Abstract
Due to its minimal systematic adverse effects, transdermal estrogen is widely used for the prevention of osteoporosis in postmenopausal women. The present meta-analysis aimed to clarify the effects of transdermal estrogen on bone mineral density (BMD) of postmenopausal women. Studies were identified by searching electronic databases including Cochrane Library, MEDLINE, Embase , and CINAHL databases, and also the Sciences Citation Index. Systematic review of articles was published between January 1989 to February 2016.Reference lists of the included articles were also evaluated and consultations were made with relevant experts. While 132 studies included the desired keywords, only nine clinical trials met the inclusion criteria and were finally reviewed. The pooled percent change in BMD was statistically significant in favor of transdermal estrogen. According to resulting pooled estimate, lumbar spine BMD one and two years after transdermal estrogen therapy was respectively 3.4% (95% CI: 1.7-5.1) and 3.7% (95% CI: 1.7-5.7) higher than the baseline values. The test for heterogeneity was not statistically significant based on the I2 heterogeneity index. One-two years of transdermal estrogen delivery can effectively increase BMD and protect the bone structure in postmenopausal women.
Key Words: Menopause, Bone mineral density, Transdermal estrogen
Introduction
Menopause predisposes women to osteoporosis is a major public health issue (1). Due to concerns about women’s bone health, several efforts have been made to effectively prevent or treat osteoporosis in postmenopausal women all around the world(2-4). Reduced estrogen levels during menopause can increase the risk of trabecular bone loss and lead to multiple bone fractures (5). According to the results of the National Osteoporosis Risk Assessment (NORA) study (6), 7.2% of the 200160 assessed postmenopausal women suffered from undiagnosed osteoporosis that predisposed them to a four-fold increased risk of bone fractures. Bone fractures due to estrogen deficiency cause disability in about two-thirds of women and increase the risk of mortality during the year after diagnosis by 20% (7). Therefore, not only bone loss prevention, but also timely diagnosis of osteoporosis is required to decrease the risk of disability and life-threatening events in postmenopausal women. Hormone replacement therapy (HRT) has thus been recommended as a proper approach to prevent bone loss during the postmenopausal period (8, 9). Steroidal hormone use has been found to change bone mineral density (BMD) in women (10). Despite its advantages, HRT might increase the risk of gall-bladder stones, endometrial cancer (especially in women with an intact uterus), and breast cancer (following estrogen therapy). Such disadvantages and limitations have raised serious concerns which have not been fully resolved.
The metabolic and therapeutic effects of estrogens depend on their type, dosage, form, and route of administration (11). Several alternative therapeutic options have been applied to provide optimal level of estrogen and reduce the risk of bone loss in the postmenopausal period (12, 13). Some systematic reviews and meta-analyses have confirmed the beneficial effects of bisphosphonates on preserving BMD and decreasing the risk of early osteoporosis in postmenopausal women (14, 15). However, gastrointestinal complications of these agents limit their use (16). Furthermore, while some selective estrogen receptor modulators, such as raloxifene, have been applied to increase bone turnover by elevating BMD, their use has been hindered by the elevated risk of thromboembolic events following their application (13).
Delivery of estrogens, either alone or in combination with progesterone, by different routes is currently the most accepted method for the prevention of osteoporosis in postmenopausal women (17). Although oral estrogens have long been the commonest regimen for the mentioned purpose (18, 19), transdermal estrogen delivery has recently received growing attention since it can provide its therapeutic effects by a very low dose of hormones and with the fewest side effects. The possibility of gradual delivery of hormones (20-22), particularly through novel nanoparticle delivery systems (23), along with beneficial effects on coagulation processes and lipid metabolism (11) are other advantages of this method. Ultra low-dose hormone therapy can alleviate the clinical symptoms of menopause by ensuring effective protection against the postmenopausal reduction in BMD (24).However, limited knowledge is presently available on the advantages and disadvantages of transdermal estrogen therapy in postmenopausal women (22). To the best of our knowledge, no systematic review has evaluated the effects of transdermal estrogen delivery on BMD in menopausal women. Prior studies have used transdermal estrogen therapy on BMD, but results have been inconsistent partly because of limited statistical power. The objective of the present meta-analysis was to evaluate the efficacy of transdermal estrogen therapy in maintaining BMD among postmenopausal women.
