In clinical practice, many centers continue temozolomide maintenance chemotherapy beyond six cycles, a practice that is controversial. This study investigated the effect of prolonged temozolomide maintenance therapy on progression‐free survival and overall survival in newly diagnosed glioblastoma.
Keywords: Glioblastoma, Maintenance chemotherapy, Temozolomide, Risk factors
Abstract
Background.
The impact of prolonging temozolomide (TMZ) maintenance beyond six cycles in newly diagnosed glioblastoma (GBM) remains a topic of discussion. We investigated the effects of prolonged TMZ maintenance on progression‐free survival (PFS) and overall survival (OS).
Patients and Methods.
In this retrospective single‐center cohort study, we included patients with GBM who were treated with radiation therapy with concomitant and adjuvant TMZ. For analysis, patients were considered who either completed six TMZ maintenance cycles (group B), continued with TMZ therapy beyond six cycles (group C), or stopped TMZ maintenance therapy within the first six cycles (group A). Patients with progression during the first six TMZ maintenance cycles were excluded.
Results.
Clinical data from 107 patients were included for Kaplan‐Meier analyses and 102 for Cox regressions. Median PFS times were 8.1 months (95% confidence interval [CI] 6.1–12.4) in group A, 13.7 months (95% CI 10.6–17.5) in group B, and 20.9 months (95% CI 15.2–43.5) in group C. At first progression, response rates of TMZ/lomustine rechallenge were 47% in group B and 13% in group C. Median OS times were 12.7 months (95% CI 10.3–16.8) in group A, 25.2 months (95% CI 17.7–55.5) in group B, and 28.6 months (95% CI 24.4–open) in group C. Nevertheless, multivariate Cox regression for patients in group C compared with group B that accounted for imbalances of other risk factors showed no different relative risk (RR) for OS (RR 0.77, p = .46).
Conclusion.
Our data do not support a general extension of TMZ maintenance therapy beyond six cycles. The Oncologist 2017;22:570–575
Implications for Practice.
Radiation therapy with concomitant and adjuvant temozolomide (TMZ) maintenance therapy is still the standard of care in patients below the age of 65 years in newly diagnosed glioblastoma. However, in clinical practice, many centers continue TMZ maintenance therapy beyond six cycles. The impact of this continuation is controversial and has not yet been addressed in prospective randomized clinical trials. We compared the effect of more than six cycles of TMZ in comparison with exactly six cycles on overall survival (OS) and progression‐free survival (PFS) by multivariate analysis and found a benefit in PFS but not OS. Thus, our data do not suggest prolonging TMZ maintenance therapy beyond six cycles, which should be considered in neurooncological practice.
Introduction
Glioblastoma (GBM) is an aggressive primary brain tumor with an incidence of 3–4 cases per 100,000 persons each year [1]. The current standard of care after neurosurgical intervention is radiotherapy (RT) with concomitant daily temozolomide (TMZ) followed by TMZ maintenance cycles (5 days of a 28‐day cycle) [2]. Four phase III trials investigating either a dose intensification of TMZ in the maintenance phase [3], the addition of bevacizumab [4], [5], or the addition of cilengitide in O6‐methylguanine‐DNA‐methyltransferase (MGMT)‐methylated GBM [6]. Recent interim data of the EF14 trial using tumor‐treating fields in addition to TMZ maintenance cycles indicate an increase in progression‐free survival (PFS) and overall survival (OS) [7]. The median OS remains in the range of 1.5 years even in these highly selected clinical trial populations [4], [5], [6]. Age, neurological status assessed by Karnofsky performance status and Mini Mental State, extent of resection (EOR), isocitrate dehydrogenase (IDH)‐mutations, and methylation of the MGMT promotor region are established prognostic factors in GBM patients [8], [9].
Radiation therapy with concomitant and adjuvant TMZ was initially introduced with six TMZ maintenance cycles [2]. In clinical practice, however, many centers continue TMZ maintenance therapy beyond six cycles. The impact of this continuation is controversial and has not yet been addressed in prospective randomized clinical trials.
Some retrospective studies suggested a benefit in OS after extension of TMZ maintenance cycles [10], [11], [12], [13]. Limitations of these studies included the following: (a) comparison of patients who only received more than six cycles of TMZ and up to six cycles but not exactly six cycles of TMZ [10], [13]; (b) missing information on MGMT methylation [11], [13]; and (c) univariate Kaplan‐Meier description of OS for patients who received six cycles or more than six cycles of TMZ but no investigation of significance by multivariate Cox regression [10], [11], [12], [13].
