Abstract
Rett syndrome (RTT), caused by loss-of-function mutations in the MECP2 gene, is a neurological disorder characterized by severe impairment of motor and cognitive functions. The aim of this study was to investigate the impact of vector design, dosage, and delivery route on the efficacy and safety of gene augmentation therapy in mouse models of RTT. Our results show that AAV-mediated delivery of MECP2 to Mecp2 null mice by systemic administration, and utilizing a minimal endogenous promoter, was associated with a narrow therapeutic window and resulted in liver toxicity at higher doses. Lower doses of this vector significantly extended the survival of mice lacking MeCP2 or expressing a mutant T158M allele but had no impact on RTT-like neurological phenotypes. Modifying vector design by incorporating an extended Mecp2 promoter and additional regulatory 3′ UTR elements significantly reduced hepatic toxicity after systemic administration. Moreover, direct cerebroventricular injection of this vector into neonatal Mecp2-null mice resulted in high brain transduction efficiency, increased survival and body weight, and an amelioration of RTT-like phenotypes. Our results show that controlling levels of MeCP2 expression in the liver is achievable through modification of the expression cassette. However, it also highlights the importance of achieving high brain transduction to impact the RTT-like phenotypes.
Keywords: Rett syndrome. MECP2, adeno-associated virus, gene therapy, neurodevelopmental disorder, autism, vector
Introduction
Rett syndrome (RTT; OMIM 312750) is a neurological disorder characterized by a constellation of clinical diagnostic and associated features and with overt onset occurring several months postnatally.1 Typical RTT is almost exclusively caused by de novo germline mutations in the X-linked gene, MECP22 (as reviewed elsewhere3, 4). Several mouse models of RTT have been generated that harbor Mecp2 deletions5, 6, 7 or knocked-in mutations.8, 9, 10, 11 Many of these models recapitulate the principal features that characterize RTT in humans, although there are differences that reflect the phenotypic variability seen in patients.12, 13, 14 Despite the severity of RTT-like phenotypes, genetic reactivation of silenced Mecp2 in conditional knockout mice resulted in a robust and enduring reversal of phenotypes.15, 16, 17
This inherent reversibility of the phenotype, added to the lack of obvious targets for pharmacotherapy, makes gene therapy an obvious therapeutic strategy in RTT. However, there are significant challenges to a gene transfer approach, including the requirement to transduce sufficient numbers of neurons in the brain16 and the avoidance of deleterious overexpression.18
Previous attempts at MECP2 gene transfer using AAV9 vectors were confounded by limited brain transduction efficiency and toxicity,19, 20 while efficacy in other studies using self-complementary adeno-associated virus (AAV) (scAAV)21 may have been compromised by the use of a construct exceeding the packaging capacity of the vector.
The aim of the present study was to assess the therapeutic impact of dose, route of administration, and expression cassette design in mice modeling RTT. Our results show that modification of the vector design by incorporating more regulatory elements is able to reduce peripheral expression of vector-derived MeCP2 and prevent liver toxicity. We also show that using the same vector design by direct brain injection in mouse neonates resulted in higher brain transduction and improved the RTT-like phenotype.
Results
Dose Escalation with AAV/MECP2 Revealed a Narrow Therapeutic Window following Systemic Administration
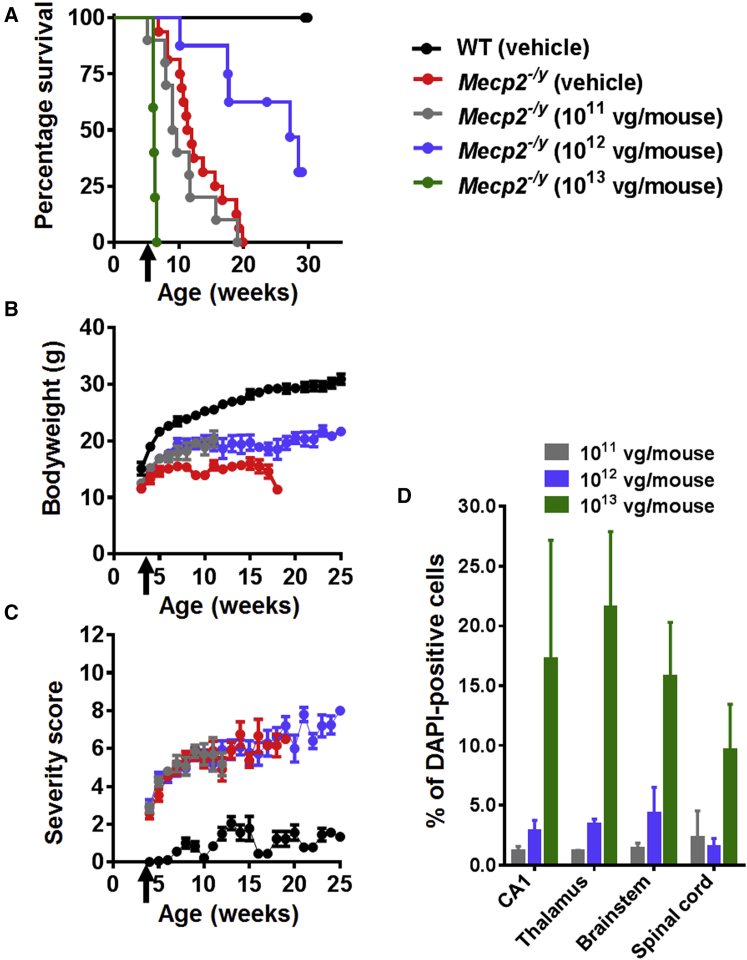
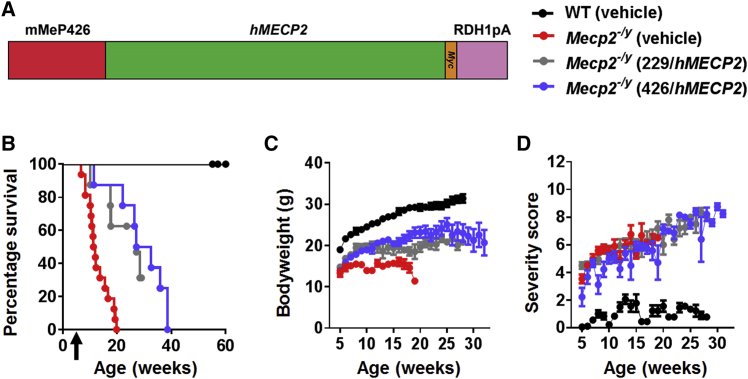
In order to explore the relationship between vector dose and therapeutic benefits, we conducted a dose escalation experiment in which an scAAV2/9 vector was used to deliver a Myc-tagged human MECP2_e1 cDNA under the control of a short, 229-bp region of the murine Mecp2 endogenous core promoter (MeP229),19, 22 hereinafter referred to as the “first-generation vector”. Juvenile male Mecp2−/y and wild-type (WT) mice were injected at the age of 4–5 weeks in the tail vein either with vehicle or with 1 × 1011 (low dose), 1 × 1012 (moderate dose), or 1 × 1013 (high dose) viral genomes (vg) per mouse (dose range, ∼1 × 1013–1 × 1015 vg/kg). As expected from previous studies of this knockout line,6, 7, 15 onset of RTT-like phenotypic signs in control vehicle-treated Mecp2−/y mice15 was observed from 4 to 5 weeks of age, and severity progressively increased until death or censoring of all mice by 20 weeks of age (Figures 1A–1C). Mecp2−/y mice treated with the low dose were indistinguishable from vehicle-treated Mecp2−/y mice in terms of survival (median survival = 9.36 weeks versus 11.64 weeks, respectively; p = 0.2, Mantel-Cox test,) and severity score (Figures 1A and 1C). However, when measured at 11 weeks (the median survival time for the control vehicle-treated Mecp2−/y mice), the mean body weight of the treated Mecp2−/y mice was significantly (p < 0.05) higher than that of the Mecp2−/y vehicle controls (Figure 1B).
