Abstract
Macrophage inflammatory protein (MIP)-2 is one of the CXC chemokines and is also known as chemokine CXC ligand (CXCL2). MIP-2 affects neutrophil recruitment and activation through the p38 mitogen-activated-protein-kinase-dependent signaling pathway, by binding to its specific receptors, CXCR1 and CXCR2. MIP-2 is produced by a variety of cell types, such as macrophages, monocytes, epithelial cells, and hepatocytes, in response to infection or injury. In liver injury, activated Kupffer cells are known as the major source of MIP-2. MIP-2-recruited and activated neutrophils can accelerate liver inflammation by releasing various inflammatory mediators. Here, we give a brief introduction to the basic molecular and cellular sources of MIP-2, and focus on its physiological and pathological functions in acute liver injury induced by concanavalin A, lipopolysaccharides, irradiation, ischemia/reperfusion, alcohol, and hypoxia, and hepatectomy-induced liver regeneration and tumor colorectal metastasis. Further understanding of the regulatory mechanisms of MIP-2 secretion and activation may be helpful to develop MIP-2-targeted therapeutic strategies to prevent liver inflammation.
Keywords: Macrophage inflammatory protein-2, Liver injury, Polymorphonuclear neutrophils, Macrophages, Inflammation
Core tip: Macrophage inflammatory protein (MIP)-2 is produced by a variety of cell types in response to infection or injury, and affects neutrophil recruitment and activation by binding to chemokine CXC receptor (CXCR)1 and CXCR2. MIP-2 plays a complex dual role in the development of liver diseases by mediating liver inflammation at a high concentration and promoting liver regeneration at a low concentration. Here, we review its physiological and pathological functions in various types of liver damage. Further understanding of the regulatory mechanisms of MIP-2 may be helpful to develop MIP-2-targeted therapeutic strategies.
INTRODUCTION
The chemokine family with its ability to mediate leukocyte chemotaxis can be classified into four subgroups according to the polypeptide chain cysteine location: C, CC, CXC, and CX3C[1,2]. To date, about 50 chemokines that exhibit various physiological and pathological properties have been discovered, and most of them belong to the CC and CXC families[3]. Macrophage inflammatory protein (MIP)-2, also known as CXC ligand (CXCL)2, is one of the CXC chemokines. It assists in the recruitment of polymorphonuclear neutrophils (PMNs) to sites of injury or infection and thereby modulates immune and inflammatory responses.
MIP-2 is released by a variety of cells in response to infection or injury, and was originally detected in macrophages as a part of their response to inflammatory stimuli. Kupffer cells contribute as potent effectors of inflammation in acute liver injury[4]. In contrast to Kupffer-cell-sufficient mice, Kupffer-cell-ablated mice are resistant to lipopolysaccharide (LPS)-induced mortality and acute liver injury[5]. Accumulation of neutrophils, which may drive inflammation in liver injury by releasing cytokines, in the liver of Kupffer-cell-ablated mice is significantly reduced. Since MIP-2 and its related molecules regulate neutrophil infiltration and microabscess formation, further understanding of MIP-2 function and its signaling network may provide new ideas for control of liver inflammation. The present review summarizes the basic molecular and cellular sources of MIP-2, and focuses on MIP-2 production and function in acute liver injury.
BASIC MOLECULAR AND RECEPTORS OF MIP-2
The murine (mu) MIP-2 genomic clone has four exons and three introns, which is the typical structure of platelet factor (PF)4 chemokine sub-family[6-8]. Murine keratinocyte chemoattractant (KC)[9] and rat gene product/cytokine-induced neutrophil chemoattractant[10] are involved in the sub-family[6]. There are many human homologs of rodent MIP-2, including human platelet basic protein[11], human growth-related oncogenes/melanoma growth stimulating activity, MIP-2α, and MIP-2β[6,12]. Although MIP-2 is a distinct member of the PF4 family, its sequence is closely related to that of the growth-related oncogene KC cytokines[12]. MIP-2γ is a novel CXC chemokine from a human dendritic cell cDNA library, and has no known ELR motif and shares greatest homology with MIP-2α/β. Murine MIP-2γ is highly homologous to human MIP-2γ[13].
MIP-2 family members are potent chemotactic factors for neutrophils. MIP-2α/β affect neutrophil recruitment and activation through the p38 mitogen-activated protein kinase (MAPK)-dependent signaling pathway, by binding to two specific receptors belonging to the G-protein-coupled receptor family, CXC chemokine receptor (CXCR)1 and CXCR2[14]. MIP-2γ can mediate neutrophil recruitment by binding to a novel CXC chemokine receptor, other than CXCR1 or CXCR2[13].
MIP-2 SECRETING CELLS
MIP-2 is produced by a variety of cell types, such as macrophages, monocytes, epithelial cells, and hepatocytes, in response to infection or injury[14]. In liver injury, activated Kupffer cells are the major source of MIP-2. Lentsch et al[15] and Mosher et al[16] reported that the level of plasma MIP-2 in GdCl3-pretreated mice, in which Kupffer cell activity was inhibited, was significantly reduced in a model of hepatic ischemia/reperfusion (IR) injury. The extent of liver injury and neutrophil infiltration was also significantly decreased in GdCl3-treated mice, which might have been associated with the decreased levels of MIP-2. Kupffer cell blockade by GdCl3 treatment significantly reduced liver MIP-2 gene expression and liver inflammation after the administration of high doses of adenovirus vectors, which can induce innate immune responses in mice[17].
The production of MIP-2 is regulated by multiple factors. Synthesis of chemokines is regulated at the transcriptional level by signaling through Toll-like receptor (TLR)2, TLR3, and TLR4 in response to diverse pathogens[18]. MIP-2 production can be effectively inhibited in LPS-stimulated mouse peritoneal macrophage cell line, RAW 264.7, through downregulating mRNA accumulation and protein expression of membrane TLR4/mCD14. This indicates that upstream inhibition of the TLR4/CD14-mediated inflammation pathway may be an effective therapeutic approach for attenuating damaging immune activation[19]. So et al[20] found that Scutellariae Radix and Liriopis Tuber (SL) significantly inhibited the release of MIP-2 in LPS-induced RAW 264.7 cells. Another study showed that histone deacetylase modulated MIP-2 secretion. The secretion of MIP-2 was enhanced in LPS-stimulated and interleukin (IL)-1β-stimulated rat small intestinal epithelial cells by butyrate, a bacterial metabolite, through modulating histone acetylase. Furthermore, acetylation of histones by a specific inhibitor of histone deacetylase enhanced MIP-2 expression in IL-1β-stimulated cells[21].
MIP-2 MEDIATES INFLAMMATION BY NEUTROPHIL RECRUITMENT
Neutrophils are the most abundant circulating white blood cell type and a major innate immune cell subset in humans. Inappropriate activation and recruitment of neutrophils to the microvasculature contributes to the pathological manifestations of many types of inflammation[22]. In the liver, the recruitment of neutrophils to the sites of injury or infection is MIP-2 dependent.
