Abstract
Antibodies against the toxin portion of recombinant immunotoxins (RIT) reduce their efficacy and pose a potential safety risk. To overcome this problem we mutated the very immunogenic immunotoxin SS1P to produce LMB-T20, a de-immunized RIT that has the eight human T-cell epitopes in SS1P modified or removed. To determine the effect of T-cell epitope removal in vivo we mapped the T-cell epitopes in immune-competent BALB/c mice and found that these mice recognize two epitopes. One corresponds to the human immunodominant T-cell epitope and the other to a human subdominant epitope; both were eliminated in LMB-T20. We found that mice immunized with LMB-T20 did not have T-cell activation and did not develop anti-drug antibodies (ADA), whereas mice immunized with SS1P, showed T-cell activation, and developed ADA detected by both ELISA and drug neutralizing assays. The ability of the mice treated with LMB-T20 to respond to other antigens was not compromised. We conclude that elimination of T-cell epitopes is sufficient to prevent formation of antibodies to an immunogenic foreign protein.
Keywords: anti-drug antibodies, deimmunization, immunogenicity, mouse epitopes, T-cell epitopes
Introduction
Immunogenicity against therapeutic proteins compromises drug efficacy and can affect patient safety. Safety concerns include anaphylactic shock, hypersensitivity reactions, and cross reactions to endogenous proteins that can result in morbidity and mortality.1,2,3 Alternatively, some immunogenicity responses do not affect patient safety but can accelerate clearance of the drug by formation of immune complexes.
Recombinant immunotoxins (RIT) are chimeric proteins designed to treat cancer. They contain a portion of a bacterial protein (Pseudomonas exotoxin A [PE38]) whose targeting domain is replaced with an antibody fragment. PE38 has been used as a payload in several immunotoxins in clinical trials. These include Moxetumomab Pasudotox (targeting CD22 for hematological malignancies treatment), SS1P (targeting mesothelin in mesothelioma, ovarian and pancreatic cancers), and LMB-2 (targeting IL2 receptor in T-cell malignancies).4,5,6,7
Due to the bacterial origin of PE38, many patients treated with these immunotoxins develop antibodies against the drug. The anti-drug antibodies (ADA) neutralize the RIT and prevent further treatment cycles.8,9
To reduce the immunogenicity of protein therapeutics, protein engineers have employed several strategies to hide the protein from the immune system. An approach that is becoming more popular is the identification and elimination of T-cell epitopes.10,11,12,13,14,15,16,17 This approach is based on the fact that most immunogenicity responses are categorized as T-cell-dependent immune responses18 and prevention of the recognition of T-cell epitopes in the protein will prevent T cell help and Ab class switching, which is required for high-affinity IgG responses.
To identify the T-cell epitopes in PE38, we characterized the immune response of 50 donors using in vitro expansion of PBMCs from these donors. To do this, the cells were first incubated with whole protein and the reacting T cells were re-stimulated with peptides spanning the sequence of PE38. We identified one immunodominant promiscuous epitope that was restricted to HLA class II-DR19 and seven weaker epitopes.10 Using this information we designed a new de-immunized toxin that has a large deletion and six-point mutations that eliminate or suppress all eight T-cell epitopes in the toxin. In these studies, PE38 present in Moxetumomab Pasudotox and in SS1P was replaced with the de-immunized payload resulting in two highly active 49 kDa immunotoxins. One is LMB-T18 that targets CD2210 and the other is LMB-T20 that targets mesothelin.20
To test the effectiveness of the de-immunization of PE38 in an animal model, we used BALB/c mice because even though the structure/sequence of the MHC proteins and T-cell receptors differs between mice and humans, BALB/c mice recognize two human T-cell epitopes, one of which is an important immune-dominant epitope. We show here that immunotoxin SS1P (SS1dsFv-PE38) induces a strong T-cell and antibody response in mice but that the mutant immunotoxin LMB-T20 does not.
Materials and methods
PE38 and alanine variant peptides
Three sets of peptides were synthesized by American Peptide, including (1) a set of 111 peptides (15-mer in length) spanning the sequence of PE38 with offsets of three amino acids. The sequence was previously described.19 (2) A set of 19 peptides, 21-mer in length spanning the sequence of peptides 13, 14, and 15 and variants of this peptide in which one amino acid at a time was substituted with alanine. In cases where the wild-type (WT) sequence contained alanine, the amino acid was replaced with glycine. (3) A set of 15 peptides (18-mer in length) corresponding to peptides 57 and 58 with alanine substitutions as described above. The amino acid sequence of the alanine variants is shown in Supplementary Table S1. All Peptides were purified to >95% homogeneity by HPLC, and their composition was confirmed by mass spectrometry. Peptides were dissolved in DMSO at 10 mM and stored at −20 °C. For initial screening, the first set of peptides was pooled into groups of five consecutive peptides, with the exception of pool 22, which contained six peptides.
SS1P and LMB-T20 proteins
SS1P and LMB-T20 were produced in Escherichia coli, refolded, and purified as previously described.21 Endotoxin was removed by high capacity endotoxin removal spin columns (Thermo Fisher Scientific, Waltham, MA). Both proteins were tested for endotoxin content and had less than 5 EU/ml.
Mouse IL2 ELISpot assays
Mice were purchased from Charles River Laboratories (Frederick, MD). Female BALB/cAnNCr mice (6–8 weeks of age) were immunized i.p. with 5 μg of SS1P or LMB-T20 in alum on days 1, 3, and 10. On day 15, mice were euthanized and spleens were extracted. Splenocytes were isolated by re-suspending the cells in 500 μl ACK lysis buffer (GIBCO) for 1 minute followed by two washes with HBSS media. Splenocytes were diluted in RPMI media containing heat-inactivated fetal calf serum and plated in a concentration of 3 × 105 cells/well in ELISpot plates that were pre-coated with anti-mouse IL2 antibodies (Mabtech) and blocked with 200 μl of the RPMI media. Cells were stimulated with the different peptides or peptide pools and incubated for 18 hours in 5% CO2 at 37 °C. Negative controls included cells with DMSO (no peptide) and positive controls included Concanavalin A (Sigma). Next, plates were washed five times with PBS and cytokine spots were detected using a biotinylated secondary Ab (Mabtech) and ALP. Spots were counted by computer-assisted image analysis (Immunospot5.0; Cellular Technology Limited). Results are shown in SFC/1E6 cells. Each assay was performed in quadruplicate. All mouse experiments followed NIH guidelines approved by the Animal Care and Use Committee of the NCI.