Figure 1.
Systemic Delivery of the First-Generation Vector to Mecp2−/y Mice Revealed Therapeutic Efficacy and a Narrow Therapeutic Window
(A) Kaplan-Meier survival plot for Mecp2−/y mice injected with different doses (1 × 1011 [n = 10], 1 × 1012 [n = 8], and 1 × 1013 [n = 5] vg per mouse] of first-generation vector compared to vehicle-treated animals (WT; n = 9, Mecp2−/y; n = 16). The median survival period in Mecp2−/y mice treated with 1 × 1012 vg per mouse was significantly higher than that in vehicle-treated controls (27.14 versus 11.64 weeks; p = 0.001, Mantel-Cox test). (B and C) Plots showing mean (B) body weight and (C) aggregate severity scores for Mecp2−/y mice treated with 1 × 1011 and 1 × 1012 vg per mouse or vehicle. Arrows indicate age at injection; data are presented as mean ± SEM. (D) Dose-dependent transduction efficiency (Myc-positive nuclei as a proportion of DAPI-positive nuclei) across different brain regions. Data are presented as mean ± SEM (n = 3 mice per group). CA1 indicates hippocampal region CA1.
In contrast, Mecp2−/y mice treated with the moderate dose (1 × 1012) showed significantly increased survival and body weight compared to the vehicle controls (median survival = 27.3 weeks versus 11.64 weeks; p = 0.001, Mantel-Cox test [Figure 1A]; p < 0.05 for mean body weight measured at 11 weeks of age [Figure 1B]). However, there was no difference in the RTT-like phenotype severity score at this dose (Figure 1C). Finally, the cohort receiving the highest dose showed acute toxicity and lethality at 10–15 days post-injection (Figure 1A). Overall, vehicle-treated WT mice differed from Mecp2−/y cohorts across all measures (all ps < 0.001).
Patterns of transduction in treated Mecp2−/y mice were assessed within the CNS by anti-Myc antibody immunofluorescence labeling (Figure S1), which revealed vector-derived MeCP2 protein expression distributed in a punctate pattern within cell nuclei corresponding to that observed for endogenous MeCP2 in WT mice. Samples from the low-dose cohort revealed low transduction efficiencies across brain regions (0.5% to 1%). The moderate dose resulted in ∼3%–5% transduction efficiency, whereas the efficiency for the high dose was 10%–22% (Figure 1D).
In order to measure cellular levels of vector-derived MeCP2 relative to native levels, WT mice were treated with vector as described earlier. The low and moderate doses were tolerated and had no observable effect on body weight or phenotypic severity score (Figures S2A–S2C). However, WT mice treated with the high dose exhibited the acute toxicity and rapid lethality observed in the knockout mice (Figures S2A–S2C). Quantification of cellular levels of MeCP2 in mice given this high dose revealed that transduced hippocampal pyramidal cells expressed vector-derived MeCP2 at a mean level equivalent to 120% of the endogenous level, resulting in total cellular levels of MeCP2 just over 2-fold higher than normal for these cells (Figures S2D–S2F).
Systemic Delivery of First-Generation Vector Resulted in Liver Toxicity
To further investigate toxic effects encountered after systemic injection of the first-generation vector at high doses, levels of vector-derived MeCP2 expression were tested in a range of peripheral tissues. Bio-distribution of the vector genome in different organs was quantified using qPCR at the end of experiment (Figure S3) and revealed, along with immunohistochemistry, that the proportion of Myc-positive cells in the liver was high (Figure S4). Endogenous MeCP2 levels are known to be much lower in liver cells than in brain neurons23, 24 and are typically below the detection threshold for immunohistochemistry using available antibodies (Figure S4A). However, vector-derived MeCP2 levels in a subset of liver cells (using anti-Myc-immunolabeling) of treated WT mice were found to be higher than MeCP2 levels seen in neurons (Figures S4B and S4C) and were thus ∼20 times higher than levels found endogenously in such cells. Myc-positive cells were detected also in the heart, kidney, and other peripheral tissues in treated Mecp2−/y mice (data not shown).
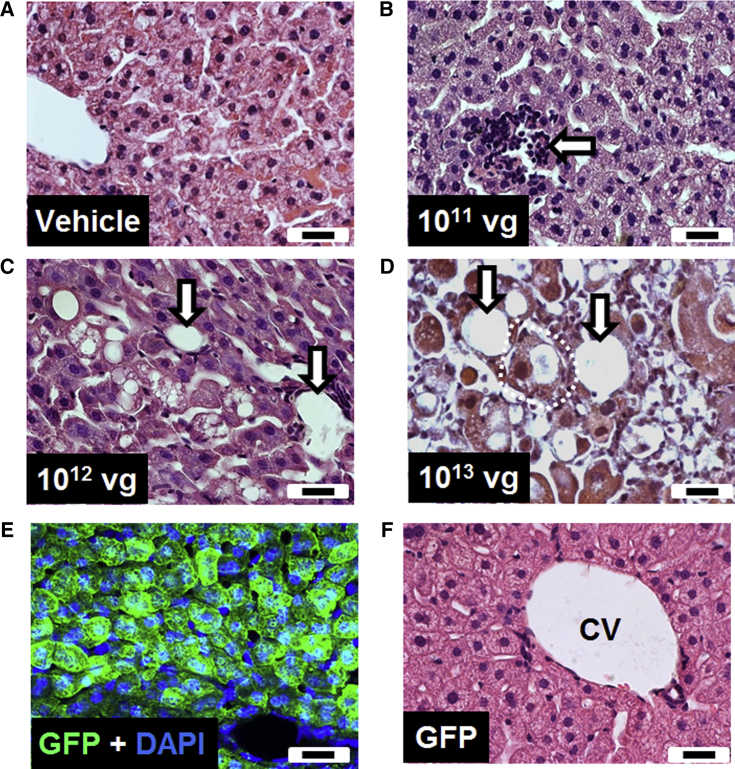
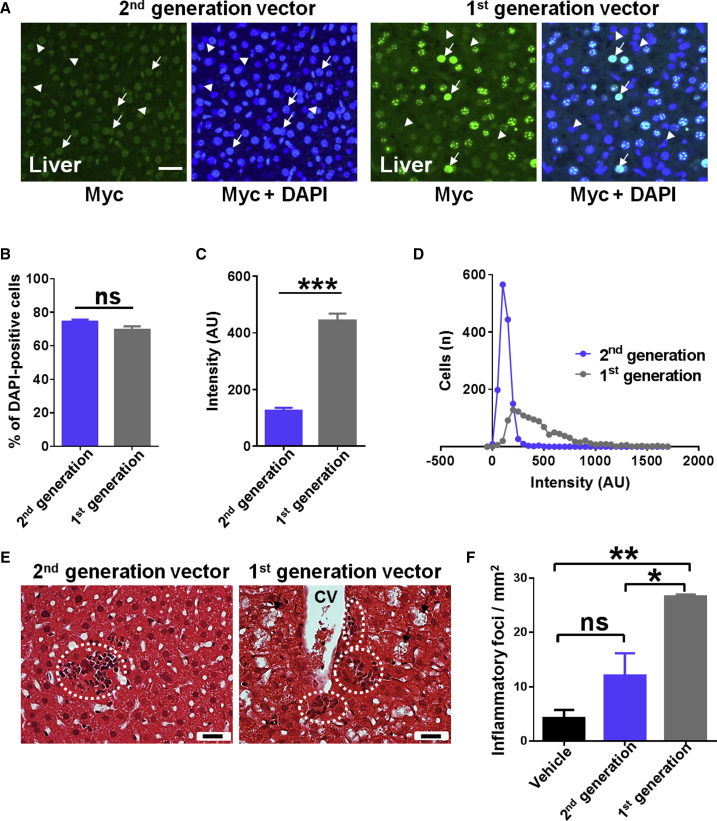
Histological investigation of liver sections from mice injected with vehicle or a low dose of the vector showed a largely normal liver structure with occasional areas of mononuclear infiltration (Figures 2A and 2B). In contrast, mice injected with higher doses of the vector showed a dose-dependent increase in pathological features, including cellular destruction and vacuolation, loss of hepatocytes, and mononuclear cell infiltration (Figures 2C and 2D).