MIP-2 as potent neutrophil chemotactic factor
MIP-2 is a potent chemotactic and activation factor of neutrophils and plays a critical role in neutrophil recruitment during acute inflammation in rat disease models[23]. It was found that corneal MIP-2 levels were correlated with persistence of PMNs in the cornea of susceptible (cornea perforates) mice after Pseudomonas aeruginosa challenge. By treating systemically with recombinant MIP-2, the number of corneal PMNs was significantly increased, and resulted in exacerbated corneal disease in resistant (cornea heals) mice[24]. In the cecal ligation and puncture (CLP) model for sepsis, MIP-2 mRNA and protein were significantly upregulated after CLP in mice, while the neutralization of MIP-2 by anti-MIP-2 antibody reduced peritoneal PMN migration. Mercer-Jones et al[25] also found that mast cells were necessary for PMN migration into the peritoneum, and significantly less migration of PMNs into the peritoneal cavity in the mast-cell-deficient mice after MIP-2 injection. MIP-2 was also involved in neutrophil recruitment in the central nervous system during experimental bacterial meningitis. The kinetics of MIP-2 mRNA expression are paralleled by the recruitment of inflammatory cells and disease severity. Blocking of MIP-2 bioactivity by anti-MIP-2 antibodies results in significantly decreased neutrophil influx[26,27]. When injected in vivo as recombinant chemokines, KC and MIP-2 in models of inflammation, can cause neutrophil influx[28,29]. The results of other studies have highlighted MIP-2 as the major chemoattractant[29]. In liver injury, neutralizing KC and MIP-2 result in less neutrophil extravasation and reduce neutrophil-induced injury in a mouse model of cholestatic liver damage[30]. Further studies have shown that neutrophil extravasation into the parenchyma requires a chemotactic signal such as MIP-2 and KC from macrophages, hepatocytes, or already-extravasated neutrophils. Tissue damage and cell necrosis often result in the release of damage-associated molecular patterns, which lead to intercellular adhesion molecule-1 upregulation on sinusoidal endothelial cells. Neutrophils are then recruited to endothelial cells or hepatocytes via a β2 integrin macrophage antigen (Mac)-1-dependent adhesion mechanism[24,31-35].
Neutrophils drive inflammation in liver injury by releasing inflammatory mediators
The recruitment of neutrophils to target cells triggers full activation of the neutrophils with a long-lasting adherence dependent oxidative stress and degranulation. The activated neutrophils release various inflammatory mediators, including proteolytic enzymes, lipocalin 2, arachidonic acid metabolites, and reactive oxygen species (ROS)[36-40].
Several mechanisms of neutrophil-mediated tissue injury have been proposed. One is the production of reactive oxygen intermediates, which may directly induce hepatic endothelial damage or indirectly induce tissue injury by triggering other inflammatory mediators[41,42]. Neutrophil-derived proteases facilitate extravasation and are involved in the regulation of inflammatory mediator production. The adhesion via Mac-1 triggers superoxide formation by NADPH oxidase and degranulation with the release of myeloperoxidase (MPO) and proteases[43]. Optimal oxygen-dependent microbicidal activity depends on MPO as the critical enzyme for the generation of hypochlorous acid and other toxic oxygen products. Although the proteases appear to be mainly involved in the promotion of chemokines, hydrogen peroxide, and MPO-derived hypochlorite, they also induce intracellular oxidative stress in hepatocytes and eventually cause oncotic necrosis[44,45].
Liver dysfunction and cell injury induced by neutrophils have been demonstrated in several experimental models including hepatic IR injury[46], endotoxic shock[47], sepsis[48], alcoholic hepatitis[49], obstructive cholestasis[50], LPS injury[51], remote organ trauma[52], and concanavalin A (ConA)-induced liver injury[53]. Neutrophil-mediated injury was also demonstrated in two-hit models of IR injury or drug hepatotoxicity in combination with endotoxemia[54].
Recruitment of neutrophils to the sites of liver injury is MIP-2 dependent, and the activated neutrophils can accelerate liver inflammation by releasing various inflammatory mediators.
MIP-2 PRODUCTION IN MURINE MODELS OF ACUTE LIVER INJURY
MIP-2 plays an important role in the progression of inflammation. Some clinical studies have shown the correlation between MIP-2 and organ inflammation, such as in pneumonia[55]. However, there are few clinical reports about the effect of MIP-2 in acute liver injury. At present, most of the studies on the mechanism of MIP-2 secretion and regulation in acute liver injury have been in animal models.
ConA-induced acute liver injury
ConA-induced hepatitis is a well-characterized form of autoimmune hepatic damage in murine models, with a pathophysiology similar to that of human viral and autoimmune hepatitis[56]. T cells, particularly CD4+ cells, play an essential role in the development of ConA-induced hepatitis. Activated T lymphocyte infiltration induces hepatocyte apoptosis and necrosis and provokes production and secretion of a series of proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, interferon (IFN)-γ, IL-6 and ROS[57,58]. Besides that, MIP-2 is another major mediator of ConA-mediated inflammation[53]. The infiltration of F4/80+ macrophages and the mRNA level of MIP-2 were dramatically increased in the liver of ConA-treated mice[53,54]. The plasma MIP-2 level was elevated and reached a peak value at 2 h after ConA injection[59]. Pretreatment with anti-MIP-2 antibody suppressed the elevation of plasma alanine aminotransferase (ALT) levels and reduce hepatic necrosis in a dose-dependent manner[20,32,60]. Hepatic infiltration of neutrophils was also attenuated by MIP-2 blockade[60,61]. Another study showed that antithrombin III, an important physiological inhibitor of the coagulation cascade, prevents ConA-induced liver injury through inhibition of MIP-2 release[59]. Our previous study showed that emodin pretreatment protects against ConA-induced liver injury in mice, and this effect may occur partially through inhibition of macrophages infiltration and activation of the p38 MAPK/nuclear factor (NF)-κB pathway in macrophages[53].
LPS-induced acute liver injury
LPS is one of the most potent innate immune-activating stimuli. LPS binds to TLR4 to induce macrophage activation, which results in the secretion of the CXC chemokines and proinflammatory mediators. Inflammatory cells such as neutrophils, T lymphocytes, multiple leukocyte subtypes, monocytes, eosinophils, basophils, dendritic cells, and natural killer cells can be attracted into the injured tissue sections through the portal vessels and play a pivotal role in initiating an inflammatory response[23,62-64]. Some studies have shown that MIP-2 is one of the key mediators in LPS-induced liver injury[65,66]. MIP-2 expression was induced in freshly isolated rat hepatocytes following treatment with LPS[67]. The hepatic expression of chemokine mRNAs was elevated after LPS exposure, with the maximal expression of MIP-2 mRNA at 1 h after LPS treatment[66,68]. LPS in vivo also induced high levels of chemokine MIP-2 mRNA in mouse liver and lung, with a concomitant increase in circulating MIP-2 protein[69]. MIP-2 production was inhibited in LPS-stimulated RAW 264.7 though downregulation of mRNA accumulation and protein expression of TLR4[19], indicating that TLR4 is the important receptor in LPS-induced liver injury. Further studies have shown that the LPS-induced MIP-2 production is dependent on NF-κB activation via inhibition of the IKK pathway[70-72]. Intraperitoneal injection of anti-MIP-2 antibody significantly decreases the influx of neutrophils in the liver of rats after LPS injection[73]. Interference with MIP-2 function protects against septic liver damage, which may constitute a potential therapeutic strategy to control pathological inflammation in endotoxemia[74].
Irradiation-induced acute liver injury
Liver is considered to be a radiosensitive organ. Radiation therapy involves the use of high-energy rays to treat local or regional malignancies. Free radicals such as ROS containing unpaired electrons are generated after irradiation in the irradiated tissue, and cells that are chemically active are prone to oxidative stress. Liver damage is a serious clinical complication of radiation therapy[64,75]. Single dose γ-irradiation (25 Gy) focused on the liver recruits neutrophils attached to the portal vessels and to portal (myo) fibroblasts in the liver, and several chemokines may be necessary in their recruitment, adhesion, and transmigration. Rapid and early induction of expression of several chemokines and chemokine receptor CXCR2 genes in irradiated liver tissue and portal area has been observed. MIP-2 has been detected in the portal vessel walls, and CXCR2 in the portal area but not in the parenchyma[64,76]. The induction of the mediators in cells of portal area (mainly myofibroblasts) may happen through ROS[39].