Antibody response
Female BALB/c mice were immunized weekly i.v. with 5 μg of SS1P, LMB-T20, or vehicle (PBS with 0.2% human serum albumin [HSA] or PBS alone). Serum samples were obtained 4 days after each immunization by submandibular bleeding according to ACUC approved protocols. Enzyme immunoassay plates (80040LE Thermo scientific) were coated overnight at 4 °C with 2 μg/ml SS1P or LMB-T20 in PBS. Plates were blocked with 0.05% PBS-Tween 20 (Bio-Rad) solution containing 3% BSA for 2 hours at room temperature and washed with PBS-T three times. Serum samples were serially diluted in PBS-T for a total of eight dilution points and allowed to bind to the coated plates for 2 hours at RT. Plates were subsequently washed three times and incubated with 266 ng/ml HRP conjugated goat anti-mouse IgG (Jackson lab 115-035-146) diluted in PBS-T for 1 hour. Plates were again washed three times and developed for 5 minutes using TMB substrate kit (Thermo-scientific 34021). Color reactions were stopped using 50 μl of 1M H2SO4 and read at 450 nm o.d. IP12 monoclonal Ab (mAb) (lab stock) that binds to an epitope on both SS1P and LMB-T20 was used to make a standard curve and quantify the antibody titer.22 Each serum sample was fitted to a four parameter curve and titer interpolation was calculated at the 50% of max o.d. for that sample.
Neutralization assay
Mice were immunized nine times (on weeks 1, 2, 4, 5, 6, 14, 15, 16, and 29) i.v. with RIT, LMB-T20, and vehicle (PBS-0.2% HSA). Mice were euthanized and post-mortem cardiac bleeds were obtained. A431/H9 cells (lab collection) were plated in flat-bottom Costar plates (Corning Inc.) at a concentration of 2500 cells/well and incubated for 18 hours in 5% CO2 at 37 °C. Serum was diluted 1:50 with assay media and mixed with various concentrations of SS1P, LMB-T20, or cycloheximide (CHX) for 1 hour at room temperature. Cells were incubated with the RITs for 72 hours (5% CO2 37 °C) and then evaluated for cytotoxic activity by addition of a 10% volume of WST-8 (Dojindo CK04) to the wells and measuring o.d. 450. Cell viability was calculated by normalization of the o.d. values to CHX-treated cells and no treatment controls. Normalized values were fitted to a nonlinear curve using a three parameter curve fit formula.
Statistical analysis
Statistical analysis and graphing were calculated using graph pad prism software. For multiple comparison of parametric variable, one-way analysis of variance (ANOVA) was used. For comparison of multiple non-parametric variables, Friedman test with Dunn's multiple comparisons were used.
Results
Identification of T-cell epitopes in PE38 in BALB/c mice
BALB/c mice were immunized with SS1P in alum. The structure of SS1P is shown in Figure 1.
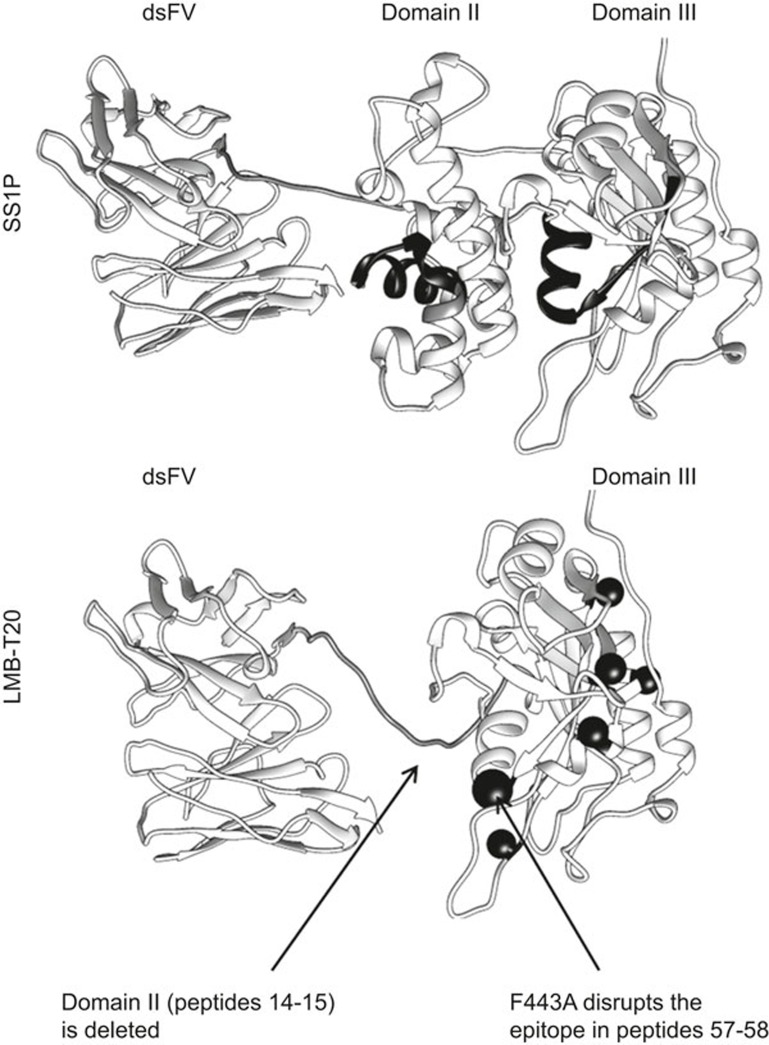
Figure 1.
Structural models of SS1P and LMB-T20. The RITs consists of a targeting dsFv that targets mesothelin on the left and a fragment of Pseudomonas exotoxin A on the right. (a) Peptides 14-15 in domain II and 67-68 in domain III are marked with black ribbons. (b) The targeting dsFv is conjugated to domain III of the toxin with a linker. The six-point mutations that eliminate human T-cell epitopes are labeled with balls (order from top left to bottom right: F443A, R494A, R505A, L552E, L477H, and R427A). The mutation in F443A which also diminishes the mouse T-cell epitope in peptides 57-58 is labeled with a larger ball.
