:101. doi: 10.3390/nu8020101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ramos-Lopez O, Roman S, Martinez-Lopez E, Fierro NA, Gonzalez-Aldaco K, Jose-Abrego A, Panduro A. CD36 genetic variation, fat intake and liver fibrosis in chronic hepatitis C virus infection. World J Hepatol. 2016;8:1067–1074. doi: 10.4254/wjh.v8.i25.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ramos-Lopez O, Panduro A, Martinez-Lopez E, Fierro NA, Ojeda-Granados C, Sepulveda-Villegas M, Roman S. Genetic Variant in the CD36 Gene (rs1761667) is Associated with Higher Fat Intake and High Serum Cholesterol among the Population of West Mexico. J Nutr Food Sci. 2015;5:2. [Google Scholar]
- 110.Ramos-Lopez O, Roman S, Martinez-Lopez E, Gonzalez-Aldaco K, Ojeda-Granados C, Sepulveda-Villegas M, Panduro A. Association of a novel TAS2R38 haplotype with alcohol intake among Mexican-Mestizo population. Ann Hepatol. 2015;14:729–734. [PubMed] [Google Scholar]
- 111.Martinez-Lopez E, Curiel-Lopez F, Hernandez-Nazara A, Moreno-Luna LE, Ramos-Marquez ME, Roman S, Panduro A. Influence of ApoE and FABP2 polymorphisms and environmental factors in the susceptibility to gallstone disease. Ann Hepatol. 2015;14:515–523. [PubMed] [Google Scholar]
- 112.Ojeda-Granados C, Panduro A, Rebello Pinho JR, Ramos-Lopez O, Gleyzer K, Malta FM, Gonzalez-Aldaco K, Roman S. Association of Lactase Persistence Genotypes with High Intake of Dairy Saturated Fat and High Prevalence of Lactase Non-Persistence among the Mexican Population. J Nutrigenet Nutrigenomics. 2016;9:83–94. doi: 10.1159/000446241. [DOI] [PubMed] [Google Scholar]
- 113.Panduro A, Ramos-Lopez O, Campollo O, Zepeda-Carrillo EA, Gonzalez-Aldaco K, Torres-Valadez R, Roman S. High frequency of the DRD2/ANKK1 A1 allele in Mexican Native Amerindians and Mestizos and its association with alcohol consumption. Drug Alcohol Depend. 2017;172:66–72. doi: 10.1016/j.drugalcdep.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 114.Ramos-Lopez O, Roman S, Ojeda-Granados C, Panduro A. Patrón de ingesta alimentaria y actividad física en pacientes hepatópatas en el Occidente de México. Rev Endocronol Nutr. 2013;21:7–15. [Google Scholar]
- 115.Roman S, Ojeda-Granados C, Ramos-Lopez O, Panduro A. Genome-based nutrition: an intervention strategy for the prevention and treatment of obesity and nonalcoholic steatohepatitis. World J Gastroenterol. 2015;21:3449–3461. doi: 10.3748/wjg.v21.i12.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schultz W. Neuronal Reward and Decision Signals: From Theories to Data. Physiol Rev. 2015;95:853–951. doi: 10.1152/physrev.00023.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