Figure 2.
Intravenous Injection of the First-Generation Vector Resulted in Pathological Changes in the Liver
(A–D) Representative H&E-stained liver sections from WT mice injected with (A) vehicle or (B–D) different doses of vector. (E) Liver section from a mouse injected intravenously with a GFP control vector, counterstained with DAPI. (F) Representative H&E-stained liver section from a GFP vector-treated mouse. Arrows indicate mononuclear cell infiltration, vacuolation, and/or loss of hepatocytes. Dashed white line indicates cellular swelling. Scale bars indicate 20 μm. CV, central vein.
To address whether the observed liver pathology was due to the high copy number of viral particles per se or was a consequence of MeCP2 overexpression, we injected mice with a vector driving expression of GFP but otherwise identical to the first-generation vector. Despite detection of widespread GFP expression in the liver (Figure 2E), histological examination of liver sections revealed no evidence of cellular damage or immune cell infiltration (Figure 2F). In addition, no changes in RTT aggregate severity score were observed with this vector (data not shown).
Systemic Administration of First-Generation Vector Improves Survival in Mecp2T158M/y Knockin Mice
An important question for gene transfer in RTT is whether the presence of endogenous mutant MeCP2 might reduce the therapeutic effect of vector-derived wild-type MeCP2. Male mice expressing native MeCP2 tagged with GFP as a fusion protein and harboring the common RTT-causing p.T158M mutation,9 Mecp2T158M/y, display a phenotype very similar to that of Mecp2 null mice (Figure S5), but with somewhat enhanced survival (median survival = 20.3 weeks and 12.4 weeks, respectively; p = 0.0016, Mantel-Cox test).
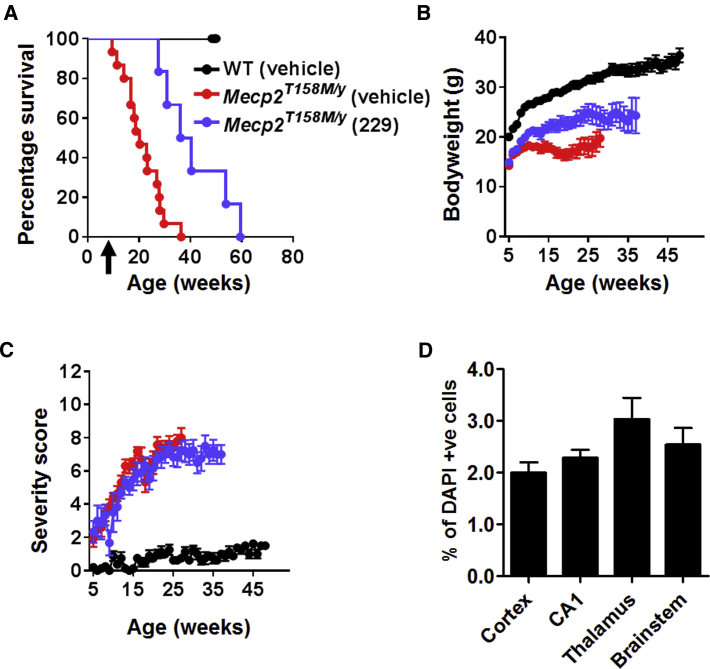
Intravenous delivery of a moderate dose (1 × 1012 vg per mouse) of the first-generation vector to 4- to 5-week-old Mecp2T158M/y mice resulted in significantly increased survival (Figure 3A; median survival = 38.3 weeks in vector-treated mice versus 20.3 weeks in vehicle-treated mice; p = 0.0019, Mantel-Cox test, n = 8–15 per group). There was a modest increase in body weight in the vector-treated cohort (Figure 3B; p < 0.05, one-way ANOVA using data at 20 weeks of age). However, there was no difference in RTT-like aggregate severity score between groups (Figure 3C), consistent with a low brain transduction efficiency (∼2%–4%) as revealed by anti-Myc labeling (Figure 3D). Overall, vehicle-treated WT mice differed from Mecp2T158M/y cohorts across all measures (all ps < 0.01).
Figure 3.
Improved Survival and Body Weight of Mecp2T158M/Y Mice after Systemic Delivery of the First-Generation Vector
(A) Survival plot for treated Mecp2T158M/y mice. Arrow indicates age at injection. (B and C) Plots of (B) body weight and C) aggregate severity score, respectively, for Mecp2T158M/y mice treated with 1 × 1012 vg per mouse of first-generation vector and control groups (Mecp2T158M/y and WT) treated with vehicle. Data presented as mean ± SEM. (D) Transduction efficiency in the brain of treated mice (Myc-positive nuclei as a proportion of DAPI-positive nuclei; n = 3 mice).
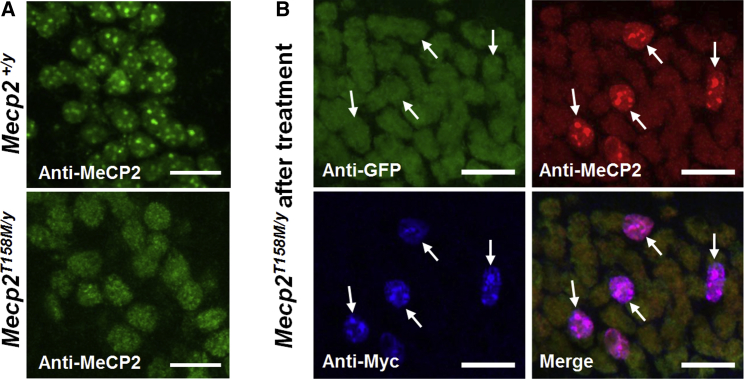
The p.T158M mutation affects the chromatin-binding capacity of MeCP2, leading to loss of the punctate element of MeCP2 labeling in the nucleus (Figure 4A).9 Immunolabeling of hippocampal neurons from treated Mecp2T158M/y mice showed WT patterns of MeCP2 expression, with restored localization to DAPI bright spots, only in transduced (Myc-positive) cells (Figure 4B). This is consistent with vector-derived MeCP2 being able to localize normally to heterochomatin, despite the presence of mutant endogenous MeCP2 protein within the same nucleus.
Figure 4.
Nuclear Localization of MeCP2 in Untreated and Treated Mecp2T158M/Y Mice
Representative confocal images of the CA1 region of the hippocampus. (A) Endogenous MeCP2 exhibits heterochromatin-enriched localization in WT nuclei, while GFP-tagged MeCP2 exhibits decreased heterochromatin localization (i.e., more diffuse labeling) in nuclei from Mecp2T158M/y mice. (B) Images demonstrating heterochromatin-enriched localization of vector-derived MeCP2 in nuclei of transduced cells in Mecp2T158M/y mice treated with the first-generation vector. White arrows indicate transduced cells (Myc-positive). Scale bars indicate 20 μm.
Development of a Second-Generation Vector that Reduced Liver Toxicity after Systemic Administration
In light of the data described earlier, it was evident that a higher AAV vector dose is required to achieve therapeutically relevant levels of brain transduction after systemic delivery. However, severe toxicity after delivery of high doses of our first-generation cassette necessitated a new design. We tested a range of modifications to the expression cassette and capsid that were predicted to result in lower cellular expression levels and/or reduce liver tropism. This included the use of expression cassettes utilizing (1) an alternative, compact, and, presumably, weaker JeT promoter25; (2) a short synthetic polyadenylation (SpA) signal (Figure S6A)26; and (3) the original first-generation expression cassette packaged in a scAAV9.47 capsid, which emerged from an in vivo screen for liver de-targeted capsid sequences relative to AAV9.27, 28 Systemic injection of these vectors at the moderate dose (1 × 1012 vg per mouse) into 4- to 5-week-old Mecp2−/y mice resulted in significantly extended survival and improved body weight, but there was no impact on the RTT-like aggregate severity score (Figure S6B). In summary, none of these modifications resulted in any significant improvements over the first-generation vector (p > 0.05 for all measures, ANOVA and Mantel-Cox tests). Importantly, these modified vectors all caused the development of liver pathology similar to that observed with the first-generation vector (as previously shown in Figure 2; Figure S6C).