Methods
Literature Search strategy
The methods of the systematic review were specified in advance and documented in a published protocol in the Prospective Register of Systematic Reviews (PROSPERO).To ensure the rigor of this meta-analysis, we designed and reported it adhering to the criteria set out by PRISMA statement. Relevant clinical trial studies were identified by searching a number of key terms, i.e. “menopause”,“bone mineral density”and “transdermal estrogen” in electronic databases including the Cochrane Library, MEDLINE, Embase, and CINAHL databases, and the Sciences Citation Index. Reference lists of the included articles were also scanned and consultations were made with experts. Clinical trials published in English during January 1989 to February 2016 were reviewed. Articles from any country with relevant information on prevalence were eligible for full review. Studies were excluded if (1) Full text was not available (2) they had case series or case studies(3) studies which did not measure changes in BMD after one-two years and (4)studies without quantitative outcome data.
Study Selection and Data Extraction
Studies were included regardless of study quality. First, two researchers (F.A.and F.R.T) independently evaluated all potentially suitable articles based on their title and abstract. Clearly ineligible articles were discarded and full texts of eligible articles were obtained and assessed independently by the two reviewers. Cases of disagreement about the eligibility of studies were resolved by consulting a third reviewer. The papers’ author list, study design, publication date, country, number of participants in each group, drug regimens for hormone therapy, lengh of follow up, and percent change in BMD were extracted. Control groups in trials were receiving either placebo or routine checkup.
Quality Appraisal
The meta-analysis was reported following the PRISMA checklist. All eligible studies were carefully reviewed. Of the 132 studies which included the desired keywords, nine clinical trials met the study criteria and were finally reviewed (25-34).The quality of trials was assessed using the Cochrane Collaboration’s tool for assessing risk of bias in randomized trials.
Data analysis
Pooled changes in BMD by prescribing transdermal estrogen was assessed using the random effects model. This statistical technique weights individual studies by sample size and variance (both within- and between study variance) and yields a pooled point estimate and a 95% confidence interval (CI). This technique was considered as an appropriate pooling technique due to the relative heterogeneity of the source population in each study. The existence of heterogeneity across trials was evaluated using the I2 statistic, i.e. P > 0.05 and/or I2< 30% indicated homogeneity. Funnel plot and Egger’s test were used to estimate potential publication bias. P-values less than 0.05 from Egger’s test suggested the presence of publication bias. All statistical analyses were performed with STATA 13.1 (StataCorp, College Station, TX).
Main results
A flow chart of the literature search and its results is presented in Figure 1. Initially, the search yielded titles and abstracts from all databases. Studies were reviewed in full and nine papers were ultimately included. All these nine studies enjoyed high quality and fulfilled the inclusion criteria, i.e. they assessed the beneficial effects of transdermal estrogen on preserving BMD in postmenopausal women. Since the participants of different studies were followed up for one or two years, we conducted subgroups analysis according to the study follows. The papers were categorized based on their follow-up period. Table 1 summarizes the characteristics of the selected studies. Results of the analysis of quality assessment are shown in Table 2. None of studies had selection bias and attrition bias. There was unclear risk in performance bias in three included studies (25,28,30).Also, two studies had unclear risk in detection bias (31) and reporting bias(28). A total of 643 women were assessed to receive transdermal estrogen. All of studies had control groups receiving either placebo or other routine checkups. Six studies were published after 2001(25-30), and three studies were published before 2000 (31-33).
Figure 1.
The flow diagram of study selection for the meta-analysis
Table 1.