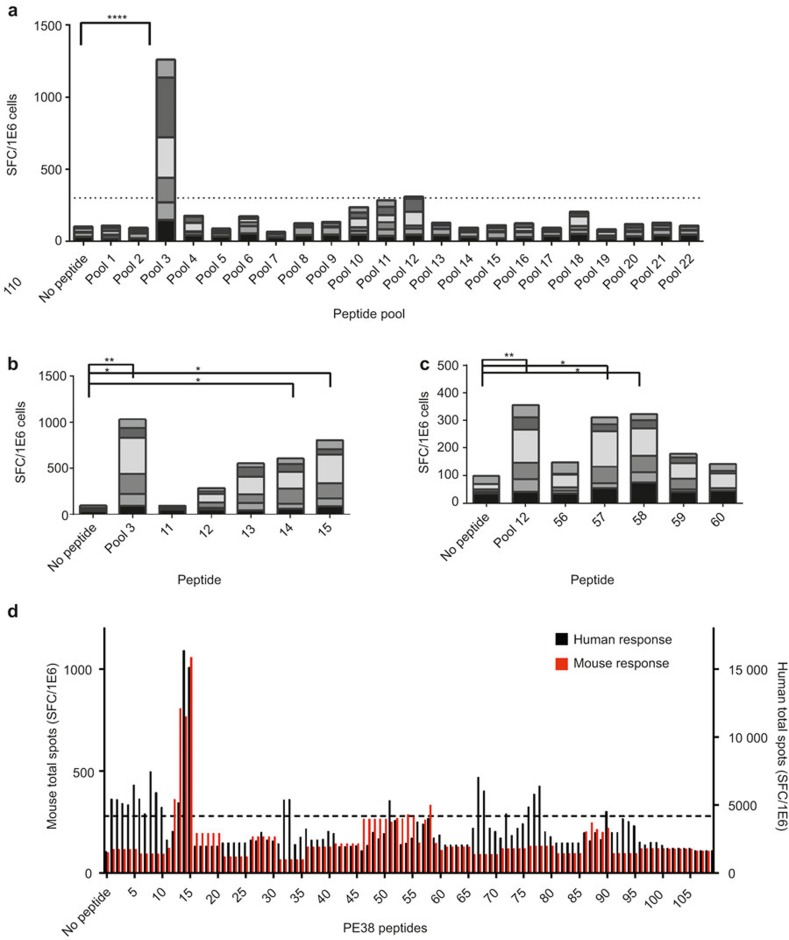
Splenocytes from six mice were isolated and stimulated with 22 peptide pools spanning the amino acid sequence of PE38. T-cell activation as a response to the peptides was measured using IL2 ELISpot. We found that pool 3 had a strong response that was significantly higher than the no peptide control (p < 0.0001; Figure 2a) and that pool 12 had a lower response that, although not significantly different from the control, was threefold over background (no peptide control), which is commonly used as a threshold for T-cell epitope mapping studies.11,19 Pool 3 is composed of peptides 11–15 and pool 12 is composed of peptides 56–60. The positive pools were further assayed for the individual peptides that stimulated the response (Figure 2b and c). Peptides 14 and 15 in pool 3 had a significantly stronger response than other peptides in the pool. Peptide 13 also had a strong response compared with peptides 11 and 12; however, that response was not significantly different. Similarly, peptides 57 and 58 in pool 12 had a greater response than other peptides comprising the pool indicating that these are the peptides that contain the epitopes. These peptides correspond to amino acids 288-308 and 436-453 in PE38, respectively.
Figure 2.
T-cell epitope mapping of PE38 peptides in BALB/c. Six mice were immunized with SS1P in 50% Alum i.p. on days 1, 3, and 10. Mice were euthanized and splenocytes were isolated. Splenocytes were plated in IL2 ELISpot coated plates and stimulated with peptides. (a) ELISpot response to peptide pools spanning the sequence of PE38. Pools that had a total response more than three times background (shown in dotted line) were fine screened using frozen autologous splenocytes. Each column contains the total spots from all six mice. (b) IL2 response to the five peptides that comprise pool 3 (n = 6). (c) IL2 response to the five peptides that comprise pool 12 (n = 6). (d) Comparison of BALB/c epitopes to human epitopes identified in 50 human donors. All peptides and peptide pools were tested in four replicas. Statistical analysis includes Friedman test, *, **, and **** indicate p < 0.05, 0.01, and 0.0001, respectively. The dotted line represents three times background.
We previously performed T-cell epitope mapping of PE38 using samples from 50 human donors10 and identified eight major epitopes. A comparison between the BALB/c epitopes and the human epitopes is shown in Figure 2d. The comparison was done by calculation of the total spots for all 50 donors and all six mice for 111 peptides. Surprisingly, both murine epitopes were also human epitopes; peptides 14 and 15 had strong and immunodominant responses in both humans and mice. Peptides 57 and 58 had a subdominant response in both. Six additional epitopes were identified in the human cohorts that were not identified in the BALB/c mapping, which demonstrates the polymorphic human immune response.
Alanine screening of the two epitopes
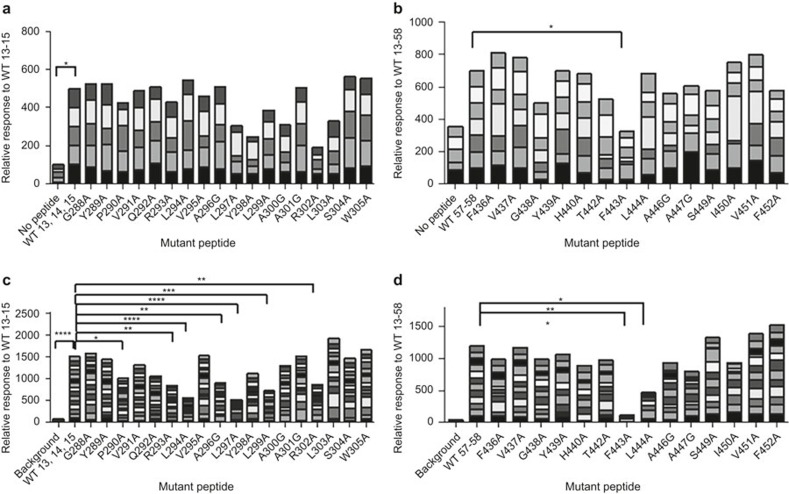
To identify critical residues that contribute to the T-cell response of the two epitopes, we synthesized a set of 19 peptides (21-mer) that spans the combined sequence of peptides 13, 14, and 15 and a set of 15 peptides (18-mer) that span the amino acid sequence of peptides 57-58 with alanine substitutions in different locations (Supplementary Table S1). In places where alanine was in the original sequence, glycine was used. We stimulated the splenocytes with each of the alanine variants and compared the T-cell activation response to that of the WT parent peptide. None of the point mutations in peptides 13–15 significantly reduced T-cell activation. Three-point mutations slightly reduced T-cell activation in WT 13-15: R302A, Y298A, and L297A (Figure 3a; p = 0.07, 0.12, and 0.12, respectively). F443A is the most critical residue in the epitope in peptides 57–58 (Figure 3b; p = 0.01). Interestingly, alanine scanning using PBMC samples from 15 human donors that had positive responses to peptides 13–15 identified that L294A, L297A, R302A, and several other mutations are critical residues that reduce T-cell activation (Figure 3c). Furthermore, stimulation of PBMC from12 donors that responded to the epitope in peptides 57–58identified F443A as the most critical residue (Figure 3d). However, not all critical residues were in common; L444A had a significant reduction in T-cell activation using human cells but not in mouse cells.