The rationale for using an endogenous Mecp2 core promoter fragment (MeP229) in the first-generation vector was that it had been shown largely to recapitulate the endogenous tissue-level pattern of MeCP2 expression.22 However, this core promoter fragment is missing a number of predicted upstream regulatory elements that may be important in cell-type-specific regulation of MeCP2 expression.29, 30, 31 Therefore, we designed a second-generation vector in which we used an extended promoter fragment (MeP426) incorporating additional promoter regulatory elements and a putative silencer element (Figure S7). We predicted that this might better enable the regulation of vector-derived MeCP2 levels in transduced cells. In addition to the extended promoter, we also incorporated a novel 3′ UTR, consisting of a fragment of the endogenous MECP2 3′ UTR together with a selected panel of binding sites for microRNAs (miRNAs) known to be involved in regulation of Mecp232, 33, 34, 35 (Figure S7).
In order to test the therapeutic efficacy of the second-generation vector, a moderate dose (1 × 1012 vg per mouse) was injected intravenously into 4- to 5-week-old Mecp2−/y mice. There was a significant extension of survival in the vector-treated mice compared to the vehicle-treated mice (median survival = 29.9 weeks and 11.6 weeks, respectively; p < 0.0001, Mantel-Cox; Figure 5B). There was also significant improvement in body weight at the age of 11 weeks (p < 0.05, one-way ANOVA, with Tukey’s post hoc pairwise comparison test; Figure 5C). In contrast, there was no effect on RTT-like aggregate severity score (Figure 5D). The second-generation vector, thus, showed no therapeutic advantages over the first-generation vector after systemic delivery (Figures 5B–5D). Again, vehicle-treated WT mice differed from Mecp2−/y cohorts across all measures (all p < 0.001). In order to compare this vector head-to-head with the first-generation vector in terms of liver safety, mice were injected intravenously with either the first- or the second-generation vector at a dose of 1 × 1012 vg per mouse. These mice were sacrificed after 30 days, and tissues were analyzed for vector-derived MeCP2 expression (using anti-Myc tag antibody) and signs of liver pathology (Figure 6). There was no significant difference in transduction efficiency between vector constructs (Figure 6B), but cellular levels of vector-derived MeCP2 (anti-Myc) in mice treated with first-generation vector were significantly higher than those in mice treated with second-generation vector (Figure 6C; p < 0.001, unpaired t test). Analysis of the distribution of cellular MeCP2 expression levels in transduced cells showed that MeCP2 expression was more tightly regulated in mice injected with the second-generation vector (Figure 6D), with fewer cells exhibiting very high expression levels. Moreover, there was none of the disrupted hepatic architecture or vacuolation previously observed with the first-generation vector (Figure 6E). The density of inflammatory foci was significantly higher in liver samples from mice injected with first-generation vector than from those injected with the second-generation vector (Figure 6F).
Figure 5.
Therapeutic Efficacy of Second-Generation Vector after Systemic Delivery to Mecp2−/y Mice
(A) Design features of our second-generation vector summarized (see Results and Figure S7 for details). (B) Survival plot for Mecp2−/y mice treated intravenously with 1 × 1012 vg per mouse of the second-generation vector (median survival = 29.9 weeks) or an identical dose of first-generation vector (median survival = 27.1 weeks) or vehicle (median survival = 11.6 weeks). Arrow indicates age at injection. (C and D) Plots showing (C) mean body weight and (D) aggregate severity scores, respectively, of Mecp2−/y mice treated as in (B). See also Figure S7.
Figure 6.
Reduced Expression of Vector-Derived MeCP2 in the Livers of Mice Treated with Second-Generation Vector
(A) Flattened confocal stack images from livers of mice 1 month after being injected intravenously at 5 weeks of age with the second-generation vector or first-generation vector at 1 × 1012 vg per mouse; confocal settings were the same in each case. Tissues were immunolabeled with anti-Myc and DAPI nuclear stain. Arrows indicate transduced cells (Myc-positive), and arrowheads indicate non-transduced cells. (B) Transduction efficiencies in the liver for both vectors. (C) Quantification of cellular levels of vector-derived MeCP2 measured as anti-Myc immunofluorescence in transduced cells in the liver (n = 3 mice, 1,400 transduced cells). Data are presented as mean ± SEM. (D) Frequency distribution of cellular levels of vector-derived MeCP2 in the liver, measured as in (C). (E) Liver sections stained with H&E showing vacuolation of hepatocytes (arrows) and sites of mononuclear cell infiltration (dashed circles). CV, central vein. White scale bar indicates 20 μm. (F) Quantification of density of inflammatory foci in the livers of treated mice (n = 3 per group). Data are presented as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001; ns, not significant.
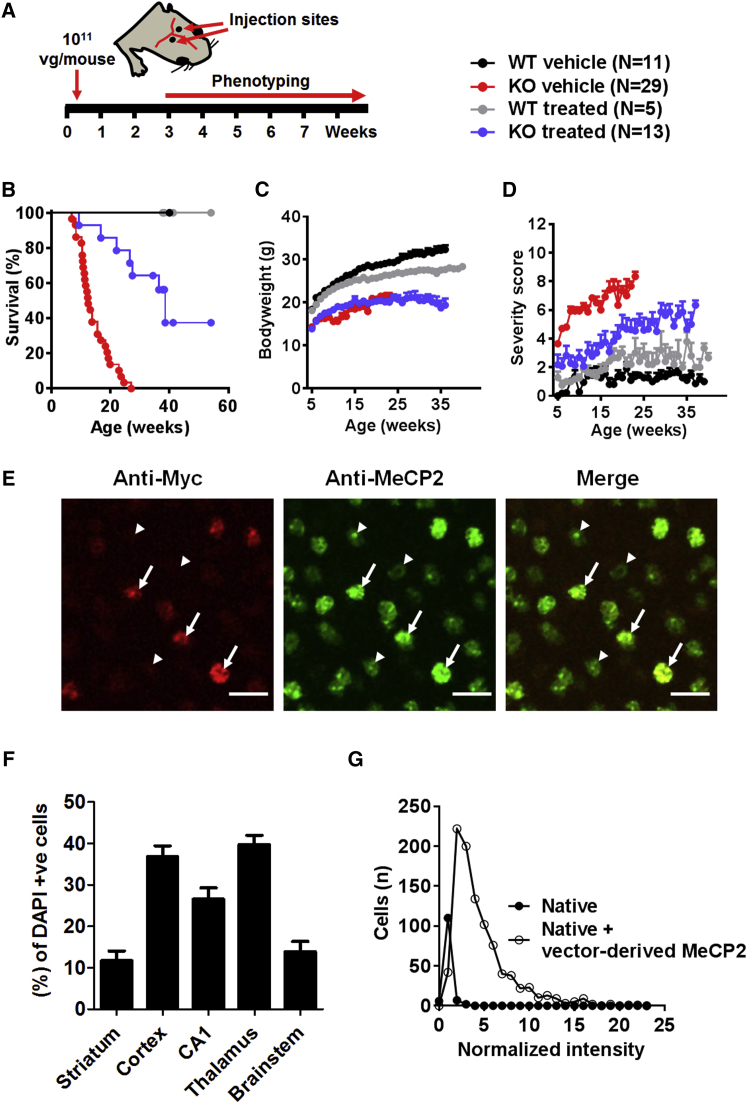
Neonatal Cerebroventricular Injection of the Second-Generation Vector Improved the RTT-like Aggregate Severity Score
The lack of impact on the phenotype after systemic administration is consistent with the low brain transduction efficiencies observed, as it has been established that phenotype severity and degree of improvement after gene restoration correlate with the proportion of MeCP2-expressing cells in the brain.16 Therefore, we decided to test the second-generation vector by direct cerebroventricular injection in mouse neonates, a delivery route that is known to afford widespread transgene expression.19 When delivered at a dose of 1 × 1011 vg per mouse (Figure 7A), there was a pronounced extension in the survival of Mecp2−/y mice treated with the second-generation vector in comparison to vehicle-treated mice (median survival = 38.5 and 12.4 weeks, respectively; p < 0.0001, Mantel-Cox test; Figure 7B). While there was a negligible effect of vector on body weight (Figure 7C), an important observation was the clear improvement in the RTT-like aggregate severity score compared to that of vehicle-treated Mecp2-null mice (Figure 7D; p < 0.01 at 11 weeks, one-way ANOVA, with Tukey post hoc pairwise comparison). Vector-derived MeCP2 (revealed by anti-Myc tag immunolabeling) was detectable in all brain regions, with transduction efficiencies across brain regions ranging from ∼10%–40% (Figures 7E and 7F). Distribution analysis revealed that the modal cellular MeCP2 level in transduced cells in cortex was approximately twice that of endogenous MeCP2 (consistent with a vector-derived expression level equal to the endogenous level), with some cells expressing higher levels of vector-derived MeCP2 (Figure 7G).