Review of studies related to the effects of transdermal estrogen delivery on BMD in postmenopausal women
| Author | Year | Country | Study design | participants | Drug regimens | Follow-up | Increase in BMD |
|---|---|---|---|---|---|---|---|
| Kim(25) | 2014 | South Korea | Comparative retrospective clinical trial | N=149 (100: HRT) (49: control) |
Transdermal estrogens were a patch (estradiol 1.5 mg patch, Estran-50 patch, twice a week, n = 21) or gel (0.1% estradiol gel, 1.5 mg once daily | 2 years | 4.9% (lumbar spine), 4.2% (hip) |
| Stanosz(26) | 2009 | Poland | Randomized controlled trail | N=75 (25: HRT) (25: control) (25:HST) |
Micronized 17β- estradiol (molar mass, 272.39 g/mol) in the form of patches at increasing-decreasing doses (25, 50, 75, and 75 μg per dose)and progesterone in the second phase of the therapeutic cycle. |
1 year | 3.8% (lumbar spine L2-L4 (g/cm2) |
| Ettinger(27) | 2004 | USA | Randomized,placebo-controlled trial | N=417 (208: HRT) (209: control) |
Unopposed transdermal estradiol at 0.014 mg/day |
2 years | 2.6% (lumbar spine) 0.4% (total hip) |
| Davas(28) | 2003 | Turkey | Comparative prospective clinical trial | N=160 (80: HRT) (80: routine checkups) |
Transdermal estrogen 0.05 mg twice weekly, and daily MPA, 5 mg orally or and alendronate, 10 mg orally | 1 year | 4.1% (lumbar spine) |
| Pereda (29) | 2002 | UK | Randomized placebo-controlled trial | N=21 (10: HRT) (11: routine checkups) |
25 mg estradiol implant inserted subcutaneously beneath the skin of the abdomen |
1 year | 5.4% (lumbar spine) 6.0% (total hip) 3.7% (femoral neck |
| Yang (30) | 2007 | Taiwan | Comparative Prospective clinical trial | N=120 (90: HRT) (30: routine checkups) |
Transdermal administration of estradiol gel at a daily dosage of 1.25, 2.5 and 5.0 g (containing 0.75, 1.5, and 3 mg of 17beta-estradiol/day) | 1 year | 4.8% (lumbar) |
| Adami(31) | 1989 | Italy | Randomised controlled trial | N=68 (34: HRT) (34:control ) |
Transdermal estradiol (ESTRADERM TTS-50), 50 micrograms/day and medroxyprogesterone (10 mg/day for 12 days) | 2 years | 4.3% (lumbar) |
| Alexandersen(32, 34) | 1999 | Denmark | Randomized placebo-controlled trail | N=100 (51: HRT) (49: control) |
Transdermal 17beta-estradiol, releasing 50 microg/day; plus oral norethisterone acetate (NETA), 1 mg/day | 2 years | 4.0% (spinal) |
| Gonnelli(33) | 1997 | Italy | Randomized controlled trail | N=90 (45: HRT) (45: control) |
transdermal estrogen( 0.05 mg/day 17 beta-estradiol) and calcium | 1 year 2 years |
5.7% (lumbar) 6.6% (lumbar) |
HRT: Hormone replacement therapy; MPA: Medroxyprogesterone acetate; HST: Hormonal supplementary therapy; NETA: Norethisterone acetate
Table 2.
Quality appraisal by Cochrane Collaboration’s tool for assessing risk of bias
| No. | Domain | Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | Other bias |
|---|---|---|---|---|---|---|---|
| Reference | |||||||
| 1 | Kim,2014 | + | ? | + | + | + | ? |
| 2 | Stanosz,2009 | + | + | + | + | + | ? |
| 3 | Ettinger,2004 | + | + | + | + | + | ? |
| 4 | Davas,2003 | + | ? | + | + | ? | ? |
| 5 | Pereda,2002 | + | + | + | + | + | ? |
| 6 | Yang,2007 | + | ? | + | + | + | ? |
| 7 | Adami ,1989 | + | + | ? | + | + | ? |
| 8 | Alexandersen,1999 | + | + | + | + | + | ? |
| 9 | Gonnelli,1997 | + | + | + | + | + | ? |