Figure 3.
T-cell responses to alanine variant peptides. Splenocytes from mice were stimulated with a set of alanine variants spanning the sequences of the epitopes. (a) Mouse T-cell response to alanine variants of 21-mer peptide 13, 14, 15 (n = 5). (b) Mouse T-cell response to alanine variants of 18-mer peptide 57-58 (n = 7). (c) Response of human PBMC from 15 naive donors to alanine variants of 21-mer peptide 13, 14, 15. (d) Response of human PBMC from 12 naive donors to alanine variants of 18-mer peptide 57-58. All responses were normalized to the response to WT. Statistical analysis includes Friedman test followed by Dunn's multiple comparisons test., *, **, ***, and **** indicate p < 0.05, 0.01, 0.001, and 0.0001, respectively.
Immunization with the LMB-T20 abrogates T-cell activation
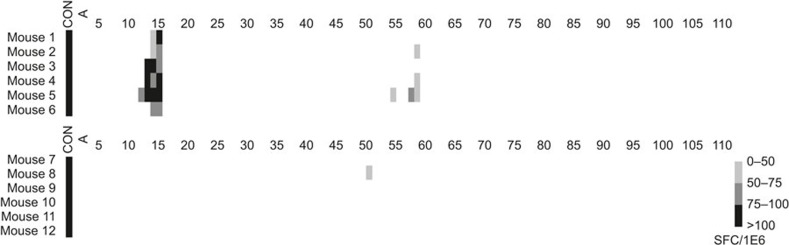
BALB/c mice were immunized with SS1P and LMB-T20 (that has both T-cell epitopes eliminated by deletion of epitope 13–15 and a point mutation in the critical residue of epitope 57-58; Figure 1) in alum adjuvant to ensure a strong response. Spleens were isolated and splenocytes were stimulated with the appropriate peptides so that cells from six mice immunized with SS1P were stimulated with 111 peptides spanning the sequence of PE38 and cells from six mice immunized with LMB-T20 were stimulated with 74 peptides spanning the sequence of the shorter de-immunized toxin. Peptides were first tested in pools and de-convolution of positive pools followed. Figure 4 shows T-cell activation in a heat map format. There is a strong response to SS1P, whereas the response to LMB-T20 was greatly reduced. There was no response to the immunodominant epitope that was deleted in LMB-T20. One mouse had a weak response to peptide 51; however, this response was not detected in the other five mice. In addition, no new T-cell epitopes were created by the mutations in LMB-T20 and no response to cryptic epitopes was observed.
Figure 4.
Immunization with the LMB-T20 variant results in abrogation of T-cell activation. BALB/c mice were immunized with SS1P and LMB-T20 in alum. Spleens were isolated and splenocytes were stimulated with the appropriate peptides so that cells from six mice immunized with SS1P were stimulated with 111 peptides spanning the sequence of PE38 (top panel, mice# 1-6) and cells from six mice immunized with LMB-T20 were stimulated with 74 peptides spanning the sequence of the shorter de-immunized toxin (bottom panel, mice# 7-12). Peptides were first tested in pools and de-convolution of positive pools followed. Concanavalin A was used as a non-specific control.
ADA response to SS1P and LMB-T20
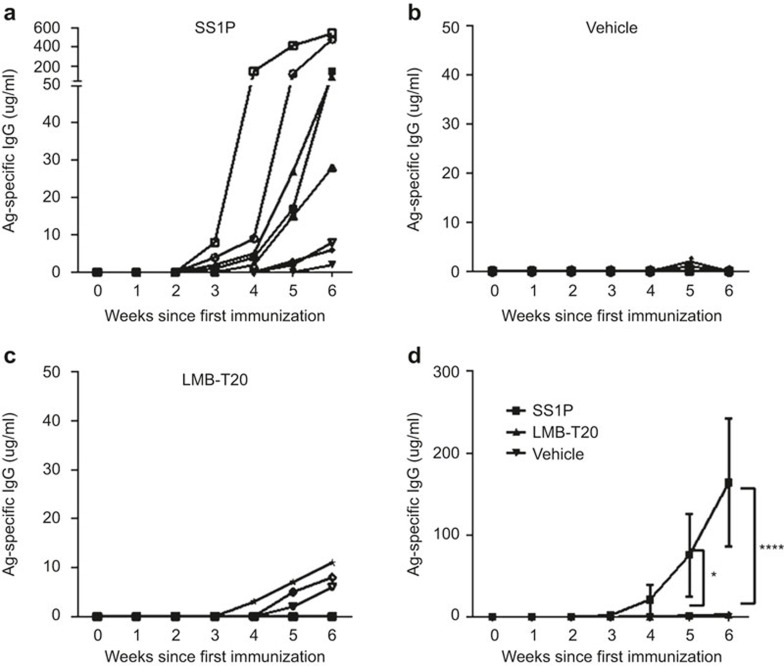
To test whether the reduction in T-cell activation in response to T-cell de-immunization also reduced the level of Ag-specific Ab, mice were immunized weekly over a course of 6 weeks with SS1P, LMB-T20, or PBS. Vehicle contained PBS with no alum to resemble the RIT treatment given to patients. Serum samples were taken 2 days before every immunization and the presence of Ag-specific Ab was measured in all serum samples using ELISA. Ab titer was interpolated based on comparison to a standard curve of IP12, a mouse monoclonal IgG that binds both RITs with similar affinity.22 We found that four out of eight mice immunized with SS1P developed a detectable IgG response starting at the third week of immunization (Figure 5a). By the fourth week of immunization, Ag-specific Ab was significantly higher compared with PBS-immunized mice (p < 0.0001; Figure 5b) and after week 6, all eight mice had detectable IgG (Figure 5a and d) with an average of 164 µg/ml. In contrast, most of the mice immunized with LMB-T20 did not develop a detectable Ab titer during the entire course of immunization (Figure 5c). The highest titer was observed on the last week of immunization when three out of eight mice had detectable titers; however, those titers were lower than 10 µg/ml and significantly lower than SS1P mice (p < 0.0001; Figure 5d).These results were then repeated with another set of mice (n = 24) with similar results.