Figure 7.
Direct Brain Delivery of Second-Generation Vector to Neonatal Mecp2−/y Mice Revealed Therapeutic Efficacy
(A) Experimental design. KO, knockout. (B) Survival plot showing extended survival of neonatally treated Mecp2−/y mice (median survival = 38.6 weeks; p < 0.0001, Mantel-Cox test) compared with vehicle-treated animals (median survival = 12.4 weeks). (C and D) Plots showing mean (C) body weight and (D) aggregate severity scores, respectively, for the mice shown in (B). (E) Representative confocal images from the cortex of injected wild-type mice. White arrows indicate transduced cells; arrowheads indicate non-transduced cells; scale bars indicate 20 μm. (F) Graph showing transduction efficiency in different brain regions (n = 3 mice). (G) Frequency distribution of MeCP2 levels in transduced and non-transduced (“native”) cells in the mouse cortex (n = 3 mice; 954 transduced cells) data presented as mean ± SEM.
Discussion
The reversal of a wide range of RTT-like phenotypes in mice following the delayed unsilencing of Mecp2 provides a strong rationale for gene transfer as a therapeutic strategy in RTT.15, 16 There are likely to be a variety of barriers to translational success that will need to be identified and addressed in order to secure optimal outcomes in human clinical trials. In the present study, we identified particular challenges associated with the systemic delivery of a MECP2-bearing gene therapy vector in terms of a narrow therapeutic window driven by low brain transduction efficiency and the appearance of peripheral overexpression toxicity upon further dose escalation. However, peripheral overexpression can be reduced by refining the cassette design. We show that direct brain delivery of vector in neonatal mice can achieve therapeutically relevant levels of transduction that result in phenotype amelioration. We also show that the vector has similar effectiveness in mice expressing the most common RTT-causing mutation, suggesting that the presence of existing mutant forms of MeCP2 is unlikely to be an obstacle to translational success. These results are consistent with experiments in transgenic mice expressing both mutant and WT forms of the protein.36
Recent attempts to deliver MECP2 exogenously in mouse models of RTT used widely varying vector doses but are difficult to compare based on additional differences in cassette design and other variables, including viral production, dosing protocol, and phenotype measures.19, 20, 21 In the present study, we used our previously published cassette design (human MECP2_e1, under the control of a MeP229 core promoter fragment)19 to directly investigate the effect of dose in terms of efficacy and safety. A notable finding was the overall lack of efficacy across the range of doses tested in terms of an effect on RTT-like phenotype severity score. This is not due to such phenotypes being inherently resistant to reversal15, 16 but is instead most likely explained by the low levels of brain transgene expression afforded by this route of delivery. In contrast to the phenotype severity score, there was a clear dose-response relationship for survival, with the intermediate dose causing a modest increase in mean body weight and a significant extension in survival. It is not clear whether the survival and body weight effects are due to sufficient (if low) transduction levels in critical brain regions or to expression of MeCP2 in peripheral tissues relevant to mortality. Recent evidence suggests that MeCP2 levels in peripheral tissues can subtly affect body weight,23 and it is possible that this may indirectly affect survival measures, as we are obliged to use the acute loss of body weight as an endpoint criterion. However, there was a clear divergence in survival between the 1011- and 1012-vg doses without overt differences in mean body weight between groups (Figure 1). Another potential explanation is that we were underestimating levels of transduction efficiency related to survival, based on the sensitivity of our immunohistochemical detection. However, vector biodistribution validation using qPCR was consistent with our measurements, confirming very modest transduction efficiencies following systemic delivery. Only the highest dose tested produced appreciable levels of brain transduction (>10%–20%), and, unfortunately, the severe liver pathology and lethality associated with this dose precluded assessment of the potential for brain-specific therapeutic effects in this situation. Liver cells normally express relatively low levels of MeCP2 compared to neurons,23 and identical doses of a GFP-expressing vector were not toxic, so the dose-dependent liver pathology is likely to be attributed to the overexpression of vector-derived MeCP2. The difference in the severity score observed in WT mice treated with the moderate dose (which showed no apparent toxicity) and with the high dose (which showed high levels of lethality) can potentially be explained in terms of the cellular levels of MeCP2 that can be tolerated by liver cells—this tolerability threshold may lie between the levels of MeCP2 achieved by the two vector doses.
Previous preclinical RTT gene therapy studies19, 20, 21 have focused on using the Mecp2−/y model to screen for vector efficacy and potential toxicity. However, the presence of mutant endogenous MeCP2 could potentially produce a quasi-dominant negative action on the vector-derived MeCP2. We have shown here that, although this knockin line9 exhibits RTT-like phenotype severity scores similar to those observed in Mecp2−/y mice, it also exhibits prolonged survival, thus indicating that the mutant allele may produce MeCP2 with some residual function. Interestingly, AAV-mediated systemic delivery of MECP2 to these mice resulted in a therapeutic effect similar to that achieved in the Mecp2−/y mice treated with the same vector dose. Therefore, we conclude that presence of the mutant protein does not impede the functionality of vector-derived MeCP2. This finding supports the potential translational application of augmentation gene therapy in patients with missense MECP2 mutations.
Our initial attempts to lower toxic MeCP2 expression and/or reduce liver tropism involved modifications to the expression cassette and capsid. However, the use of putative weaker synthetic promoters and polyadenylation signals was not sufficient to avoid liver toxicity. Surprisingly, the use of an AAV9.47 capsid, which is purported to de-target the liver relative to AAV9,27, 28 resulted in liver pathology similar to that seen with AAV9. Therefore, we focused efforts on a second-generation vector, whose design was based on the inclusion of endogenous regulatory elements that may better regulate levels of vector-derived MeCP2 in transduced cells. This included the incorporation of an extended endogenous promoter and an endogenous 3′ UTR fragment. Studies analyzing the well-conserved human MECP2 and mouse Mecp2 promoter regions indicated the presence of a number of putative regulatory elements within a 1-kb window immediately upstream of the transcription start site.29, 30, 31 Consequently, our extended endogenous promoter (426 bp) in the second-generation vector comprised a putative silencer element at position −274 to −335, with respect to the RefSeq transcription start site (Figure S7).