In this study, we investigated the effect of prolonged TMZ maintenance therapy on PFS and OS in a retrospective single‐center analysis. We compared the effect of more than six cycles of TMZ in comparison with exactly six cycles on OS and PFS by univariate Kaplan‐Meier and multivariate Cox regression and adjusted survival curve analysis by inverse probability weights accounting for potential unequal distributions of the other predictors of OS and PFS.
Materials and Methods
Study Design
This retrospective observational single‐center study included patients with newly diagnosed GBM that were treated in routine clinical practice outside clinical trials at the University Hospital of Tübingen in Baden‐Württemberg, Germany. The clinical endpoints PFS and OS were evaluated by the Kaplan‐Meier‐method. Risk ratios (RR) for the standard (six cycles) and prolonged TMZ maintenance therapy (beyond six cycles) and the known covariates (age, EOR, MGMT status and Karnofsky performance score [KPS]) were determined by Cox regression. Survival curves were adjusted with inverse probability weights accounting for the aforementioned covariates.
Patients and Data Collection
The patient selection is outlined in the CONSORT diagram (Fig. 1). All adult patients (age ≥18 years) included in the study had surgery in our department from January 2006 to December 2014. Only patients who were assigned to standard RT with concomitant and TMZ maintenance were considered and identified by an electronic database search. Patients received TMZ maintenance therapy according to physicians’ choice. We collected data on general patient characteristics, neuropathological diagnosis, including molecular markers (mutations of IDH, MGMT), EOR, performance status (KPS), treatment parameters (radiation dose, TMZ dose and cycles, adverse events), and key chronological parameters (time of surgery, time of progression, time of death, time of last monitoring, time of starting treatment). Postoperative magnetic resonance imaging (MRI) within 48 hours was used to assess the EOR, and follow‐up MRIs were performed every 3 months. Patients who discontinued concomitant radio‐chemotherapy for any reason were excluded. Approval of the study was obtained from the institutional ethics committee of the University of Tübingen.
Figure 1.
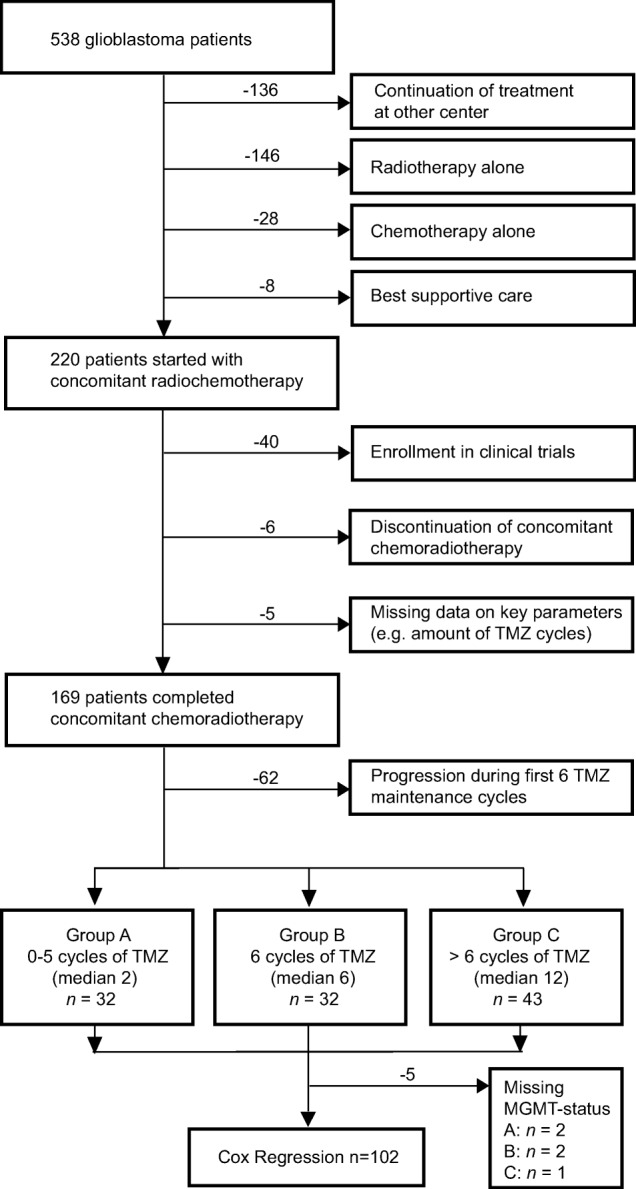
CONSORT diagram of all glioblastoma patients. CONSORT diagram outlining the patient flow and cohort in our retrospective analysis.
