Abstract
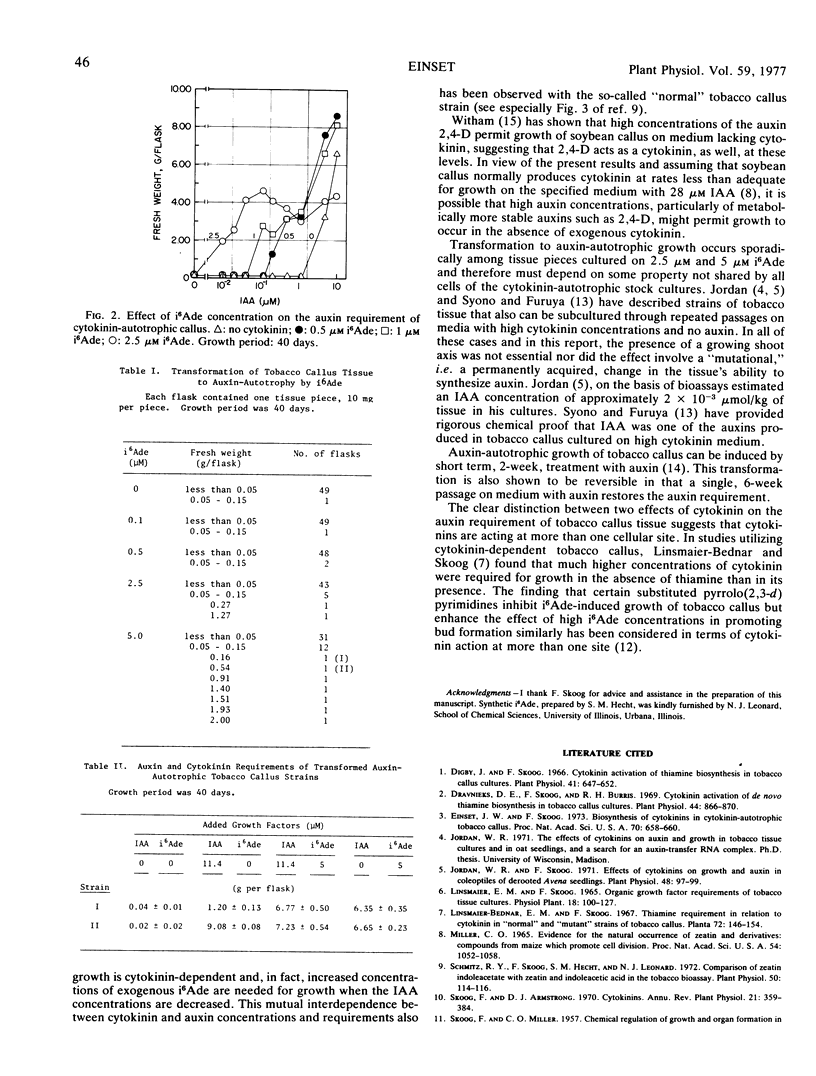
Cytokinin affects the requirement for auxin of a strain of tobacco callus (Nicotiana tabacum) which is cytokinin-autotrophic when grown on Murashige and Skoog medium with 11.4 μm of indole-3-acetic acid but requires cytokinin 6-(3-methyl-2-butenylamino)purine (i6 Ade) when grown on the same medium with <3 μm indole-3-acetic acid. As the exogenous concentration of cytokinin (i6 Ade) is increased, the concentration of indole-3-acetic acid required for growth is decreased. A second effect of cytokinin, observed sporadically in cultures with 2.5 μm or 5 μm i6 Ade, is the transformation of some of the callus pieces to auxin-autotrophic growth. Strains, both callus-forming and bud-forming tissues, that arise in this manner are not permanently altered in their auxin requirement because subcultures on medium without cytokinin still require exogenous auxin.