Figure 5.
Immunization with LMB-T20 impedes formation of Ag-specific IgG. BALB/c mice were immunized with SS1P, LMB-T20, or PBS. Serum samples were taken 2 days before every immunization. Ag-specific Ab titer was evaluated using direct ELISA as a response to (a) SS1P (n = 8), (b) PBS (n = 8), and (c) LMB-T20 (n = 8). Each line represents the Ag-specific antibody titer for one mouse over the course of 6 weeks. (d) Average titer for all treatment groups. Ab titer was interpolated based on comparison to a standard curve of IP12. Each line represents the Ag specific antibody titer for one mouse over the course of 6 weeks. Error bars for SEM. Significance in two-way ANOVA. * and **** indicate p < 0.05 and 0.0001, respectively.
To confirm that the treatment with SS1P and LMB-T20 did not have a non-specific effect on the immune status of the mice, mice were immunized with a combination of the RIT with HSA. Levels of HSA specific antibodies in serum samples from one representative mouse in each treatment group and were measured using ELISA and are shown in Supplementary Figure S1. All three mice, regardless of the immunotoxin treatment did not have pre-existing Ab to HSA (Supplementary Figure S1a) and all developed an Ab response after 2, 5, and 6 immunizations (Supplementary Figure S1b–d). The response to HSA was very similar in the three treatment groups.
ADA memory response to SS1P and LMB-T20
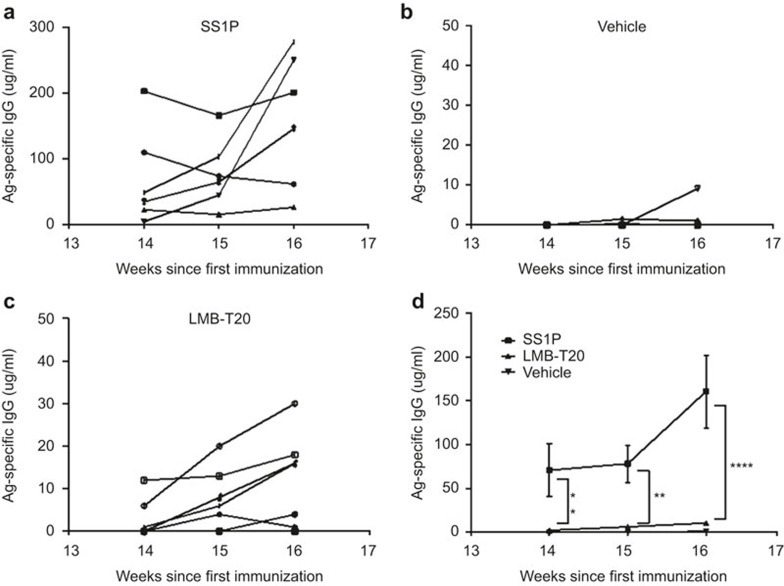
To address the possibility that low and undetectable levels of activated T cells and antibody can create immune memory that will be manifest in a strong recall response, we immunized 18 mice with SS1P, LMB-T20, or vehicle on weeks 1, 2, 4, 5, 6, and 7 and re-immunized the mice on weeks 14, 15, and 16. Serum taken from the mice after the delayed immunizations was used to assess Ag-specific Ab. We found that five out of six mice immunized with SS1P had a rapid and significant boost in IgG titer (Figure 6a) compared to mice immunized with vehicle or LMB-T20 (p < 0.01; Figure 6b and c) resulting in an average titer of 161 µg/ml. However, mice immunized with LMB-T20 had a very low titer throughout the three immunizations with an average titer of 11 ug/ml after the final immunization, which was not significantly different from mice immunized with PBS (p = 0.89; Figure 6d).
Figure 6.
Delayed immunization with LMB-T20 diminishes Ab-specific memory recall response. BALB/c mice were immunized with SS1P, LMB-T20, or PBS six times in weeks 1–7 and then immunized again two times on weeks 14, 15, and 16. Serum samples were taken 2 days before every immunization. Ag-specific Ab titer was evaluated using direct ELISA as a response to (a) SS1P (n = 6), (b) PBS (n = 8), (c) LMB-T20 (n = 8). Each line represents the Ag-specific antibody titer for one mouse over the course of 3 weeks. (d) Average titer for all treatment groups. Ab titer was interpolated based on comparison to a standard curve of IP12. Each line represents the Ag-specific antibody titer for one mouse over the course of 6 weeks. Error bars for SEM. Significance in two way ANOVA. ** and **** indicate p < 0.01 and 0.0001, respectively.
Neutralization assay
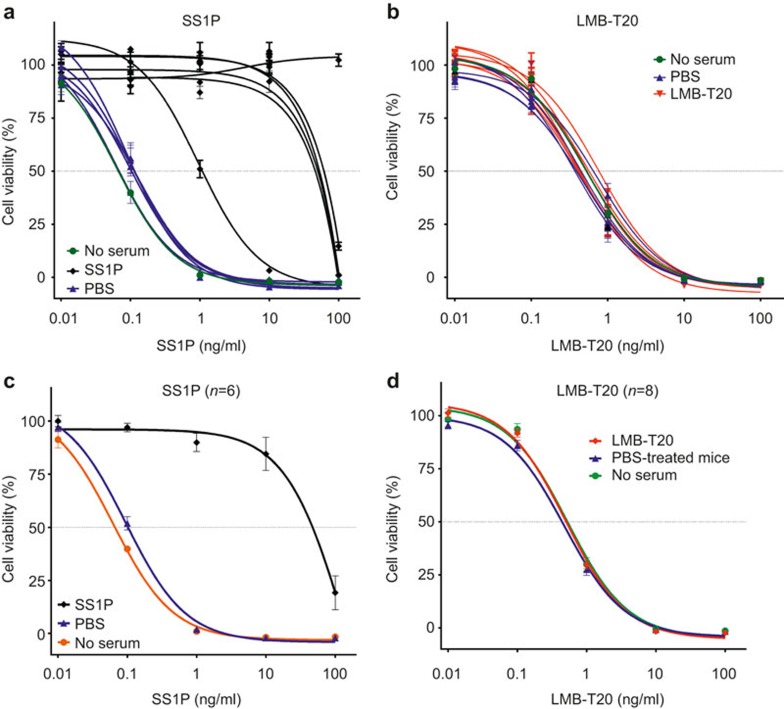
Because the detection of ADAs by ELISA does necessarily indicate that those antibodies will neutralize the activity of the immunotoxin, we evaluated the ability of the serum to neutralize the activity of the RITs in vitro using a cytotoxicity assay in which RITs kill cells. To assess the neutralization activity, the mice shown in Figure 5c were immunized one more time on week 29 and serum was taken the day after. Various concentrations of SS1P and LMB-T20 were mixed with 1:50 diluted serum from mice that were immunized with SS1P or LMB-T20. The mixtures were incubated for 1 hour and added to A431/H9 cells. Addition of the mixture to the cells resulted in an additional twofold dilution of the serum.SS1P has an IC50 of 0.05 ng/ml (Figure 7a). Incubation of SS1P with serum taken from six mice that were immunized with SS1P neutralized the activity of SS1P, so that the IC50 was shifted more than 200-fold to 10 ng/ml in five out of six mice. The mean IC50 of SS1P after neutralization by serum from six mice is 146 ng/ml (Figure 7b), demonstrating 1500-fold loss in activity compared with cells treated with no serum or serum from mice treated with vehicle. In contrast, serum from mice immunized with LMB-T20 did not neutralize the activity of LMB-T20 (Figure 7c and d). The IC50 was 0.5 ng/ml in all the groups.