An endogenous 3′ UTR was also incorporated, containing the distal MECP2 polyadenylation signal and a number of clustered putative regulatory elements.37, 38, 39 In addition, we performed an analysis of miRNA binding sites in the 3′ UTR of MECP2, using a number of bioinformatic tools,40, 41, 42 and incorporated a compact sequence containing the binding sites of three highly conserved miRNAs known to be involved in regulation of MeCP2 in the brain; miR-22,32 miR-19,33 and miR-132.34 Combined, these modifications significantly reduced MeCP2 expression in the liver, with subsequent reduction of the hepatotoxicity encountered with the first-generation vector. The relative importance of different modifications (elements within the extended promoter and novel 3′ UTR) was not investigated. However, the efficacy of both vectors after systemic injection of moderate doses was not significantly different. The important advantage of the second-generation vector is the lack of prominent liver pathology at a dose that provides some therapeutic benefit (i.e., 1 × 1012 vg per mouse). The improved survival after systemic injection, despite low brain transduction efficiency, could be due to restoration of MeCP2 levels in sufficiently numerous critical cells in the brain or due to restoration in important peripheral tissues. Targeting more cells in the brain through direct brain injection in mouse neonates, along with potentially greater impact via earlier intervention, led to pronounced survival enhancement at a dose (1 × 1011 vg per mouse) approximately equivalent to the 1012 systemic dose. Delivery by this direct brain injection route was associated with an improvement in body weight but, importantly, also with an improvement in RTT-like phenotype score. The improvement was not as profound as that reported in genetic reversal experiments,16 and this is likely to be due to the combined effects of (1) the relative inefficiency of MeCP2 re-expression across the brain (10%–40%), compared to genetic reversal experiments (up to 90%), and (2) the possible deleterious counteracting effects of overexpressing MeCP2 in a proportion of transduced cells. Analysis of MeCP2 levels, indeed, indicates a significant pool of cells overexpressing MeCP2, presumably transduced with multiple copies of vector delivering MECP2. This may also account for the slightly elevated severity score in vector-treated WT mice (Figure 7D) in the form of mild hindlimb clasping. We cannot rule out very subtle consequences of MeCP2 overexpression that may be revealed by fine-grained behavioral testing. Overall, the proof-of-concept experiments involving direct brain delivery in neonatal mice suggest that, if transduction efficiency across the brain can reach sufficiently high levels, then a behavioral improvement is conferred by this vector design.
Conclusions
The results of the present study highlight the challenges associated with both systemic and direct brain delivery of MECP2. The findings suggest that achieving widespread brain expression, while at the same time maintaining cell-type appropriate control of MeCP2 levels, will be essential requirements for the successful development of a translational therapy. The development of expression cassettes capable of producing effective and sub-toxic levels of MeCP2 may overcome issues of cellular overexpression and enable direct delivery via the cerebrospinal fluid compartment. While AAV9 appears to be insufficiently efficient in terms of brain transduction after systemic delivery of MECP2 to achieve the desired therapeutic benefit, combining the safer second-generation cassette together with capsids with improved brain penetrance43 may effectively pair effective CNS gene transfer with safe levels of peripheral MeCP2 transgene expression. Such a combination would hold enhanced translational promise.
Materials and Methods
Animals
All experiments were carried out in accordance with the European Communities Council Directive (86/609/EEC) and with the terms of a project license under the UK Scientific Procedures Act (1986). The Mecp2 null, Mecp2tm1.1Bird, and Mecp2T158M mice, originally provided as a kind gift from Professor Adrian Bird, were maintained on a C57BL/6 background. Animals were maintained on 12-hr:12-hr light/dark cycles with free access to normal mouse food. Mice were genotyped as described previously.9, 15
Viral Vector Preparation
Recombinant AAV vector particles were generated at the University of North Carolina (UNC) Gene Therapy Center Vector Core facility. The scAAV particles (AAV2 ITR [inverted terminal repeat]-flanked genomes packaged into AAV9 or AAV9.47 serotype capsids) were produced from suspension HEK293 cells transfected using polyethyleneimine (Polysciences) with helper plasmids (pXX6-80 and pGSK2/9) and a plasmid containing the appropriate ITR-flanked transgene construct. All MeCP2-expressing constructs utilized the human MECP2_e1 coding region with a C-terminal Myc epitope tag unless stated otherwise. Virus production was performed as previously described,44 and the vectors were prepared in a final formulation of high-salt PBS (containing 350 mmol/L total NaCl) supplemented with 5% sorbitol.
scAAV Vector Injection and Mouse Phenotyping
Frozen scAAV9 viral particle aliquots were thawed and diluted to 100 μL in PBS/350 mmol/L NaCl containing 5% sorbitol. Control injections were made using the same diluent lacking vector (“vehicle control”). For direct brain injection into mouse neonates, littermates were sexed at birth, and direct bilateral injections of virus (3 μL per site) were delivered into the neuropil of unanesthetized males at postnatal day (P)0–P3, as described previously.19 The injected pups were returned to the home cage containing their non-injected female littermates. Genotyping was carried out at 3 weeks, at which time phenotyping was initiated. For injection into juvenile male mice, injections were made via the tail vein at 4–5 weeks of age. Following injection, all mice were weighed weekly. Phenotyping was carried out, blind to genotype and treatment, twice a week. Mice were scored on an aggregate severity scale using an established protocol (mice were scored for RTT-like phenotypes comprising mobility, gait, breathing, hindlimb clasping, tremor, and general condition).15, 16, 19, 21 For survival analysis, mice were censored after natural death or if body weight losses exceeded 20% of peak body weight.
Vector Biodistribution Analysis
For these analyses, mice were sacrificed, blood was collected transcardially, and organs were harvested for DNA purification. Genomic DNA was recovered from tissues using the DNeasy Blood and Tissue Kit (QIAGEN). A Qiacube (QIAGEN) was used to carry out automated purifications. Genomic qPCR reactions and analysis were performed on a Roche Lightcycler 480, following the manufacturer’s instructions. For the quantification of vector biodistribution, the amount of vector genome present in each sample was standardized against an amplicon from a single-copy mouse gene, Lmnb2, amplified from genomic DNA. Lmnb2 primers and “universal” MECP2 primers (that amplify mouse and human MECP2) were published previously.19, 45
Immunohistochemistry
Mice were anesthetized with pentobarbitone (50 mg, intraperitoneally) and transcardially perfused with 4% paraformaldehyde (0.1 mol/L PBS). A vibrating microtome (Leica VT1200) was used to obtain 80-μm sections of brain, spinal cord, and liver. Sections were dehydrated by incubation in 50% ethanol in distilled water (v/v) for 30 min and then were washed three times in 0.3 mol/L PBS, followed by incubation in 10 mM sodium citrate (pH 6, 85°C, 30 min) for antigen retrieval. Sections were then incubated in the blocking solution (5% normal goat serum in 0.3mol/L PBS with 0.3% Triton X-100) for 1 hr at room temperature. Samples then were incubated for 48 hr on a shaker at 4°C with the following primary antibodies: rabbit anti-Myc (Abcam, ab9106), mouse monoclonal anti-MeCP2 (Sigma, WH0004204M1), and chicken anti-GFP (Abcam, ab13970). The primary antibodies were then washed off (3× 0.3 mol/L PBST), and secondary antibodies were applied to the sections overnight at 4°C: Alexa Fluor 488 goat anti-mouse/rabbit (Invitrogen; 1/500), Alexa Fluor 546 goat anti-mouse/rabbit (Invitrogen; 1/500), Alexa Fluor 649, goat anti-mouse (Jackson ImmunoResearch Laboratories, 112-495-003JIR). Finally, sections were incubated with DAPI nuclear stain (Sigma; 1/1,000) for 30 min at room temperature before mounting with Vectashield (Vector Laboratories).
H&E Staining
Liver samples were rinsed with 0.1 mol/L PBS and then dehydrated through ascending grades of ethanol, and they were then cleared in amyl acetate using an automated tissue processor. Specimens were embedded in Paraplast, and sections (10 μm thick) were collected on APES (aminopropyltriethoxysilane)-coated slides and dried overnight in the oven at 37°C. Sections were then deparaffinized through two changes of Histo-Clear (Agar Scientific) for 15 min and rehydrated through descending grades of alcohol (100%, 90%, and 70%). The sections were stained with Mayer’s hematoxylin for 8 min and then rinsed using tap water. The nuclei were stained blue by placing the slides into Scott’s solution for 1 min and were then rinsed using tap water. Sections were then stained with 1% eosin for 2 min and washed by water. Finally, the sections were dehydrated through ascending grades of alcohol and Histo-Clear before being mounted with DPX. Images were captured using an AxioCam MRc (Zeiss) mounted on a light microscope (Zeiss).