IR-induced acute liver injury
IR-induced hepatic injury is an important clinical problem after liver resection or transplantation. Initial IR-induced hepatic injury is reported to be mediated by activated Kupffer cells without dependence on neutrophils[77,78]. The later phase of IR injury is dependent upon hepatic neutrophil sequestration, and the subsequent increased adherence between neutrophils and endothelial cells. Depletion of neutrophils and Kupffer cells before ischemia greatly reduces reperfusion injury[79,80]. MIP-2 is also known as an important mediator in IR-induced liver injury by regulating hepatic neutrophil accumulation[41,42,73,81,82]. Expression of MIP-2 mRNA was induced within 3 h after reperfusion, before neutrophil accumulation in the liver, and was increased to a greater extent in the ischemic liver lobe at 9 h post-reperfusion[15]. The anti-inflammatory cytokine IL-10 affects inflammatory reactions partly through inhibitory effects on NF-κB. Yoshidome et al[83] found that IL-10 protects against hepatic IR injury by suppressing NF-κB activation as well as hepatic mRNA expression and the serum level of MIP-2. A recent study showed that Pin1, as a critical regulator for NF-κB/DNA binding and activation, might be an important protective factor for hepatocytes against IR injury by reducing serum MIP-2 level after reperfusion[84].
Alcohol-induced acute liver injury
Alcohol is a well-known risk factor related to liver injury. Excessive alcohol exposure leads to alcohol liver disease; a major cause of morbidity and mortality worldwide. Alcohol abuse also causes hepatic steatosis. Alcohol combination with a high-fructose diet could aggravate alcoholic fatty liver disease[85]. The major pathogenetic factors are multifactorial and complex, involving increased hepatic de novo lipogenesis and triglyceride synthesis, impaired mitochondrial fatty acid β-oxidation, decreased very low-density lipoprotein secretion, and increased levels of chemokine secretion and adhesion molecule expression[86-88].
MIP-2 also plays a potent role in alcohol-induced liver injury. In alcohol-fed male Sprague-Dawley rats, alcohol intoxication induced hepatic injury through endotoxin influx in the circulation, and stimulated the Kupffer cells to produce MIP2 and upregulated expression of adhesion molecules on hepatic cells, which resulted in altered hepatic function and hepatotoxicity by hepatic neutrophils recruitment[89]. Nanji et al[90] showed that alcohol-induced liver injury was more severe in female than in male rats. Female rats had higher levels of endotoxin, lipid peroxidation, non-heme iron, and chemokines MCP-1 and MIP-2 after alcohol intake. The upregulation of MIP-2 in alcohol-induced liver injury has also been shown to be NF-κB dependent[90,91]. Inhibition of NF-κB activation by treatment with a phenolic antioxidant, curcumin, prevented the pathological and biochemical changes induced by alcohol, and enhanced MIP-2 expression[91,92].
Hypoxia-induced liver injury
In cellular responses to hypoxia, hypoxia-inducible factors as well as proinflammatory cytokine/chemokines are released. Oxygen consumption by hepatocytes and infiltrating inflammatory leukocytes is dramatically increased. Mice exposed to chronic intermittent hypoxia (CIH) exhibited lobular inflammation and fibrosis in the liver. CIH caused significant increases in lipid peroxidation in serum and liver, and increased hepatic levels of proinflammatory cytokines IL-1β, IL-6 and CXC chemokine MIP-2[93]. An in vitro study showed that MIP-2 expression was prominently induced by hypoxia both at the mRNA and protein level in RAW264.7 cells, while it was abolished by a mutation targeted to an NF-κB binding site in the MIP-2 promoter, suggesting that hypoxia-induced MIP-2 expression occurs exclusively via the NF-κB pathway. Further study of the mechanism by using inhibitors of signaling kinases have shown that the induction of MIP-2 is correlated with p42/p44 and PI3 kinase but not p38 kinase signaling in hypoxia[94].
OTHER FUNCTIONS OF MIP-2
Apart from its major role in mediating inflammation, MIP-2 also plays important roles in liver regeneration and engraftment of colorectal metastasis at extrahepatic sites. Administration of exogenous MIP-2 after 70% hepatectomy dramatically increases hepatocyte proliferation. Inhibition of the MIP-2 receptor, CXCR2, decreases baseline hepatocyte proliferation in the setting of partial hepatectomy. These data suggest that MIP-2 is important for hepatocyte proliferation and pharmacological doses of MIP-2 after hepatic injury may accelerate hepatic regeneration[95]. Adenovirus-mediated gene therapy or acetaminophen ingestion often produces profound hepatocellular injury. MIP-2 has a protective role in both adenovirus- and acetaminophen-mediated hepatotoxicity, suggesting that MIP-2 promotes hepatic regeneration following acute hepatic injury[96,97]. CCR2 is the primary receptor for chemokine MCP-1, which mainly attracts macrophages to secrete MIP-2. It was shown that CCR2-deficient mice had increased hepatic toxicity after acetaminophen exposure[98]. Further studies have shown that the properties in liver regeneration of ELR-CXC chemokines, such as MIP-2, in acetaminophen challenge are attributed mainly to the ELR motif[77,99,100].
Other studies have demonstrated the role of MIP-2 in the hepatectomy-induced acceleration of tumor growth[101,102]. Major liver resection often initiates rapid regeneration of the remnant liver to restore functional hepatic capacity. Besides parenchymal regeneration, hepatectomy also accelerates tumor growth in the remaining liver and remote organ sites[6]. MIP-2 contributes to liver-resection-induced acceleration of colorectal metastasis at extrahepatic sites. Blockade of MIP-2 decreases the hepatectomy-induced increase of CXCR2 expression on tumor cells, thus attenuating the augmentation of angiogenesis and metastatic tumor growth after hepatectomy[103]. A further study showed that liver-resection-associated MIP-2 upregulation stimulates extrahepatic tumor cell engraftment but not the growth of established metastases[101].
MIP-2 SIGNALING PATHWAYS
A variety of cytokines and signal pathways regulate the production level of MIP-2 in macrophages (Figure 1). MIP-2 expression is partially inhibited by intradermal injection of a neutralizing antibody against IL-1, which has modest stimulus activity for MIP-2[103]. Fas ligation induces MIP-2 expression in the liver through activation of caspase-3 and nuclear translocation of activator protein-1[104]. The increased production of ROS by ATP-stimulated macrophages activates the signaling pathways that promote MIP-2 production, which, in turn, induces neutrophil migration[105]. Cotreatment with IL-17A synergistically enhances the upregulation of MIP-2 in taurocholic-acid-treated primary mouse hepatocytes, suggesting that IL-17A promotes hepatic inflammation by enhancing bile-acid-induced production of MIP-2[106]. Treatment of RAW264.7 cells with an inhibitor of p38 attenuated the synergistic effects of C5a and MIP-2 on cells primed with muramyl dipeptide[107]. Another study has shown that inhibiting ELR-CXC chemokines can block ELR+ CXC chemokines neutrophil recruitment and activation in vitro. In IR-induced hepatic injury, the hepatic levels of ELR+ chemokines, including MIP-2, were decreased in response to IFN-γ, which is known to upregulate ELR-CXC chemokines[108]. IL-10 also shown protects against hepatic IR injury by suppressing NF-κB activation and subsequent expression of MIP-2[83,109].