Figure 7.
RIT is neutralized by serum from mice treated with SS1P but not LMB-T20. Serum from mice that were treated with SS1P, PBS, or LMB-T20 was diluted in 1:50 in PBS and mixed with either SS1P or LMB-T20 immunotoxin for 1 hour at room temperature. A431/H9 cells were placed in 96-well plates and treated with the serum-immunotoxin mixture. After 72 hours, cell viability was assessed using WST-8. (a) Viability of A431/H9 cells after treatment with SS1P that was incubated with serum from six mice that were previously immunized with SS1P (black curves) and four mice immunized with PBS (blue curves). The orange curve indicates cell viability after treatment with SS1P that was not incubated with serum. (b) Cell viability after treatment with LMB-T20 that was incubated with serum from eight mice that were immunized with LMB-T20 and four mice treated with PBS. The green curve indicates cell viability after treatment with SS1P that was not incubated with serum. (c) Average of the curves shown in A. (d) Average of the curves shown in C. All experiments were in six replicas. Error bars show standard deviation.
Discussion
We show here that the elimination of T-cell epitopes in an immunogenic therapeutic protein results in a significant disruption of ADA formation in mice.
Mice and human common T-cell epitopes
We mapped the murine T-cell epitopes in PE38 using an immunocompetent BALB/c model and found that mice recognized two epitopes: an immunodominant epitope and a subdominant epitope. Both epitopes correspond to human epitopes; one corresponds to the immunodominant human epitope and the other to a weaker epitope.10 Commonality between human and murine epitopes is not rare. Brocke et al. found that three strains of mice (SJL, C3H/He, and BALB/c) responded to immunogenic HLA-DR restricted peptides derived from acetylcholine receptor23 and similarly, Yeung et al. found that the immunodominant T-cell epitope in IFNβ was common for both humans and BALB/c mice.24 It seems likely that the similar structures of human and murine MHC molecules and also the similar structures of T-cell receptors make it possible to find common epitopes among different species. Common epitopes can also result from common pathogens prevalent in both mice and humans, which cause co-evolution of the immune system to recognize similar epitopes.
Use of mouse models to predict immunogenicity
The use of mice and other animal models to predict immunogenicity has been shown to be ambiguous in some instances,25 mostly due to the fact that recognition of a protein or an epitope as “self” in one species cannot assure tolerance in a different species. Nevertheless, it has been shown that transgenic tolerant mice can predict one variant to be more immunogenic than another.26 The approach of T-cell epitope mapping in human cells and complementary mapping in animal models should be useful in cases where the epitopes are the same and thus the animal model can be used as a proof of concept for de-immunization strategies.
Immunodominant epitope
The immunodominant epitope in position 291-306 of PE was previously identified as a immunodominant epitope that promoted T-cell responses in 21/50 naive donors and six out of nine patients that were treated with an immunotoxin containing PE38.10 This epitope also promotes T-cell response in C57/B6 mice (data not shown) and is predicted to bind to numerous HLA DR molecules with high affinity.27 Interestingly, this epitope is conserved in Pseudomonas aeruginosa and does not resemble any epitopes from other species based on a search in IEDB epitope database.28
Deimmunization of T-cell epitopes in LMB-T20
The immunodominant epitope in position 291-306 is located in domain II. We previously showed that a deletion of this domain, except for 11 amino acids that are required for furin cleavage resulted in an active immunotoxin with much less non-specific toxicity.29 Deletion of domain II also resulted in complete removal of the immunodominant T-cell epitope. To identify the critical residues for the immune response in the subdominant epitope in position 236-451, we performed alanine mutagenesis and found that change of a phenylalanine to alanine in position 443 interrupts the immune response of both humans and BALB/c mice. However, other residues that affected immunogenicity were not shared between the two species. This indicates that the F443 is very critical for the immune response. Interestingly, MHC II-binding algorithm predictions for the murine presentation molecules IAd and IEd predict that the F443 is not a critical residue for binding to the MHC II molecule and that mutation of the phenylalanine to alanine should actually result in a sixfold stronger presentation.30 Therefore, it is possible that the phenylalanine plays a role in binding to the T-cell receptor.
As a result of the commonality of the human and murine epitopes in BALB/c, LMB-T20 that was originally designed to eliminate human T-cell epitopes is also de-immunized for BALB/c and can provide insight on the immune response to a de-immunized foreign protein.