Image Analysis
Analysis of expression patterns, transduction efficiency, and quantification of vector-derived MeCP2 levels within nuclei was carried out on image stacks captured using a Zeiss LSM710 or Zeiss Axiovert LSM510 laser confocal microscope (Zeiss). The z series were taken at 1-μm intervals through the section of interest using a 40× objective. To account for antibodies’ penetrability, stack images were taken close to the surface of sections to a maximum depth of 20 μm. To estimate transduction efficiency, images were captured as described earlier, and the ratio of Myc-immunopositive nuclei to DAPI-stained nuclei was calculated for random fields (n = 12 images per region:4 images from each of three mice) from sections of hippocampus (CA1 region), layer 5 of primary motor cortex, thalamus, hypothalamus, brain stem, and striatum. To quantify levels of vector-derived MeCP2 per nucleus in WT mice, confocal stacks (20 μm thick) were obtained as described earlier, and ImageJ software (http://rsbweb.nih.gov/ij/) was used to determine mean MeCP2-channel fluorescence intensity within transduced (Myc +ve) and non-transduced (Myc −ve) cells. Fluorescence in the DAPI channel was used to define the nuclear boundary.
Statistical Analysis
Tests for differences between treatment groups were carried out in GraphPad PRISM using one-way ANOVA, Student’s t test, and the Mantel-Cox test (survival curves), as appropriate. p < 0.05 was used to define statistical significance. In multi-group comparisons, multiple testing correction for pairwise tests among groups was applied using Tukey’s post hoc analysis.
Author Contributions
Conceptualization, S.R.C., M.E.S.B., and S.J.G.; Methodology, S.R.C., K.K.E.G., N.G.B., and R.D.H.; Investigation, K.K.E.G., T.V., S.S., N.G.B., and R.D.H.; Writing – Original Draft, S.R.C., K.K.E.G., R.D.H., M.E.S.B., and S.J.G.; Funding Acquisition, S.R.C., S.J.G., M.E.S.B., and K.K.E.G.; Resources, R.D.H. and S.S.; Supervision, S.R.C. and M.E.S.B.
Conflicts of Interest
S.J.G. declares a conflict of interest with Asklepios Biopharma, from which he has received patent royalties for intellectual property that is not used in this study.
Acknowledgments
This work was funded by grants from the Rett Syndrome Research Trust (to S.R.C., M.E.S.B., and S.J.G.), the Chief Scientist Office (ETM/334 to S.R.C. and M.E.S.B.) and the NIH (4T32HD040127-15 to S.S.). We are also grateful to the Rett Syndrome Association Scotland, the R.S. MacDonald Charitable Trust, the Stoneygate Trust, and the Rosetrees Trust for additional support (to S.R.C., M.E.S.B., and K.K.E.G.). T.V. received a Thailand government studentship. Indirect administrative support for S.J.G. was provided by Research to Prevent Blindness to the UNC-CH Department of Ophthalmology. The authors thank John Craig at Glasgow University and Daphne Chen, Clifford Heindel, and Violeta Zaric at UNC for their technical assistance. We also thank the B. Kaspar and G. Mandel labs and other members of the Rett Syndrome Research Trust Gene Therapy Consortium for helpful discussions.
Footnotes
Supplemental Information includes seven figures and can be found with this article online at http://dx.doi.org/10.1016/j.omtm.2017.04.007.
Supplemental Information
References
- 1.Neul J.L., Kaufmann W.E., Glaze D.G., Christodoulou J., Clarke A.J., Bahi-Buisson N., Leonard H., Bailey M.E., Schanen N.C., Zappella M., RettSearch Consortium Rett syndrome: revised diagnostic criteria and nomenclature. Ann. Neurol. 2010;68:944–950. doi: 10.1002/ana.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amir R.E., Van den Veyver I.B., Wan M., Tran C.Q., Francke U., Zoghbi H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 3.Bienvenu T., Chelly J. Molecular genetics of Rett syndrome: when DNA methylation goes unrecognized. Nat. Rev. Genet. 2006;7:415–426. doi: 10.1038/nrg1878. [DOI] [PubMed] [Google Scholar]
- 4.Lyst M.J., Bird A. Rett syndrome: a complex disorder with simple roots. Nat. Rev. Genet. 2015;16:261–275. doi: 10.1038/nrg3897. [DOI] [PubMed] [Google Scholar]
- 5.Chen R.Z., Akbarian S., Tudor M., Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat. Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- 6.Guy J., Hendrich B., Holmes M., Martin J.E., Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- 7.Shahbazian M., Young J., Yuva-Paylor L., Spencer C., Antalffy B., Noebels J., Armstrong D., Paylor R., Zoghbi H. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–254. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- 8.Goffin D., Allen M., Zhang L., Amorim M., Wang I.T., Reyes A.R., Mercado-Berton A., Ong C., Cohen S., Hu L. Rett syndrome mutation MeCP2 T158A disrupts DNA binding, protein stability and ERP responses. Nat. Neurosci. 2011;15:274–283. doi: 10.1038/nn.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown K., Selfridge J., Lagger S., Connelly J., De Sousa D., Kerr A., Webb S., Guy J., Merusi C., Koerner M.V., Bird A. The molecular basis of variable phenotypic severity among common missense mutations causing Rett syndrome. Hum. Mol. Genet. 2016;25:558–570. doi: 10.1093/hmg/ddv496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyst M.J., Ekiert R., Ebert D.H., Merusi C., Nowak J., Selfridge J., Guy J., Kastan N.R., Robinson N.D., de Lima Alves F. Rett syndrome mutations abolish the interaction of MeCP2 with the NCoR/SMRT co-repressor. Nat. Neurosci. 2013;16:898–902. doi: 10.1038/nn.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitcher M.R., Herrera J.A., Buffington S.A., Kochukov M.Y., Merritt J.K., Fisher A.R., Schanen N.C., Costa-Mattioli M., Neul J.L. Rett syndrome like phenotypes in the R255X Mecp2 mutant mouse are rescued by MECP2 transgene. Hum. Mol. Genet. 2015;24:2662–2672. doi: 10.1093/hmg/ddv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Archer H., Evans J., Leonard H., Colvin L., Ravine D., Christodoulou J., Williamson S., Charman T., Bailey M.E., Sampson J. Correlation between clinical severity in patients with Rett syndrome with a p.R168X or p.T158M MECP2 mutation, and the direction and degree of skewing of X-chromosome inactivation. J. Med. Genet. 2007;44:148–152. doi: 10.1136/jmg.2006.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh R.P., Horowitz-Scherer R.A., Nikitina T., Gierasch L.M., Woodcock C.L. Rett syndrome-causing mutations in human MeCP2 result in diverse structural changes that impact folding and DNA interactions. J. Biol. Chem. 2008;283:20523–20534. doi: 10.1074/jbc.M803021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leonard H., Cobb S., Downs J. Clinical and biological progress over 50 years in Rett syndrome. Nat. Rev. Neurol. 2016;13:37–51. doi: 10.1038/nrneurol.2016.186. [DOI] [PubMed] [Google Scholar]
- 15.Guy J., Gan J., Selfridge J., Cobb S., Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson L., Guy J., McKay L., Brockett E., Spike R.C., Selfridge J., De Sousa D., Merusi C., Riedel G., Bird A., Cobb S.R. Morphological and functional reversal of phenotypes in a mouse model of Rett syndrome. Brain. 2012;135:2699–2710. doi: 10.1093/brain/aws096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jugloff D.G., Vandamme K., Logan R., Visanji N.P., Brotchie J.M., Eubanks J.H. Targeted delivery of an Mecp2 transgene to forebrain neurons improves the behavior of female Mecp2-deficient mice. Hum. Mol. Genet. 2008;17:1386–1396. doi: 10.1093/hmg/ddn026. [DOI] [PubMed] [Google Scholar]