Figure 1.
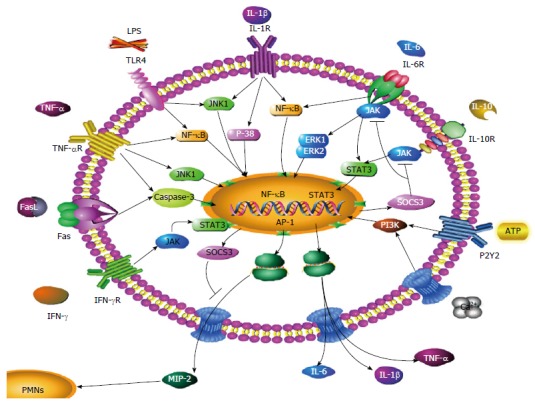
Molecular mechanisms involved in macrophage inflammatory protein-2 secretion of macrophages. The Figure illustrates several reciprocal molecular pathways for the secretion of MIP-2. These include: LPS-mediated induction of MIP-2, IL-6, IL-1β, TNF-α through the NF-κB/MAPK signaling pathway; IL-6-mediated induction of MIP-2 through the NF-κB/MAPK signaling pathway; TNF-α-mediated induction of MIP-2 through the NF-κB/MAPK, caspase-3 signaling pathway; FasL-mediated induction of MIP-2 through the caspase-3 signaling pathway; Ca2+- and ATP-mediated induction of MIP-2 through the PI3K signaling pathway; IL-1β-mediated induction of MIP-2 through the NF-κB/MAPK signaling pathway; IFN-γ and IL-10-mediated inhibition of MIP-2, IL-6, IL-1β and TNF-α through the JAK/STAT3 signaling pathway; SOCS3-mediated inhibition of JAK/STAT3. MIP-2: Macrophage inflammatory protein-2; TNF-α: Tumor necrosis factor-α; IFN-γ: Interferon-γ; IL: Interleukin; LPS: Lipopolysaccharide.
CONCLUSION
MIP-2 plays a dual role in mediating liver inflammation and promoting liver regeneration. Liver regeneration depends on the physiological concentration of MIP-2, however, excessive elevation of MIP-2 induced by acute liver injury promotes liver inflammation by neutrophil recruitment. An imbalance of MIP-2 secretion resulting in a disorder between the pro- and anti-inflammatory mediators may be vital in determining the outcome of liver injury. Since the signaling mechanisms of MIP-2 secretion remain to be elucidated, further understanding of the regulation mechanism of MIP-2 secretion is helpful to develop MIP-2-targeted therapeutic strategies for preventing liver inflammation.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors declare that there are no conflicts of interest related to this study.
Peer-review started: November 3, 2016
First decision: December 19, 2016
Article in press: February 8, 2017
P- Reviewer: Erin N, Karmouty-Quintana H S- Editor: Yu J L- Editor: A E- Editor: Zhang FF
References
- 1.Miller MD, Krangel MS. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit Rev Immunol. 1992;12:17–46. [PubMed] [Google Scholar]
- 2.Nomiyama H, Mera A, Ohneda O, Miura R, Suda T, Yoshie O. Organization of the chemokine genes in the human and mouse major clusters of CC and CXC chemokines: diversification between the two species. Genes Immun. 2001;2:110–113. doi: 10.1038/sj.gene.6363742. [DOI] [PubMed] [Google Scholar]
- 3.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 4.Tsutsui H, Nishiguchi S. Importance of Kupffer cells in the development of acute liver injuries in mice. Int J Mol Sci. 2014;15:7711–7730. doi: 10.3390/ijms15057711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imamura M, Tsutsui H, Yasuda K, Uchiyama R, Yumikura-Futatsugi S, Mitani K, Hayashi S, Akira S, Taniguchi S, Van Rooijen N, et al. Contribution of TIR domain-containing adapter inducing IFN-beta-mediated IL-18 release to LPS-induced liver injury in mice. J Hepatol. 2009;51:333–341. doi: 10.1016/j.jhep.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 6.Anisowicz A, Bardwell L, Sager R. Constitutive overexpression of a growth-regulated gene in transformed Chinese hamster and human cells. Proc Natl Acad Sci USA. 1987;84:7188–7192. doi: 10.1073/pnas.84.20.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deuel TF, Keim PS, Farmer M, Heinrikson RL. Amino acid sequence of human platelet factor 4. Proc Natl Acad Sci USA. 1977;74:2256–2258. doi: 10.1073/pnas.74.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doi T, Greenberg SM, Rosenberg RD. Structure of the rat platelet factor 4 gene: a marker for megakaryocyte differentiation. Mol Cell Biol. 1987;7:898–904. doi: 10.1128/mcb.7.2.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oquendo P, Alberta J, Wen DZ, Graycar JL, Derynck R, Stiles CD. The platelet-derived growth factor-inducible KC gene encodes a secretory protein related to platelet alpha-granule proteins. J Biol Chem. 1989;264:4133–4137. [PubMed] [Google Scholar]
- 10.Watanabe K, Konishi K, Fujioka M, Kinoshita S, Nakagawa H. The neutrophil chemoattractant produced by the rat kidney epithelioid cell line NRK-52E is a protein related to the KC/gro protein. J Biol Chem. 1989;264:19559–19563. [PubMed] [Google Scholar]
- 11.Holt JC, Harris ME, Holt AM, Lange E, Henschen A, Niewiarowski S. Characterization of human platelet basic protein, a precursor form of low-affinity platelet factor 4 and beta-thromboglobulin. Biochemistry. 1986;25:1988–1996. doi: 10.1021/bi00356a023. [DOI] [PubMed] [Google Scholar]
- 12.Tekamp-Olson P, Gallegos C, Bauer D, McClain J, Sherry B, Fabre M, van Deventer S, Cerami A. Cloning and characterization of cDNAs for murine macrophage inflammatory protein 2 and its human homologues. J Exp Med. 1990;172:911–919. doi: 10.1084/jem.172.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolpe SD, Sherry B, Juers D, Davatelis G, Yurt RW, Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci USA. 1989;86:612–616. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rittner HL, Labuz D, Richter JF, Brack A, Schäfer M, Stein C, Mousa SA. CXCR1/2 ligands induce p38 MAPK-dependent translocation and release of opioid peptides from primary granules in vitro and in vivo. Brain Behav Immun. 2007;21:1021–1032. doi: 10.1016/j.bbi.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and Kupffer cells. Hepatology. 1998;27:507–512. doi: 10.1002/hep.510270226. [DOI] [PubMed] [Google Scholar]
- 16.Mosher B, Dean R, Harkema J, Remick D, Palma J, Crockett E. Inhibition of Kupffer cells reduced CXC chemokine production and liver injury. J Surg Res. 2001;99:201–210. doi: 10.1006/jsre.2001.6217. [DOI] [PubMed] [Google Scholar]
- 17.Liu Q, Zaiss AK, Colarusso P, Patel K, Haljan G, Wickham TJ, Muruve DA. The role of capsid-endothelial interactions in the innate immune response to adenovirus vectors. Hum Gene Ther. 2003;14:627–643. doi: 10.1089/104303403321618146. [DOI] [PubMed] [Google Scholar]
- 18.De Filippo K, Henderson RB, Laschinger M, Hogg N. Neutrophil chemokines KC and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. J Immunol. 2008;180:4308–4315. doi: 10.4049/jimmunol.180.6.4308. [DOI] [PubMed] [Google Scholar]
- 19.Lei M, Jiao H, Liu T, Du L, Cheng Y, Zhang D, Hao Y, Man C, Wang F. siRNA targeting mCD14 inhibits TNF-α, MIP-2, and IL-6 secretion and NO production from LPS-induced RAW264.7 cells. Appl Microbiol Biotechnol. 2011;92:115–124. doi: 10.1007/s00253-011-3371-7. [DOI] [PubMed] [Google Scholar]