Key: Low risk: +; Unclear risk:? ; High risk: -
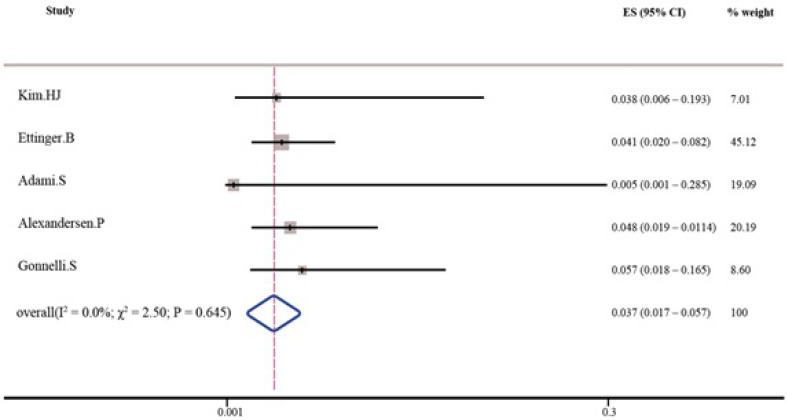
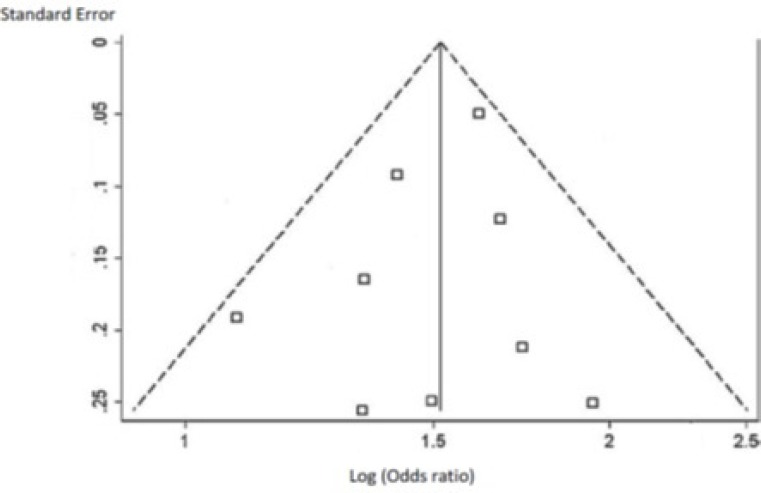
As shown in Tables 3 and 4, the pooled percent change in BMD was statistically significant in favor of transdermal estrogen. According to the resulting pooled estimate, BMD one and two years after transdermal estrogen therapy was respectively 3.4% (95% CI: 1.7-5.1) and 3.7% (95% CI: 1.7-5.7) higher than the baseline values. The test for heterogeneity was not statistically significant in studies with either one-year follow-up (I2 = 0.0%; χ2 = 1.82; P = 0.768; Figure 2) or two-year follow-up (I2 = 0.0%; χ2 = 2.50; P = 0.645; Figure 3). Moreover, Egger’s test and developed funnel plots did not suggest publication bias(P = 0.524)(Figure 4).
Table 3.
Increase in BMD following one year of using transdermal estrogen
Table 4.
Increase in BMD following two years of using transdermal estrogen
Figure 2.
Percent increase in BMD following one year of using transdermal estrogen
Figure 3.
Percent increase in BMD following two years of using transdermal estrogen
Figure 4.
Funnel plot for publication bias.
Discussion
The present research highlighted an agreement between the reviewed studies with regard to the effectiveness of transdermal estrogen in improving BMD. In fact, one-two years of receiving transdermal estrogen were associated with 3.4%-3.7% increase in BMD. Moreover, in all trials, the drug was well tolerated with no major adverse events. Our analysis also indicated the homogeneity and lack of publication bias across the studies. Minimal side effects and a potential for the gradual delivery of the drug at selective dosages have turned transdermal estrogen therapy as a favorable method for the prevention of osteoporosis in postmenopausal women. Several trials have attempted to assess the efficacy of this method in the improvement and preservation of BMD. Our review of literature confirmed the beneficial effects of transdermal estrogen therapy on increasing BMD during the postmenopausal period. Transdermal estrogen delivery offers some important benefits over oral administration of the drug. Since transdermal estrogen can systemically circulate before reaching the liver, the first-pass metabolism of estrogen in the liver is prevented. Therefore, the desired effects can be obtained with lower doses of the drug (35). This will, in turn, inhibit the overproduction of triglyceride, which is generally seen following the oral administration of estrogen (36-38). In fact, transdermal estrogen delivery has been found to reduce triglyceride levels by 33.7% (38) and is thus believed to decrease the risk of cardiovascular events in postmenopausal women (39, 40). Based on previous research, transdermal estrogen delivery can decrease the incidence of coronary artery disease by reducing systolic blood pressure and vascular resistance while elevating cardiac stroke volume and cardiac output (41-43). In fact, because of simultaneous effects of transdermal estrogen delivery on both BMD preservation and cardioprotection, this therapeutic regimen is now superseding other treatment modalities. However, as mentioned earlier, some long-term side effects of transdermal estrogen delivery, including the increased risk of endometrial and breast cancers, have limited its application.