Fewer T-cell epitopes in this study compared to human study
In this study, we only identified two T-cell epitopes, whereas in the human study we identified eight. The reason for the larger number of epitopes in the human study is that it included 50 donors with various HLA haplotypes, while in this study all mice have identical MHC haplotypes. The mild variations in the magnitude of the mice responses can be attributed to differences in their T-cell receptor repertoire such that some mice have higher affinity T-cell receptors or higher frequency of T cells with the specific receptors.31
Possible reasons for reduction in response to LMB-T20
LMB-T20 was designed to eliminate T-cell epitopes; however, as a result of the engineering of the molecules there are several differences between LMB-T20 and SS1P that may contribute to the reduction in immunogenicity in addition to the elimination of T-cell epitopes. (1) Previous characterization of PE38 identified seven B cell epitopes by generation of 60 anti-PE38 mAb from immunized mice and mutual competition of all possible pairs of those mAbs. Epitope 1A was identified as the principle neutilizing epitope by neutralization assays.32 Since two out of seven of the B cell epitopes (including the neutralizing epitope 1A) are located in domain II and were deleted in the construction of LMB-T20, deletion of these epitopes could have contributed to the reduced immunogenicity. (2) In addition to the deletion of known B cell epitopes, the molecular changes in SS1P can cause structural changes in the exposed residues and hide additional B cell epitopes that were exposed. (3) LMB-T20 is significantly smaller than SS1P (49 kD compared to 63 kD), which may affect pharmacokinetics and accelerate the clearance of LMB-T20, promoting a shorter exposure of the molecule to the immune system. However, the shorter half-life of LMB-T20 does not affect its efficacy. We found that LMB-T20 was much better tolerated than SS1P in xenografts mice and promoted complete tumor regressions in seven out of eight mice while SS1P did not.20 The deletion of most of domain II reduced the nonspecific toxicity of LMB-T20 by more than 12-fold29 which increases the therapeutic window and makes it possible to obtain complete tumor regressions in mice.20
No epitope spreading
In this proof of concept study, we found that changes in LMB-T20 abrogate ADA formation. Because LMB-T20 still has five B cell epitopes,33 these results indicate that the elimination of T-cell epitopes is sufficient for the prevention of neutralizing and ADA formation. Furthermore, we found that elimination of the main T-cell epitopes did not result in emergence of cryptic or de novo epitopes. These results are in agreement with the findings of Yeung et al. that showed that elimination of T-cell epitopes in the protein IFNβ resulted in elimination of ADA response in BALB/c mice.24 A significant diminish in ADA was also noted after de-immunization of T-cell epitopes in E. coli L-asparaginase II.12
An immune “recall” response
An immune “recall” response, also known as a memory response, is defined as a stronger and faster response that involves activation of long-lived memory B and T cells.34 The mice that were immunized with SS1P showed a rapid immune response and developed a high titer of IgG upon re-immunization on week 14. Mice treated with LMB-T20 did not. Since the memory response requires activation of both memory B and T cells, it is likely that the absence of effector T cells against LMB-T20 disrupted the recall response to this protein.
Neutralization
It has been shown that ADA can form without the ability to neutralize the protein.35 This can happen when the ADA is monoclonal or when it binds to an epitope that does not affect the internalization and activity of the drug.36 Characterization of the ADA response to distinguish neutralizing from non-neutralizing ADA is highly recommended by regulators because the neutralizing and non-neutralizing antibodies can have different clinical effects.37,38 Here we show that the murine ADAs against SS1P are neutralizing, which correlates with the neutralizing antibodies observed in patients treated with SS1P in clinical trials.8,9 This finding emphasizes the predictive value of this mouse model.
The fact that no neutralization was observed in the mice immunized with LMB-T20 further confirms the non-immunogenic potential of LMB-T20 and suggests that it should be more efficacious in clinical settings. To our knowledge, this is the first in vivo demonstration of prevention of neutralization and a recall response by T-cell de-immunization.
Conclusions
We conclude that the approach of de-immunization by identification and removal of T-cell epitopes can prevent the formation of neutralizing antibodies in vivo for both a naive response and a memory response.
Competing interests
I. Pastan and R. Mazor are the inventors on patents or patent applications related to de-immunizing immunotoxins. All of these have been assigned to the NIH. All authors declare no conflict.
Funding
This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, in part with Federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E, and in part with a Cooperative Research and Development Agreement (#2791) with Roche Pharmaceuticals.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Frederick National Laboratory (currently Leidos Biomedical Research, Inc.) is accredited by AAALAC International and follows the Public Health Service Policy for the Care and Use of Laboratory Animals. Animal care was provided in accordance with the procedures outlined in the “Guide for Care and Use of Laboratory Animals” (National Research Council; 2011; National Academies Press; Washington, D.C.).
Acknowledgments
The authors thank Dr. Chin-Hsien Tai for creating the structural model of RIT for figure 1. Supplementary information of this article can be found on the Cellular & Molecular Immunology's website (http://www.nature.com/cmi).
Footnotes
Supplementary information of this article can be found on the Cellular & Molecular Immunology's website (http://www.nature.com/cmi).
Supplementary Information
References
- Casadevall N, Nataf J, Viron B, Kolta A, Kiladjian JJ, Martin-Dupont P et al. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N Engl J Med 2002; 346: 469–475. [DOI] [PubMed] [Google Scholar]
- Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med 2008; 358: 1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellekens H. How to predict and prevent the immunogenicity of therapeutic proteins. Biotechnol Annu Rev 2008; 14: 191–202. [DOI] [PubMed] [Google Scholar]
- Kreitman RJ, Tallman MS, Robak T, Coutre S, Wilson WH, Stetler-Stevenson M et al. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol 2012; 30: 1822–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan R, Sharon E, Thomas A, Zhang J, Ling A, Miettinen M et al. Phase 1 study of the antimesothelin immunotoxin SS1P in combination with pemetrexed and cisplatin for front-line therapy of pleural mesothelioma and correlation of tumor response with serum mesothelin, megakaryocyte potentiating factor, and cancer antigen 125. Cancer 2014; 120: 3311–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Zhang Y, Pastan I, Kreitman RJ. Synergistic antitumor activity of anti-CD25 recombinant immunotoxin LMB-2 with chemotherapy. Clin Cancer Res 2012; 18: 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldon JE, Pastan I. A guide to taming a toxin--recombinant immunotoxins constructed from Pseudomonas exotoxin A for the treatment of cancer. FEBS J 2011; 278: 4683–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham MC et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res 2007; 13: 5144–5149. [DOI] [PubMed] [Google Scholar]