- 20.So MH, Choi YK. Anti-Inflammatory Effect of Combination of Scutellariae Radix and Liriopis Tuber Water Extract. Evid Based Complement Alternat Med. 2015;2015:203965. doi: 10.1155/2015/203965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohno Y, Lee J, Fusunyan RD, MacDermott RP, Sanderson IR. Macrophage inflammatory protein-2: chromosomal regulation in rat small intestinal epithelial cells. Proc Natl Acad Sci USA. 1997;94:10279–10284. doi: 10.1073/pnas.94.19.10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu R, Huang H, Zhang Z, Wang FS. The role of neutrophils in the development of liver diseases. Cell Mol Immunol. 2014;11:224–231. doi: 10.1038/cmi.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amanzada A, Moriconi F, Mansuroglu T, Cameron S, Ramadori G, Malik IA. Induction of chemokines and cytokines before neutrophils and macrophage recruitment in different regions of rat liver after TAA administration. Lab Invest. 2014;94:235–247. doi: 10.1038/labinvest.2013.134. [DOI] [PubMed] [Google Scholar]
- 24.Cole N, Hume EB, Khan S, Garthwaite L, Conibear TC, Willcox MD. The role of CXC chemokine receptor 2 in Staphylococcus aureus keratitis. Exp Eye Res. 2014;127:184–189. doi: 10.1016/j.exer.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Mercer-Jones MA, Shrotri MS, Heinzelmann M, Peyton JC, Cheadle WG. Regulation of early peritoneal neutrophil migration by macrophage inflammatory protein-2 and mast cells in experimental peritonitis. J Leukoc Biol. 1999;65:249–255. doi: 10.1002/jlb.65.2.249. [DOI] [PubMed] [Google Scholar]
- 26.Ramos CD, Fernandes KS, Canetti C, Teixeira MM, Silva JS, Cunha FQ. Neutrophil recruitment in immunized mice depends on MIP-2 inducing the sequential release of MIP-1alpha, TNF-alpha and LTB(4) Eur J Immunol. 2006;36:2025–2034. doi: 10.1002/eji.200636057. [DOI] [PubMed] [Google Scholar]
- 27.Feng L, Xia Y, Yoshimura T, Wilson CB. Modulation of neutrophil influx in glomerulonephritis in the rat with anti-macrophage inflammatory protein-2 (MIP-2) antibody. J Clin Invest. 1995;95:1009–1017. doi: 10.1172/JCI117745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frevert CW, Huang S, Danaee H, Paulauskis JD, Kobzik L. Functional characterization of the rat chemokine KC and its importance in neutrophil recruitment in a rat model of pulmonary inflammation. J Immunol. 1995;154:335–344. [PubMed] [Google Scholar]
- 29.Zhao Z, Hyun JS, Satsu H, Kakuta S, Shimizu M. Oral exposure to cadmium chloride triggers an acute inflammatory response in the intestines of mice, initiated by the over-expression of tissue macrophage inflammatory protein-2 mRNA. Toxicol Lett. 2006;164:144–154. doi: 10.1016/j.toxlet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Wintermeyer P, Cheng CW, Gehring S, Hoffman BL, Holub M, Brossay L, Gregory SH. Invariant natural killer T cells suppress the neutrophil inflammatory response in a mouse model of cholestatic liver damage. Gastroenterology. 2009;136:1048–1059. doi: 10.1053/j.gastro.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orlichenko LS, Behari J, Yeh TH, Liu S, Stolz DB, Saluja AK, Singh VP. Transcriptional regulation of CXC-ELR chemokines KC and MIP-2 in mouse pancreatic acini. Am J Physiol Gastrointest Liver Physiol. 2010;299:G867–G876. doi: 10.1152/ajpgi.00177.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luyendyk JP, Flanagan KC, Williams CD, Jaeschke H, Slusser JG, Mackman N, Cantor GH. Tissue factor contributes to neutrophil CD11b expression in alpha-naphthylisothiocyanate-treated mice. Toxicol Appl Pharmacol. 2011;250:256–262. doi: 10.1016/j.taap.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gujral JS, Liu J, Farhood A, Hinson JA, Jaeschke H. Functional importance of ICAM-1 in the mechanism of neutrophil-induced liver injury in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2004;286:G499–G507. doi: 10.1152/ajpgi.00318.2003. [DOI] [PubMed] [Google Scholar]
- 34.Hasan Z, Palani K, Rahman M, Thorlacius H. Targeting CD44 expressed on neutrophils inhibits lung damage in abdominal sepsis. Shock. 2011;35:567–572. doi: 10.1097/SHK.0b013e3182144935. [DOI] [PubMed] [Google Scholar]
- 35.McDonald B, McAvoy EF, Lam F, Gill V, de la Motte C, Savani RC, Kubes P. Interaction of CD44 and hyaluronan is the dominant mechanism for neutrophil sequestration in inflamed liver sinusoids. J Exp Med. 2008;205:915–927. doi: 10.1084/jem.20071765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolaczkowska E, Jenne CN, Surewaard BG, Thanabalasuriar A, Lee WY, Sanz MJ, Mowen K, Opdenakker G, Kubes P. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat Commun. 2015;6:6673. doi: 10.1038/ncomms7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang H, Tohme S, Al-Khafaji AB, Tai S, Loughran P, Chen L, Wang S, Kim J, Billiar T, Wang Y, et al. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology. 2015;62:600–614. doi: 10.1002/hep.27841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wree A, Eguchi A, McGeough MD, Pena CA, Johnson CD, Canbay A, Hoffman HM, Feldstein AE. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology. 2014;59:898–910. doi: 10.1002/hep.26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sultan S, Cameron S, Ahmad S, Malik IA, Schultze FC, Hielscher R, Rave-Fränk M, Hess CF, Ramadori G, Christiansen H. Serum Lipocalin2 is a potential biomarker of liver irradiation damage. Liver Int. 2013;33:459–468. doi: 10.1111/liv.12073. [DOI] [PubMed] [Google Scholar]
- 40.Sultan S, Pascucci M, Ahmad S, Malik IA, Bianchi A, Ramadori P, Ahmad G, Ramadori G. LIPOCALIN-2 is a major acute-phase protein in a rat and mouse model of sterile abscess. Shock. 2012;37:191–196. doi: 10.1097/SHK.0b013e31823918c2. [DOI] [PubMed] [Google Scholar]
- 41.Yasukawa K, Tokuda H, Tun X, Utsumi H, Yamada K. The detrimental effect of nitric oxide on tissue is associated with inflammatory events in the vascular endothelium and neutrophils in mice with dextran sodium sulfate-induced colitis. Free Radic Res. 2012;46:1427–1436. doi: 10.3109/10715762.2012.732698. [DOI] [PubMed] [Google Scholar]
- 42.Adam M, Gajdova S, Kolarova H, Kubala L, Lau D, Geisler A, Ravekes T, Rudolph V, Tsao PS, Blankenberg S, et al. Red blood cells serve as intravascular carriers of myeloperoxidase. J Mol Cell Cardiol. 2014;74:353–363. doi: 10.1016/j.yjmcc.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 43.El-Benna J, Dang PM, Gougerot-Pocidalo MA, Elbim C. Phagocyte NADPH oxidase: a multicomponent enzyme essential for host defenses. Arch Immunol Ther Exp (Warsz) 2005;53:199–206. [PubMed] [Google Scholar]