Several clinical trials have assessed the efficacy of estrogen-based hormone therapy in reducing the risk of bone fractures, especially in postmenopausal women. However, the efficacy of such treatments may depend on two main factors, i.e. women’s age and the minimal effective dose of estrogen (44). Early studies in this field indicated estrogen doses lower than 0.625 mg to be practically ineffective in decreasing bone fracture risk (44, 45). Later studies, however, found estrogen doses as low as 0.3 mg to be capable of preserving BMD during both premenopausal and postmenopausal periods (46). A significant increase in total BMD was also observed in postmenopausal women who took 0.25 mg/day of estrogen for three years (47). Nevertheless, the beneficial effects of transdermal estrogen therapy on the skeletal health may depend on women’s endogenous estrogen levels before treatment (48).
Finally, while transdermal estrogen therapy did not change women’s lipoprotein profile (49), it favorably modified platelets hemostasis and reversed the adverse effects of menopause (50). Moreover, transdermal estrogens might be safe with respect to thrombotic risk (51, 52). In conclusion, it seems that one-two years of transdermal estrogen delivery can increase bone density, preserve BMD, and successfully protect the bone structure in postmenopausal women. It can thus prevent single or multiple bone fractures and their consequent disability and poor quality of life in older women. There are limitations of this study that should be considered. There was a lack of articles that met the inclusion criteria for meta-analysis. As with other similar meta-analyses; this study is restricted by the heterogeneity of the included trials.
Implications of the systematic review for clinical practice:
Based on our findings, there is a need to revise recommendations about the effects of transdermal estrogen delivery on BMD in postmenopausal women. Transdermal estrogen can provide adequate skeletal loading and successfully protect the bone structure and reduce the risk of fractures in older women.
Acknowledgment
The study was approved by the Student Research Committee of Shahid Beheshti University of Medical Sciences (Tehran, Iran). The authors wish to express their gratitude to the mentioned committee.
Author Contribution
All of the authors had the same contribution in various process performed in this project.
Financial disclosure
We have no financial interests related to the material in this manuscript.
Funding/Support
This study did not receive any funding support.
Conflict of interests
Authors have no conflict of interests.
References
- 1.Abdi F, Mobedi H, Mosaffa N, Dolatian M, Tehrani FR. Hormone Therapy for Relieving Postmenopausal Vasomotor Symptoms: A Systematic Review. Archives of Iranian Medicine. 2016;19:141–6. [PubMed] [Google Scholar]
- 2.Palacios S, Currie H, Mikkola TS, Dragon E. Perspective on prescribing conjugated estrogens/bazedoxifene for estrogen-deficiency symptoms of menopause: A practical guide. Maturitas. 2015;80:435–40. doi: 10.1016/j.maturitas.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Khan A, Fortier M, Fortier M, Reid R, Abramson BL, Blake J. Osteoporosis in menopause. Journal of obstetrics and gynaecology Canada : JOGC. 2014;36:839–43. doi: 10.1016/S1701-2163(15)30489-8. [DOI] [PubMed] [Google Scholar]
- 4.Sacco SM, Ward WE. Revisiting estrogen: efficacy and safety for postmenopausal bone health. Journal of osteoporosis . 2010;2010:1–8. doi: 10.4061/2010/708931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seifert-Klauss V, Fillenberg S, Schneider H, Luppa P, Mueller D, Kiechle M. Bone loss in premenopausal, perimenopausal and postmenopausal women: results of a prospective observational study over 9 years. Climacteric . 2012;15:433–40. doi: 10.3109/13697137.2012.658110. [DOI] [PubMed] [Google Scholar]
- 6.Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. Jama . 2001;286:2815–22. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]
- 7.Eddy DM, Johnston C, Cummings S, Dawson-Hughes B, Lindsay R, Melton L. Osteoporosis: review of the evidence for prevention, diagnosis, and treatment and cost-effectiveness analysis. Osteoporosis International. 1998;8:1–4. [Google Scholar]