- Kreitman RJ, Hassan R, Fitzgerald DJ, Pastan I. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin Cancer Res 2009; 15: 5274–5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazor R, Eberle JA, Hu X, Vassall AN, Onda M, Beers R et al. Recombinant immunotoxin for cancer treatment with low immunogenicity by identification and silencing of human T-cell epitopes. Proc Natl Acad Sci USA 2014; 111: 8571–8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding FA, Liu AD, Stickler M, Razo OJ, Chin R, Faravashi N et al. A beta-lactamase with reduced immunogenicity for the targeted delivery of chemotherapeutics using antibody-directed enzyme prodrug therapy. Mol Cancer Ther 2005; 4: 1791–1800. [DOI] [PubMed] [Google Scholar]
- Cantor JR, Yoo TH, Dixit A, Iverson BL, Forsthuber TG, Georgiou G. Therapeutic enzyme deimmunization by combinatorial T-cell epitope removal using neutral drift. Proc Natl Acad Sci USA 2011; 108: 1272–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moise L, Song C, Martin WD, Tassone R, De Groot AS, Scott DW. Effect of HLA DR epitope de-immunization of Factor VIII in vitro and in vivo. Clin Immunol 2012; 142: 320–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cizeau J, Grenkow DM, Brown JG, Entwistle J, MacDonald GC. Engineering and biological characterization of VB6-845, an anti-EpCAM immunotoxin containing a T-cell epitope-depleted variant of the plant toxin bouganin. J Immunother 2009; 32: 574–584. [DOI] [PubMed] [Google Scholar]
- Salvat RS, Choi Y, Bishop A, Bailey-Kellogg C, Griswold KE. Protein deimmunization via structure-based design enables efficient epitope deletion at high mutational loads. Biotechnol Bioeng 2015; 112: 1306–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot AS, Martin W. Reducing risk, improving outcomes: bioengineering less immunogenic protein therapeutics. Clin Immunol 2009; 131: 189–201. [DOI] [PubMed] [Google Scholar]
- Tangri S, Mothe BR, Eisenbraun J, Sidney J, Southwood S, Briggs K et al. Rationally engineered therapeutic proteins with reduced immunogenicity. J Immunol 2005; 174: 3187–3196. [DOI] [PubMed] [Google Scholar]
- Baker MP, Reynolds HM, Lumicisi B, Bryson CJ. Immunogenicity of protein therapeutics: The key causes, consequences and challenges. Self Nonself 2010; 1: 314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazor R, Vassall AN, Eberle JA, Beers R, Weldon JE, Venzon DJ et al. Identification and elimination of an immunodominant T-cell epitope in recombinant immunotoxins based on Pseudomonas exotoxin A. Proc Natl Acad Sci USA 2012; 109: E3597–E3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazor R, Zhang J, Xiang L, Addissie S, Awuah P, Beers R, et al. Recombinant immunotoxin with T cell epitope mutations that greatly reduce immunogenicity for treatment of mesothelin expressing tumors. Mol Cancer Ther in press. [DOI] [PMC free article] [PubMed]
- Pastan I, Beers R, Bera TK. Recombinant immunotoxins in the treatment of cancer. Methods Mol Biol 2004; 248: 503–518. [DOI] [PubMed] [Google Scholar]
- Liu W, Onda M, Lee B, Kreitman RJ, Hassan R, Xiang L et al. Recombinant immunotoxin engineered for low immunogenicity and antigenicity by identifying and silencing human B-cell epitopes. Proc Natl Acad Sci USA 2012; 109: 11782–11787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocke S, Dayan M, Rothbard J, Fuchs S, Mozes E. The autoimmune response of different mouse strains to T-cell epitopes of the human acetylcholine receptor alpha subunit. Immunology 1990; 69: 495–500. [PMC free article] [PubMed] [Google Scholar]
- Yeung VP, Chang J, Miller J, Barnett C, Stickler M, Harding FA. Elimination of an immunodominant CD4+ T cell epitope in human IFN-beta does not result in an in vivo response directed at the subdominant epitope. J Immunol 2004; 172: 6658–6665. [DOI] [PubMed] [Google Scholar]
- Brinks V, Jiskoot W, Schellekens H. Immunogenicity of therapeutic proteins: the use of animal models. Pharm Res 2011; 28: 2379–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beers MM, Sauerborn M, Gilli F, Hermeling S, Brinks V, Schellekens H et al. Hybrid transgenic immune tolerant mouse model for assessing the breaking of B cell tolerance by human interferon beta. J Immunol Methods 2010; 352: 32–37. [DOI] [PubMed] [Google Scholar]
- Mazor R, Tai CH, Lee B, Pastan I. Poor correlation between T-cell activation assays and HLA-DR binding prediction algorithms in an immunogenic fragment of Pseudomonas exotoxin A. J Immunol Methods 2015; in press. [DOI] [PMC free article] [PubMed]
- Vita R, Overton JA, Greenbaum JA, Ponomarenko J, Clark JD, Cantrell JR et al. The immune epitope database (IEDB) 3.0. Nucleic Acids Res 2015; 43: D405–D412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldon JE, Xiang L, Chertov O, Margulies I, Kreitman RJ, FitzGerald DJ et al. A protease-resistant immunotoxin against CD22 with greatly increased activity against CLL and diminished animal toxicity. Blood 2009; 113: 3792–3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Sidney J, Dow C, Mothe B, Sette A, Peters B. A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLoS Comput Biol 2008; 4: e1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delluc S, Ravot G, Maillere B. Quantitative analysis of the CD4 T-cell repertoire specific to therapeutic antibodies in healthy donors. FASEB J 2011; 25: 2040–2048. [DOI] [PubMed] [Google Scholar]
- Onda M, Nagata S, FitzGerald DJ, Beers R, Fisher RJ, Vincent JJ et al. Characterization of the B cell epitopes associated with a truncated form of Pseudomonas exotoxin (PE38) used to make immunotoxins for the treatment of cancer patients. J Immunol 2006; 177: 8822–8834. [DOI] [PubMed] [Google Scholar]
- Onda M, Beers R, Xiang L, Lee B, Weldon JE, Kreitman RJ et al. Recombinant immunotoxin against B-cell malignancies with no immunogenicity in mice by removal of B-cell epitopes. Proc Natl Acad Sci USA 2011; 108: 5742–5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W. Fundamental Immunology. 6th ed. Philadelphia, PA: Lippincott Willians & Wilkins, 2008. [Google Scholar]
- Shankar G, Pendley C, Stein KE. A risk-based bioanalytical strategy for the assessment of antibody immune responses against biological drugs. Nat Biotechnol 2007; 25: 555–561. [DOI] [PubMed] [Google Scholar]
- Chirmule N, Jawa V, Meibohm B. Immunogenicity to therapeutic proteins: impact on PK/PD and efficacy. AAPS J 2012; 14: 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services Food and Drug Administration. Guidance for industry immunogenicity assessment for therapeutic protein products. Silver Spring, MD: Center for Drug Evaluation and Research; 2014. [Google Scholar]
- U.S. Department of Health and Human Services Food and Drug Administration. Guidance for Industry Assay Development for Immunogenicity Testing of Therapeutic Proteins. Silver Spring, MD: Center for Drug Evaluation and Research; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.