- 44.Schönberg M, Reibetanz U, Rathmann S, Lessig J. Maintenance of α(1)-antitrypsin activity by means of co-application of hypochlorous acid-scavengers in vitro and in the supernatant of polymorphonuclear leukocytes: as a basis for a new drug delivery approach. Biomatter. 2012;2:24–36. doi: 10.4161/biom.19190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amanzada A, Malik IA, Nischwitz M, Sultan S, Naz N, Ramadori G. Myeloperoxidase and elastase are only expressed by neutrophils in normal and in inflamed liver. Histochem Cell Biol. 2011;135:305–315. doi: 10.1007/s00418-011-0787-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guan LY, Fu PY, Li PD, Li ZN, Liu HY, Xin MG, Li W. Mechanisms of hepatic ischemia-reperfusion injury and protective effects of nitric oxide. World J Gastrointest Surg. 2014;6:122–128. doi: 10.4240/wjgs.v6.i7.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bergquist M, Jirholt P, Nurkkala M, Rylander C, Hedenstierna G, Lindholm C. Glucocorticoid receptor function is decreased in neutrophils during endotoxic shock. J Infect. 2014;69:113–122. doi: 10.1016/j.jinf.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 48.de Oliveira TH, Amorin AT, Rezende IS, Santos Barbosa M, Martins HB, Brito AK, Andrade EF, Gonçalves GK, Campos GB, Silva RA, et al. Sepsis induced by Staphylococcus aureus: participation of biomarkers in a murine model. Med Sci Monit. 2015;21:345–355. doi: 10.12659/MSM.892528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lazaro R, Wu R, Lee S, Zhu NL, Chen CL, French SW, Xu J, Machida K, Tsukamoto H. Osteopontin deficiency does not prevent but promotes alcoholic neutrophilic hepatitis in mice. Hepatology. 2015;61:129–140. doi: 10.1002/hep.27383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang M, Ramachandran A, Yan HM, Woolbright BL, Copple BL, Fickert P, Trauner M, Jaeschke H. Osteopontin is an initial mediator of inflammation and liver injury during obstructive cholestasis after bile duct ligation in mice. Toxicol Lett. 2014;224:186–195. doi: 10.1016/j.toxlet.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Britt RD, Locy ML, Tipple TE, Nelin LD, Rogers LK. Lipopolysaccharide-induced cyclooxygenase-2 expression in mouse transformed Clara cells. Cell Physiol Biochem. 2012;29:213–222. doi: 10.1159/000337602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jawa RS, Quist E, Boyer CW, Shostrom VK, Mercer DW. Mesenteric ischemia-reperfusion injury up-regulates certain CC, CXC, and XC chemokines and results in multi-organ injury in a time-dependent manner. Eur Cytokine Netw. 2013;24:148–156. doi: 10.1684/ecn.2014.0345. [DOI] [PubMed] [Google Scholar]
- 53.Xue J, Chen F, Wang J, Wu S, Zheng M, Zhu H, Liu Y, He J, Chen Z. Emodin protects against concanavalin A-induced hepatitis in mice through inhibiting activation of the p38 MAPK-NF-κB signaling pathway. Cell Physiol Biochem. 2015;35:1557–1570. doi: 10.1159/000373971. [DOI] [PubMed] [Google Scholar]
- 54.Tan M, Schmidt RH, Beier JI, Watson WH, Zhong H, States JC, Arteel GE. Chronic subhepatotoxic exposure to arsenic enhances hepatic injury caused by high fat diet in mice. Toxicol Appl Pharmacol. 2011;257:356–364. doi: 10.1016/j.taap.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Osakabe N, Takano H, Sanbongi C, Yasuda A, Yanagisawa R, Inoue K, Yoshikawa T. Anti-inflammatory and anti-allergic effect of rosmarinic acid (RA); inhibition of seasonal allergic rhinoconjunctivitis (SAR) and its mechanism. Biofactors. 2004;21:127–131. doi: 10.1002/biof.552210125. [DOI] [PubMed] [Google Scholar]
- 56.Fei M, Xie Q, Zou Y, He R, Zhang Y, Wang J, Bo L, Li J, Deng X. Alpha-lipoic acid protects mice against concanavalin A-induced hepatitis by modulating cytokine secretion and reducing reactive oxygen species generation. Int Immunopharmacol. 2016;35:53–60. doi: 10.1016/j.intimp.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 57.Ji YR, Kim HJ, Yu DH, Bae KB, Park SJ, Park SJ, Jang WY, Kang MC, Jeong J, Sung YH, et al. Over-expression of Roquin aggravates T cell mediated hepatitis in transgenic mice using T cell specific promoter. Biochem Biophys Res Commun. 2014;452:822–827. doi: 10.1016/j.bbrc.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 58.Pratap UP, Sharma HR, Mohanty A, Kale P, Gopinath S, Hima L, Priyanka HP, ThyagaRajan S. Estrogen upregulates inflammatory signals through NF-κB, IFN-γ, and nitric oxide via Akt/mTOR pathway in the lymph node lymphocytes of middle-aged female rats. Int Immunopharmacol. 2015;29:591–598. doi: 10.1016/j.intimp.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 59.Nakamura K, Ito T, Yoneda M, Takamoto S, Nakade Y, Okamoto S, Okada M, Yokohama S, Aso K, Makino I. Antithrombin III prevents concanavalin A-induced liver injury through inhibition of macrophage inflammatory protein-2 release and production of prostacyclin in mice. J Hepatol. 2002;36:766–773. doi: 10.1016/s0168-8278(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 60.Muruve DA, Barnes MJ, Stillman IE, Libermann TA. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum Gene Ther. 1999;10:965–976. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- 61.Ebe Y, Hasegawa G, Takatsuka H, Umezu H, Mitsuyama M, Arakawa M, Mukaida N, Naito M. The role of Kupffer cells and regulation of neutrophil migration into the liver by macrophage inflammatory protein-2 in primary listeriosis in mice. Pathol Int. 1999;49:519–532. doi: 10.1046/j.1440-1827.1999.00910.x. [DOI] [PubMed] [Google Scholar]
- 62.Tirosh O, Artan A, Aharoni-Simon M, Ramadori G, Madar Z. Impaired liver glucose production in a murine model of steatosis and endotoxemia: protection by inducible nitric oxide synthase. Antioxid Redox Signal. 2010;13:13–26. doi: 10.1089/ars.2009.2789. [DOI] [PubMed] [Google Scholar]