- 8.Fournier A, Fritel X, Panjo H, Zins M, Ringa V. Health characteristics of women beginning postmenopausal hormone therapy: have they changed since the publication of the Women›s Health Initiative? Menopause . 2014;21:687–93. doi: 10.1097/GME.0000000000000159. [DOI] [PubMed] [Google Scholar]
- 9.Sanders S, Geraci SA. Osteoporosis in postmenopausal women: considerations in prevention and treatment: (women›s health series) Southern medical journal . 2013;106:698–706. doi: 10.1097/SMJ.0b013e3182a0df8b. [DOI] [PubMed] [Google Scholar]
- 10.Lopez LM, Grimes DA, Schulz KF, Curtis KM, Chen M. Steroidal contraceptives: effect on bone fractures in women. The Cochrane database of systematic reviews. 2014;6:17–23. doi: 10.1002/14651858.CD006033.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanosz S, Jastrzebska M, Sankowski Z, Stanosz M. Anticoagulant effect of estroprogesterone therapy in women in menopausal period. Przeglad Menopauzalny . 2005;4:48–52. [Google Scholar]
- 12.Montalcini T, Migliaccio V, Ferro Y, Gazzaruso C, Pujia A. Androgens for postmenopausal women›s health? Endocrine. 2012;42:514–20. doi: 10.1007/s12020-012-9692-1. [DOI] [PubMed] [Google Scholar]
- 13.Birkhauser M. Selective Estrogen Receptor Modulators (SERMs) for prevention and treatment of postmenopausal osteoporosis. Therapeutische Umschau Revue therapeutique . 2012;69:163–72. doi: 10.1024/0040-5930/a000269. [DOI] [PubMed] [Google Scholar]
- 14.Ellis AG, Reginster JY, Luo X, Cappelleri JC, Chines A, Sutradhar S. Bazedoxifene versus oral bisphosphonates for the prevention of nonvertebral fractures in postmenopausal women with osteoporosis at higher risk of fracture: a network meta-analysis. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research . 2014;17:424–32. doi: 10.1016/j.jval.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reginster JY, Neuprez A, Beaudart C, Lecart MP, Sarlet N, Bernard D. Antiresorptive drugs beyond bisphosphonates and selective oestrogen receptor modulators for the management of postmenopausal osteoporosis. Drugs and aging. 2014;31:413–24. doi: 10.1007/s40266-014-0179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diab DL, Watts NB. Bisphosphonates in the treatment of osteoporosis. Endocrinology and metabolism clinics of North America . 2012;41:487–506. doi: 10.1016/j.ecl.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Marjoribanks J, Farquhar C, Roberts H, Lethaby A. Long term hormone therapy for perimenopausal and postmenopausal women. The Cochrane database of systematic reviews. . 2012;7:41–3. doi: 10.1002/14651858.CD004143.pub4. [DOI] [PubMed] [Google Scholar]
- 18.Davey DA. Update: estrogen and estrogen plus progestin therapy in the care of women at and after the menopause. Women›s health . 2012;8:169–89. doi: 10.2217/whe.12.1. [DOI] [PubMed] [Google Scholar]
- 19.Body JJ. How to manage postmenopausal osteoporosis? Acta clinica Belgica . 2011;66:443–7. doi: 10.2143/ACB.66.6.2062612. [DOI] [PubMed] [Google Scholar]
- 20.Kopper NW, Gudeman J, Thompson DJ. Transdermal hormone therapy in postmenopausal women: a review of metabolic effects and drug delivery technologies. Drug design, development and therapy . 2009;2:193–202. doi: 10.2147/dddt.s4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meczekalski B, Czyzyk A. New forms of estrogenotherapy in postmenopausal osteoporosis. Polski merkuriusz lekarski : organ Polskiego Towarzystwa Lekarskiego . 2009;27:77–80. [PubMed] [Google Scholar]