- 18.Gadalla K.K.E., Ross P.D., Hector R.D., Bahey N.G., Bailey M.E.S., Cobb S.R. Gene therapy for Rett syndrome: prospects and challenges. Future Neurol. 2015;10:467–484. [Google Scholar]
- 19.Gadalla K.K., Bailey M.E., Spike R.C., Ross P.D., Woodard K.T., Kalburgi S.N., Bachaboina L., Deng J.V., West A.E., Samulski R.J. Improved survival and reduced phenotypic severity following AAV9/MECP2 gene transfer to neonatal and juvenile male Mecp2 knockout mice. Mol. Ther. 2013;21:18–30. doi: 10.1038/mt.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matagne V., Ehinger Y., Saidi L., Borges-Correia A., Barkats M., Bartoli M., Villard L., Roux J.C. A codon-optimized Mecp2 transgene corrects breathing deficits and improves survival in a mouse model of Rett syndrome. Neurobiol. Dis. 2017;99:1–11. doi: 10.1016/j.nbd.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Garg S.K., Lioy D.T., Cheval H., McGann J.C., Bissonnette J.M., Murtha M.J., Foust K.D., Kaspar B.K., Bird A., Mandel G. Systemic delivery of MeCP2 rescues behavioral and cellular deficits in female mouse models of Rett syndrome. J. Neurosci. 2013;33:13612–13620. doi: 10.1523/JNEUROSCI.1854-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray S.J., Foti S.B., Schwartz J.W., Bachaboina L., Taylor-Blake B., Coleman J., Ehlers M.D., Zylka M.J., McCown T.J., Samulski R.J. Optimizing promoters for recombinant adeno-associated virus-mediated gene expression in the peripheral and central nervous system using self-complementary vectors. Hum. Gene Ther. 2011;22:1143–1153. doi: 10.1089/hum.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross P.D., Guy J., Selfridge J., Kamal B., Bahey N., Tanner K.E., Gillingwater T.H., Jones R.A., Loughrey C.M., McCarroll C.S. Exclusive expression of MeCP2 in the nervous system distinguishes between brain and peripheral Rett syndrome-like phenotypes. Hum. Mol. Genet. 2016;25:4389–4404. doi: 10.1093/hmg/ddw269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skene P.J., Illingworth R.S., Webb S., Kerr A.R., James K.D., Turner D.J., Andrews R., Bird A.P. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol. Cell. 2010;37:457–468. doi: 10.1016/j.molcel.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tornøe J., Kusk P., Johansen T.E., Jensen P.R. Generation of a synthetic mammalian promoter library by modification of sequences spacing transcription factor binding sites. Gene. 2002;297:21–32. doi: 10.1016/s0378-1119(02)00878-8. [DOI] [PubMed] [Google Scholar]
- 26.Levitt N., Briggs D., Gil A., Proudfoot N.J. Definition of an efficient synthetic poly(A) site. Genes Dev. 1989;3:1019–1025. doi: 10.1101/gad.3.7.1019. [DOI] [PubMed] [Google Scholar]
- 27.Pulicherla N., Shen S., Yadav S., Debbink K., Govindasamy L., Agbandje-McKenna M., Asokan A. Engineering liver-detargeted AAV9 vectors for cardiac and musculoskeletal gene transfer. Mol. Ther. 2011;19:1070–1078. doi: 10.1038/mt.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karumuthil-Melethil S., Nagabhushan Kalburgi S., Thompson P., Tropak M., Kaytor M.D., Keimel J.G., Mark B.L., Mahuran D., Walia J.S., Gray S.J. Novel vector design and hexosaminidase variant enabling self-complementary adeno-associated virus for the treatment of Tay-Sachs disease. Hum. Gene Ther. 2016;27:509–521. doi: 10.1089/hum.2016.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J., Francke U. Identification of cis-regulatory elements for MECP2 expression. Hum. Mol. Genet. 2006;15:1769–1782. doi: 10.1093/hmg/ddl099. [DOI] [PubMed] [Google Scholar]
- 30.Adachi M., Keefer E.W., Jones F.S. A segment of the Mecp2 promoter is sufficient to drive expression in neurons. Hum. Mol. Genet. 2005;14:3709–3722. doi: 10.1093/hmg/ddi402. [DOI] [PubMed] [Google Scholar]
- 31.Liyanage V.R., Zachariah R.M., Rastegar M. Decitabine alters the expression of Mecp2 isoforms via dynamic DNA methylation at the Mecp2 regulatory elements in neural stem cells. Mol. Autism. 2013;4:46. doi: 10.1186/2040-2392-4-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng Y., Huang W., Wani M., Yu X., Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS ONE. 2014;9:e88685. doi: 10.1371/journal.pone.0088685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jovičić A., Roshan R., Moisoi N., Pradervand S., Moser R., Pillai B., Luthi-Carter R. Comprehensive expression analyses of neural cell-type-specific miRNAs identify new determinants of the specification and maintenance of neuronal phenotypes. J. Neurosci. 2013;33:5127–5137. doi: 10.1523/JNEUROSCI.0600-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein M.E., Lioy D.T., Ma L., Impey S., Mandel G., Goodman R.H. Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nat. Neurosci. 2007;10:1513–1514. doi: 10.1038/nn2010. [DOI] [PubMed] [Google Scholar]
- 35.Visvanathan J., Lee S., Lee B., Lee J.W., Lee S.K. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 2007;21:744–749. doi: 10.1101/gad.1519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heckman L.D., Chahrour M.H., Zoghbi H.Y. Rett-causing mutations reveal two domains critical for MeCP2 function and for toxicity in MECP2 duplication syndrome mice. eLife. 2014;3:e02676. doi: 10.7554/eLife.02676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coy J.F., Sedlacek Z., Bächner D., Delius H., Poustka A. A complex pattern of evolutionary conservation and alternative polyadenylation within the long 3″-untranslated region of the methyl-CpG-binding protein 2 gene (MeCP2) suggests a regulatory role in gene expression. Hum. Mol. Genet. 1999;8:1253–1262. doi: 10.1093/hmg/8.7.1253. [DOI] [PubMed] [Google Scholar]
- 38.Bagga J.S., D’Antonio L.A. Role of conserved cis-regulatory elements in the post-transcriptional regulation of the human MECP2 gene involved in autism. Hum. Genomics. 2013;7:19. doi: 10.1186/1479-7364-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newnham C.M., Hall-Pogar T., Liang S., Wu J., Tian B., Hu J., Lutz C.S. Alternative polyadenylation of MeCP2: Influence of cis-acting elements and trans-acting factors. RNA Biol. 2010;7:361–372. doi: 10.4161/rna.7.3.11564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis B.P., Burge C.B., Bartel D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 41.Vorozheykin P.S., Titov I.I. [Web server for prediction of miRNAs and their precursors and binding sites] Mol. Biol. (Mosk.) 2015;49:846–853. doi: 10.7868/S0026898415050195. [DOI] [PubMed] [Google Scholar]
- 42.Rehmsmeier M., Steffen P., Hochsmann M., Giegerich R. Fast and effective prediction of microRNA/target duplexes. RNA. 2004;10:1507–1517. doi: 10.1261/rna.5248604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deverman B.E., Pravdo P.L., Simpson B.P., Kumar S.R., Chan K.Y., Banerjee A., Wu W.L., Yang B., Huber N., Pasca S.P., Gradinaru V. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 2016;34:204–209. doi: 10.1038/nbt.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clément N., Grieger J.C. Manufacturing of recombinant adeno-associated viral vectors for clinical trials. Mol. Ther. Methods Clin. Dev. 2016;3:16002. doi: 10.1038/mtm.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gray S.J., Blake B.L., Criswell H.E., Nicolson S.C., Samulski R.J., McCown T.J., Li W. Directed evolution of a novel adeno-associated virus (AAV) vector that crosses the seizure-compromised blood-brain barrier (BBB) Mol. Ther. 2010;18:570–578. doi: 10.1038/mt.2009.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.