- 63.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 64.Malik IA, Moriconi F, Sheikh N, Naz N, Khan S, Dudas J, Mansuroglu T, Hess CF, Rave-Fränk M, Christiansen H, et al. Single-dose gamma-irradiation induces up-regulation of chemokine gene expression and recruitment of granulocytes into the portal area but not into other regions of rat hepatic tissue. Am J Pathol. 2010;176:1801–1815. doi: 10.2353/ajpath.2010.090505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li X, Klintman D, Sato T, Hedlund G, Schramm R, Jeppsson B, Thorlacius H. Interleukin-10 mediates the protective effect of Linomide by reducing CXC chemokine production in endotoxin-induced liver injury. Br J Pharmacol. 2004;143:865–871. doi: 10.1038/sj.bjp.0706015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Massey VL, Poole LG, Siow DL, Torres E, Warner NL, Schmidt RH, Ritzenthaler JD, Roman J, Arteel GE. Chronic Alcohol Exposure Enhances Lipopolysaccharide-Induced Lung Injury in Mice: Potential Role of Systemic Tumor Necrosis Factor-Alpha. Alcohol Clin Exp Res. 2015;39:1978–1988. doi: 10.1111/acer.12855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dong W, Simeonova PP, Gallucci R, Matheson J, Fannin R, Montuschi P, Flood L, Luster MI. Cytokine expression in hepatocytes: role of oxidant stress. J Interferon Cytokine Res. 1998;18:629–638. doi: 10.1089/jir.1998.18.629. [DOI] [PubMed] [Google Scholar]
- 68.Pennington HL, Wilce PA, Worrall S. Chemokine and cell adhesion molecule mRNA expression and neutrophil infiltration in lipopolysaccharide-induced hepatitis in ethanol-fed rats. Alcohol Clin Exp Res. 1998;22:1713–1718. [PubMed] [Google Scholar]
- 69.Schmid A, Kopp A, Hanses F, Karrasch T, Schäffler A. C1q/TNF-related protein-3 (CTRP-3) attenuates lipopolysaccharide (LPS)-induced systemic inflammation and adipose tissue Erk-1/-2 phosphorylation in mice in vivo. Biochem Biophys Res Commun. 2014;452:8–13. doi: 10.1016/j.bbrc.2014.06.054. [DOI] [PubMed] [Google Scholar]
- 70.Reynaert NL, van der Vliet A, Guala AS, McGovern T, Hristova M, Pantano C, Heintz NH, Heim J, Ho YS, Matthews DE, et al. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc Natl Acad Sci USA. 2006;103:13086–13091. doi: 10.1073/pnas.0603290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cuaz-Pérolin C, Billiet L, Baugé E, Copin C, Scott-Algara D, Genze F, Büchele B, Syrovets T, Simmet T, Rouis M. Antiinflammatory and antiatherogenic effects of the NF-kappaB inhibitor acetyl-11-keto-beta-boswellic acid in LPS-challenged ApoE-/- mice. Arterioscler Thromb Vasc Biol. 2008;28:272–277. doi: 10.1161/ATVBAHA.107.155606. [DOI] [PubMed] [Google Scholar]
- 72.Sasakawa Y, Kominami A, Yamamoto K, Nakaoka F, Nakamura M, Nakao M, Abe M, Fukuhama C, Kagawa K. Effects of globin digest and its active ingredient Trp-Thr-Gln-Arg on galactosamine/lipopolysaccharide-induced liver injury in ICR mice. Life Sci. 2012;90:190–199. doi: 10.1016/j.lfs.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 73.Dorman RB, Gujral JS, Bajt ML, Farhood A, Jaeschke H. Generation and functional significance of CXC chemokines for neutrophil-induced liver injury during endotoxemia. Am J Physiol Gastrointest Liver Physiol. 2005;288:G880–G886. doi: 10.1152/ajpgi.00317.2004. [DOI] [PubMed] [Google Scholar]
- 74.Li X, Klintman D, Liu Q, Sato T, Jeppsson B, Thorlacius H. Critical role of CXC chemokines in endotoxemic liver injury in mice. J Leukoc Biol. 2004;75:443–452. doi: 10.1189/jlb.0603297. [DOI] [PubMed] [Google Scholar]
- 75.Martius G, Alwahsh SM, Rave-Fränk M, Hess CF, Christiansen H, Ramadori G, Malik IA. Hepatic fat accumulation and regulation of FAT/CD36: an effect of hepatic irradiation. Int J Clin Exp Pathol. 2014;7:5379–5392. [PMC free article] [PubMed] [Google Scholar]
- 76.Cameron S, Schwartz A, Sultan S, Schaefer IM, Hermann R, Rave-Fränk M, Hess CF, Christiansen H, Ramadori G. Radiation-induced damage in different segments of the rat intestine after external beam irradiation of the liver. Exp Mol Pathol. 2012;92:243–258. doi: 10.1016/j.yexmp.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 77.Mukhopadhyay P, Rajesh M, Horváth B, Bátkai S, Park O, Tanchian G, Gao RY, Patel V, Wink DA, Liaudet L, et al. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Radic Biol Med. 2011;50:1368–1381. doi: 10.1016/j.freeradbiomed.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ouzounidis N, Giakoustidis A, Poutahidis T, Angelopoulou K, Iliadis S, Chatzigiagkos A, Zacharioudaki A, Angelopoulos S, Papalois A, Papanikolaou V, et al. Interleukin 18 binding protein ameliorates ischemia/reperfusion-induced hepatic injury in mice. Liver Transpl. 2016;22:237–246. doi: 10.1002/lt.24359. [DOI] [PubMed] [Google Scholar]
- 79.Stewart RK, Dangi A, Huang C, Murase N, Kimura S, Stolz DB, Wilson GC, Lentsch AB, Gandhi CR. A novel mouse model of depletion of stellate cells clarifies their role in ischemia/reperfusion- and endotoxin-induced acute liver injury. J Hepatol. 2014;60:298–305. doi: 10.1016/j.jhep.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim HY, Kim SJ, Lee SM. Activation of NLRP3 and AIM2 inflammasomes in Kupffer cells in hepatic ischemia/reperfusion. FEBS J. 2015;282:259–270. doi: 10.1111/febs.13123. [DOI] [PubMed] [Google Scholar]
- 81.Ocuin LM, Zeng S, Cavnar MJ, Sorenson EC, Bamboat ZM, Greer JB, Kim TS, Popow R, DeMatteo RP. Nilotinib protects the murine liver from ischemia/reperfusion injury. J Hepatol. 2012;57:766–773. doi: 10.1016/j.jhep.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Monson KM, Dowlatshahi S, Crockett ET. CXC-chemokine regulation and neutrophil trafficking in hepatic ischemia-reperfusion injury in P-selectin/ICAM-1 deficient mice. J Inflamm (Lond) 2007;4:11. doi: 10.1186/1476-9255-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yoshidome H, Kato A, Edwards MJ, Lentsch AB. Interleukin-10 suppresses hepatic ischemia/reperfusion injury in mice: implications of a central role for nuclear factor kappaB. Hepatology. 1999;30:203–208. doi: 10.1002/hep.510300120. [DOI] [PubMed] [Google Scholar]
- 84.Kuboki S, Sakai N, Clarke C, Schuster R, Blanchard J, Edwards MJ, Lentsch AB. The peptidyl-prolyl isomerase, Pin1, facilitates NF-kappaB binding in hepatocytes and protects against hepatic ischemia/reperfusion injury. J Hepatol. 2009;51:296–306. doi: 10.1016/j.jhep.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alwahsh SM, Xu M, Schultze FC, Wilting J, Mihm S, Raddatz D, Ramadori G. Combination of alcohol and fructose exacerbates metabolic imbalance in terms of hepatic damage, dyslipidemia, and insulin resistance in rats. PLoS One. 2014;9:e104220. doi: 10.1371/journal.pone.0104220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mathews S, Feng D, Maricic I, Ju C, Kumar V, Gao B. Invariant natural killer T cells contribute to chronic-plus-binge ethanol-mediated liver injury by promoting hepatic neutrophil infiltration. Cell Mol Immunol. 2016;13:206–216. doi: 10.1038/cmi.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pritchard MT, Nagy LE. Ethanol-induced liver injury: potential roles for egr-1. Alcohol Clin Exp Res. 2005;29:146S–150S. doi: 10.1097/01.alc.0000189286.81943.51. [DOI] [PubMed] [Google Scholar]