- 22.Shulman LP. Transdermal hormone therapy and bone health. Clinical interventions in aging . 2008;3:51–4. doi: 10.2147/cia.s937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Botelho MA, Queiroz DB, Barros G, Guerreiro S, Fechine P, Umbelino S. Nanostructured transdermal hormone replacement therapy for relieving menopausal symptoms: a confocal Raman spectroscopy study. Clinics . 2014;69:75–82. doi: 10.6061/clinics/2014(02)01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gambacciani M, Cappagli B, Ciaponi M, Pepe A, Vacca Fand Genazzani AR. Ultra low-dose hormone replacement therapy and bone protection in postmenopausal women. Maturitas. . 2008;59:2–6. doi: 10.1016/j.maturitas.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Kim HJ, Oh YK, Lee JS, Lee DY, Choi D, Yoon BK. Effect of transdermal estrogen therapy on bone mineral density in postmenopausal korean women. Journal of menopausal medicine. 2014;20:111–7. doi: 10.6118/jmm.2014.20.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanosz S, Żochowska E, Safranow K, Sieja K, Stanosz M. Influence of modified transdermal hormone replacement therapy on the concentrations of hormones, growth factors, and bone mineral density in women with osteopenia. Metabolism . 2009;58:1–7. doi: 10.1016/j.metabol.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Ettinger B, Ensrud KE, Wallace R, Johnson KC, Cummings SR, Yankov V. Effects of ultralow-dose transdermal estradiol on bone mineral density: a randomized clinical trial. Obstetrics and gynecology . 2004;104:443–51. doi: 10.1097/01.AOG.0000137833.43248.79. [DOI] [PubMed] [Google Scholar]
- 28.Davas I, Altintas A, Yoldemir T, Varolan A, Yazgan A, Baksu B. Effect of daily hormone therapy and alendronate use on bone mineral density in postmenopausal women. Fertility and sterility . 2003;80:536–40. doi: 10.1016/s0015-0282(03)00757-x. [DOI] [PubMed] [Google Scholar]
- 29.Pereda CA, Hannon RA, Naylor KE, Eastell R. The impact of subcutaneous oestradiol implants on biochemical markers of bone turnover and bone mineral density in postmenopausal women. BJOG. 2002;109:812–20. doi: 10.1111/j.1471-0528.2002.01177.x. [DOI] [PubMed] [Google Scholar]
- 30.Yang TS, Chen YJ, Liang WH, Chang CY, Tai LC, Chang SP. A clinical trial of 3 doses of transdermal 17beta-estradiol for preventing postmenopausal bone loss: a preliminary study. Journal of the Chinese Medical Association . 2007;70:200–6. doi: 10.1016/S1726-4901(09)70358-2. [DOI] [PubMed] [Google Scholar]
- 31.Adami S, Suppi R, Bertoldo F, Rossini M, Residori M, Maresca V. Transdermal estradiol in the treatment of postmenopausal bone loss. Bone and mineral . 1989;7:79–86. doi: 10.1016/0169-6009(89)90064-0. [DOI] [PubMed] [Google Scholar]
- 32.Alexandersen P, Riis BJ, Christiansen C. Monofluorophosphate combined with hormone replacement therapy induces a synergistic effect on bone mass by dissociating bone formation and resorption in postmenopausal women: a randomized study. The Journal of clinical endocrinology and metabolism . 1999;84:3013–20. doi: 10.1210/jcem.84.9.5988. [DOI] [PubMed] [Google Scholar]
- 33.Gonnelli S, Cepollaro C, Pondrelli C, Martini S, Monaco R, Gennari C. The usefulness of bone turnover in predicting the response to transdermal estrogen therapy in postmenopausal osteoporosis. Journal of bone and mineral research . 1997;12:624–31. doi: 10.1359/jbmr.1997.12.4.624. [DOI] [PubMed] [Google Scholar]
- 34.Schaefers M, Muysers C, Alexandersen P, Christiansen C. Effect of microdose transdermal 17beta-estradiol compared with raloxifene in the prevention of bone loss in healthy postmenopausal women: a 2-year, randomized, double-blind trial. Menopause . 2009;16:559–65. doi: 10.1097/gme.0b013e31818ebfba. [DOI] [PubMed] [Google Scholar]
- 35.Cobin R, Bedsoe M, Futerweit W, Goldzieher J, Goodman N, Petak S. AACE medical guidelines for clinical practice for management of menopause. Endocrine Practice . 1999;5:354–64. [Google Scholar]
- 36.Crook D, Cust MP, Gangar KF, Worthington M, Hillard TC, Stevenson JC. Comparison of transdermal and oral estrogen-progestin replacement therapy: effects on serum lipids and lipoproteins. American journal of obstetrics and gynecology. 1992;166:950–5. doi: 10.1016/0002-9378(92)91370-p. [DOI] [PubMed] [Google Scholar]
- 37.Erenus M, Karakoc B, Gurler A. Comparison of effects of continuous combined transdermal with oral estrogen and oral progestogen replacement therapies on serum lipoproteins and compliance. Climacteric . 2001;4:228–34. [PubMed] [Google Scholar]