Full text
PDF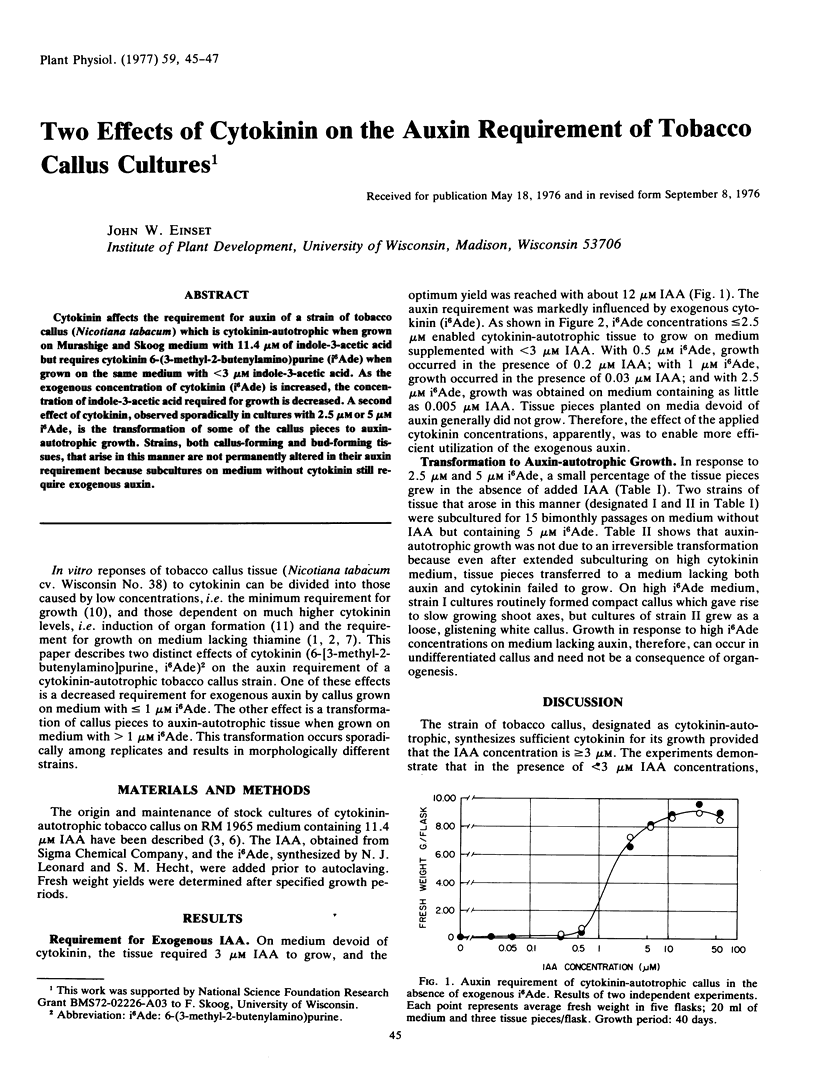

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Digby J., Skoog F. Cytokinin activation of thiamine biosynthesis in tobacco callus cultures. Plant Physiol. 1966 Apr;41(4):647–652. doi: 10.1104/pp.41.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravnieks D. E., Skoog F., Burris R. H. Cytokinin activation of de novo thiamine biosynthesis in tobacco callus cultures. Plant Physiol. 1969 Jun;44(6):866–870. doi: 10.1104/pp.44.6.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einset J. W., Skoog F. Biosynthesis of cytokinins in cytokinin-autotrophic tobacco callus. Proc Natl Acad Sci U S A. 1973 Mar;70(3):658–660. doi: 10.1073/pnas.70.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan W. R., Skoog F. Effects of cytokinins on growth and auxin in coleoptiles of derooted Avena seedlings. Plant Physiol. 1971 Jul;48(1):97–99. doi: 10.1104/pp.48.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. O. Evidence for the natural occurrence of zeatin and derivatives: compounds from maize which promote cell division. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1052–1058. doi: 10.1073/pnas.54.4.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz R. Y., Skoog F. Comparison of zeatin indoleacetate with zeatin and indoleacetic Acid in the tobacco bioassay. Plant Physiol. 1972 Jul;50(1):114–116. doi: 10.1104/pp.50.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog F., Schmitz R. Y., Hecht S. M., Frye R. B. Anticytokinin activity of substituted pyrrolo[2,3-d]pyrimidines. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3508–3512. doi: 10.1073/pnas.72.9.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]