- 88.Budick-Harmelin N, Dudas J, Demuth J, Madar Z, Ramadori G, Tirosh O. Triglycerides potentiate the inflammatory response in rat Kupffer cells. Antioxid Redox Signal. 2008;10:2009–2022. doi: 10.1089/ars.2007.1876. [DOI] [PubMed] [Google Scholar]
- 89.Bautista AP. Acute ethanol binge followed by withdrawal regulates production of reactive oxygen species and cytokine-induced neutrophil chemoattractant and liver injury during reperfusion after hepatic ischemia. Antioxid Redox Signal. 2002;4:721–731. doi: 10.1089/152308602760598864. [DOI] [PubMed] [Google Scholar]
- 90.Nanji AA, Jokelainen K, Fotouhinia M, Rahemtulla A, Thomas P, Tipoe GL, Su GL, Dannenberg AJ. Increased severity of alcoholic liver injury in female rats: role of oxidative stress, endotoxin, and chemokines. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1348–G1356. doi: 10.1152/ajpgi.2001.281.6.G1348. [DOI] [PubMed] [Google Scholar]
- 91.Nanji AA, Jokelainen K, Tipoe GL, Rahemtulla A, Thomas P, Dannenberg AJ. Curcumin prevents alcohol-induced liver disease in rats by inhibiting the expression of NF-kappa B-dependent genes. Am J Physiol Gastrointest Liver Physiol. 2003;284:G321–G327. doi: 10.1152/ajpgi.00230.2002. [DOI] [PubMed] [Google Scholar]
- 92.Jayaraman J, Jesudoss VA, Menon VP, Namasivayam N. Anti-inflammatory role of naringenin in rats with ethanol induced liver injury. Toxicol Mech Methods. 2012;22:568–576. doi: 10.3109/15376516.2012.707255. [DOI] [PubMed] [Google Scholar]
- 93.Savransky V, Bevans S, Nanayakkara A, Li J, Smith PL, Torbenson MS, Polotsky VY. Chronic intermittent hypoxia causes hepatitis in a mouse model of diet-induced fatty liver. Am J Physiol Gastrointest Liver Physiol. 2007;293:G871–G877. doi: 10.1152/ajpgi.00145.2007. [DOI] [PubMed] [Google Scholar]
- 94.Zampetaki A, Mitsialis SA, Pfeilschifter J, Kourembanas S. Hypoxia induces macrophage inflammatory protein-2 (MIP-2) gene expression in murine macrophages via NF-kappaB: the prominent role of p42/ p44 and PI3 kinase pathways. FASEB J. 2004;18:1090–1092. doi: 10.1096/fj.03-0991fje. [DOI] [PubMed] [Google Scholar]
- 95.Ren X, Carpenter A, Hogaboam C, Colletti L. Mitogenic properties of endogenous and pharmacological doses of macrophage inflammatory protein-2 after 70% hepatectomy in the mouse. Am J Pathol. 2003;163:563–570. doi: 10.1016/S0002-9440(10)63684-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hogaboam CM, Simpson KJ, Chensue SW, Steinhauser ML, Lukacs NW, Gauldie J, Strieter RM, Kunkel SL. Macrophage inflammatory protein-2 gene therapy attenuates adenovirus- and acetaminophen-mediated hepatic injury. Gene Ther. 1999;6:573–584. doi: 10.1038/sj.gt.3300858. [DOI] [PubMed] [Google Scholar]
- 97.Hogaboam CM, Bone-Larson CL, Steinhauser ML, Lukacs NW, Colletti LM, Simpson KJ, Strieter RM, Kunkel SL. Novel CXCR2-dependent liver regenerative qualities of ELR-containing CXC chemokines. FASEB J. 1999;13:1565–1574. doi: 10.1096/fasebj.13.12.1565. [DOI] [PubMed] [Google Scholar]
- 98.Hogaboam CM, Bone-Larson CL, Steinhauser ML, Matsukawa A, Gosling J, Boring L, Charo IF, Simpson KJ, Lukacs NW, Kunkel SL. Exaggerated hepatic injury due to acetaminophen challenge in mice lacking C-C chemokine receptor 2. Am J Pathol. 2000;156:1245–1252. doi: 10.1016/S0002-9440(10)64995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wilson GC, Kuboki S, Freeman CM, Nojima H, Schuster RM, Edwards MJ, Lentsch AB. CXC chemokines function as a rheostat for hepatocyte proliferation and liver regeneration. PLoS One. 2015;10:e0120092. doi: 10.1371/journal.pone.0120092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Du J, Luan J, Liu H, Daniel TO, Peiper S, Chen TS, Yu Y, Horton LW, Nanney LB, Strieter RM, et al. Potential role for Duffy antigen chemokine-binding protein in angiogenesis and maintenance of homeostasis in response to stress. J Leukoc Biol. 2002;71:141–153. [PMC free article] [PubMed] [Google Scholar]
- 101.Kollmar O, Junker B, Rupertus K, Scheuer C, Menger MD, Schilling MK. Liver resection-associated macrophage inflammatory protein-2 stimulates engraftment but not growth of colorectal metastasis at extrahepatic sites. J Surg Res. 2008;145:295–302. doi: 10.1016/j.jss.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 102.Kollmar O, Menger MD, Schilling MK. Macrophage inflammatory protein-2 contributes to liver resection-induced acceleration of hepatic metastatic tumor growth. World J Gastroenterol. 2006;12:858–867. doi: 10.3748/wjg.v12.i6.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shayakhmetov DM, Li ZY, Ni S, Lieber A. Interference with the IL-1-signaling pathway improves the toxicity profile of systemically applied adenovirus vectors. J Immunol. 2005;174:7310–7319. doi: 10.4049/jimmunol.174.11.7310. [DOI] [PubMed] [Google Scholar]
- 104.Jaeschke H. Inflammation in response to hepatocellular apoptosis. Hepatology. 2002;35:964–966. doi: 10.1053/jhep.2002.0350964. [DOI] [PubMed] [Google Scholar]
- 105.Kawamura H, Kawamura T, Kanda Y, Kobayashi T, Abo T. Extracellular ATP-stimulated macrophages produce macrophage inflammatory protein-2 which is important for neutrophil migration. Immunology. 2012;136:448–458. doi: 10.1111/j.1365-2567.2012.03601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.O’Brien KM, Allen KM, Rockwell CE, Towery K, Luyendyk JP, Copple BL. IL-17A synergistically enhances bile acid-induced inflammation during obstructive cholestasis. Am J Pathol. 2013;183:1498–1507. doi: 10.1016/j.ajpath.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tang H, Amara U, Tang D, Barnes MA, McDonald C, Nagy LE. Synergistic interaction between C5a and NOD2 signaling in the regulation of chemokine expression in RAW 264.7 macrophages. Adv Biosci Biotechnol. 2013;4:30–37. doi: 10.4236/abb.2013.48A3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Colletti LM, Green ME, Burdick MD, Strieter RM. The ratio of ELR+ to ELR- CXC chemokines affects the lung and liver injury following hepatic ischemia/ reperfusion in the rat. Hepatology. 2000;31:435–445. doi: 10.1002/hep.510310225. [DOI] [PubMed] [Google Scholar]
- 109.Colletti LM, Cortis A, Lukacs N, Kunkel SL, Green M, Strieter RM. Tumor necrosis factor up-regulates intercellular adhesion molecule 1, which is important in the neutrophil-dependent lung and liver injury associated with hepatic ischemia and reperfusion in the rat. Shock. 1998;10:182–191. doi: 10.1097/00024382-199809000-00006. [DOI] [PubMed] [Google Scholar]