- 38.Shulman LP, Yankov V, Uhl K. Safety and efficacy of a continuous once-a-week 17beta-estradiol/levonorgestrel transdermal system and its effects on vasomotor symptoms and endometrial safety in postmenopausal women: the results of two multicenter, double-blind, randomized, controlled trials. Menopause . 2002;9:195–207. doi: 10.1097/00042192-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Walsh BW, Cox DA, Sashegyi A, Dean RA, Tracy RP, Anderson PW. Role of tumor necrosis factor-alpha and interleukin-6 in the effects of hormone replacement therapy and raloxifene on C-reactive protein in postmenopausal women. The American journal of cardiology . 2001;88:825–8. doi: 10.1016/s0002-9149(01)01865-3. [DOI] [PubMed] [Google Scholar]
- 40.Renoux C, Dell›aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ . . 2010;340:19–25. doi: 10.1136/bmj.c2519. [DOI] [PubMed] [Google Scholar]
- 41.West SG, Hinderliter AL, Wells EC, Girdler SS, Light KC. Transdermal estrogen reduces vascular resistance and serum cholesterol in postmenopausal women. Am J Obstet Gynecol . 2001;184:926–33. doi: 10.1067/mob.2001.112104. [DOI] [PubMed] [Google Scholar]
- 42.Alfie J, Lugones L, Belardo A, Tutzer M, Galarza CR, Waisman GD. Hemodynamic effects of transdermal estradiol alone and combined with norethisterone acetate. Maturitas. 1997;27:163–9. doi: 10.1016/s0378-5122(97)00030-3. [DOI] [PubMed] [Google Scholar]
- 43.Cacciatore B, Paakkari I, Hasselblatt R, Nieminen MS, Toivonen J, Tikkanen MI. Randomized comparison between orally and transdermally administered hormone replacement therapy regimens of long-term effects on 24-hour ambulatory blood pressure in postmenopausal women. Am J Obstet Gynecol. . 2001;184:904–9. doi: 10.1067/mob.2001.111246. [DOI] [PubMed] [Google Scholar]
- 44.Lindsay R, Hart DM, Clark DM. The minimum effective dose of estrogen for prevention of postmenopausal bone loss. Obstetrics and gynecology. 1984;63:759–63. [PubMed] [Google Scholar]
- 45.Genant HK, Cann CE, Ettinger B, Gordan GS. Quantitative computed tomography of vertebral spongiosa: a sensitive method for detecting early bone loss after oophorectomy. Clinical orthopaedics and related research. 2000;372:3–8. doi: 10.1097/00003086-200003000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Recker RR, Davies KM, Dowd RM, Heaney RP. The effect of low-dose continuous estrogen and progesterone therapy with calcium and vitamin D on bone in elderly women. A randomized, controlled trial. Annals of internal medicine . 1999;130:897–904. doi: 10.7326/0003-4819-130-11-199906010-00005. [DOI] [PubMed] [Google Scholar]
- 47.Prestwood KM, Kenny AM, Kleppinger A, Kulldorff M. Ultralow-dose micronized 17beta-estradiol and bone density and bone metabolism in older women: a randomized controlled trial. Jama. . 2003;290:1042–8. doi: 10.1001/jama.290.8.1042. [DOI] [PubMed] [Google Scholar]
- 48.Huang AJ, Ettinger B, Vittinghoff E, Ensrud KE, Johnson KC, Cummings SR. Endogenous estrogen levels and the effects of ultra-low-dose transdermal estradiol therapy on bone turnover and BMD in postmenopausal women. Journal of bone and mineral research . 2007;22:1791–7. doi: 10.1359/jbmr.070707. [DOI] [PubMed] [Google Scholar]
- 49.Vrablik M, Fait T, Kovar J, Poledne R, Ceska R. Oral but not transdermal estrogen replacement therapy changes the composition of plasma lipoproteins. Metabolism . 2008;57:1088–92. doi: 10.1016/j.metabol.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 50.Stachowiak G, Pertynski T, Pertynska-Marczewska M. Effect of transdermal hormone therapy on platelet haemostasis in menopausal women. Annals of agricultural and environmental medicine . 2015;22:167–71. doi: 10.5604/12321966.1141389. [DOI] [PubMed] [Google Scholar]
- 51.Canonico M, Fournier A, Carcaillon L, Olie V, Plu-Bureau G, Oger E. Postmenopausal hormone therapy and risk of idiopathic venous thromboembolism: results from the E3N cohort study. Arteriosclerosis, thrombosis, and vascular biology . 2010;30:340–5. doi: 10.1161/ATVBAHA.109.196022. [DOI] [PubMed] [Google Scholar]
- 52.Abdi F, Mobedi H, Mosaffa N, Dolatian M, Ramezani Tehrani F. Effects of hormone replacement therapy on immunological factors in the postmenopausal period. Climacteric . 2016;19:234–9. doi: 10.3109/13697137.2016.1164136. [DOI] [PubMed] [Google Scholar]