Abbreviations: MGMT, O6‐methylguanine‐DNA‐methyltransferase; TMZ, temozolomide.
Data Analysis
We analyzed the outcome by OS and PFS, which were defined as the interval between initial surgery and death of the patient or the first radiologically documented tumor progression by MRI, respectively. Latency of initial therapy was defined as the time between surgery and start of RT. Patients were subdivided into three groups: patients who received up to five cycles of TMZ (group A), patients who received exactly six cycles followed by follow‐up until first progression (group B), and patients who received more than six cycles of maintenance TMZ (group C). Patients who stopped TMZ during maintenance therapy because of tumor progression were excluded from analysis (Fig. 1). Instead, only patients who stopped TMZ because of any limiting toxicity or due to patients’ wishes for any reason were included in the analysis.
Subgroup OS analyses were performed for significant covariates that were identified in Cox regression. Cox regression analyses were performed for the different therapy groups (B versus A, C versus A, and C versus B) and the known prognostic factors of age (age ≤50 years versus >50 years), KPS (<70 versus ≥70), MGMT gene promoter (unmethylated versus methylated), EOR (gross total versus subtotal and biopsy), and gender.
Statistical Analyses
Distributions of all clinical data were compared between therapy groups. Cox regressions were performed to analyze the influence of the grouped numbers of TMZ maintenance cycles (groups A to C) on OS. First, crude associations were determined to enable comparison of our results with other published univariate results. Subsequently, Cox regressions for OS were repeated after adjusting for the established factors (age, KPS, MGMT status, EOR) and gender. Continuous variables (age, KPS) were dichotomized to categorical variables using established predictive thresholds, according to the literature, as follows: age ≤50 years and >50 years and KPS <70 and ≥70. Results were reported as RR with 95% confidence intervals (CIs) and p values. The level of significance was defined as α < 0.05. OS and PFS were determined by Kaplan‐Meier methods for all therapy groups. Comparisons of OS and PFS between groups B and C were adjusted regarding the established covariates (age, KPS, MGMT status, EOR) by inverse probability weights to account for unequal distributions of covariates in this retrospective analysis. JMP (SAS Institute Inc., Cary, NC https://www.jmp.com/en_us/home.html) Statistical Discovery Software version 11.1.1. was used for statistical analyses. For adjusted Kaplan‐Meier estimation, IPW survival package in R from Le Borgne and Foucher [14] were used according to Cole and Hernán [15].
Results
Among 538 patients initially treated for GBM from January 2006 to December 2014 at our center, 220 patients with follow‐up at our hospital started radiation therapy with concomitant and adjuvant TMZ after surgery (Fig. 1). Fifty‐one patients were excluded because they (a) were enrolled in prospective studies with experimental therapies (n = 40), (b) did not complete concomitant radio‐chemotherapy because of adverse events (n = 6), or (c) had missing data on key parameters, for example, number of TMZ cycles (n = 5). Overall, 169 patients were included in this retrospective single‐center study. At the time of the final analysis in April 2016, 120 (71%) patients had died and 49 (29%) patients were still alive (n = 33) or were treated outside our institution (n = 16). Characteristics of the eligible patients related to therapy groups are shown in supplemental online Table 1. Seventy‐five (44%) patients out of our cohort (n = 169) completed six or more cycles of TMZ maintenance therapy. For Kaplan‐Meier analyses and Cox regression, 107 and 102 patients, respectively, were eligible after exclusion of 62 patients because of progression during the first 6 cycles of TMZ maintenance therapy and 5 patients because of missing MGMT status. In the overall analysis, group A (n = 32) completed a median of 1 TMZ cycles, group B (n = 32) completed 6 cycles, and group C (n = 43) completed a median of 12 cycles.
Interestingly, the likelihood of receiving prolonged TMZ maintenance treatment changed between 2006 and 2014 (p = .02). Of the patients who completed 6 cycles of TMZ maintenance therapy, 60% (6/10) in 2006–2008, 79% (19/24) in 2009–2011, and 44% (18/41) in 2012–2014 received additional TMZ cycles. The EOR, age, and adverse events were obviously not balanced among groups. Adverse events were observed more often in the groups that received less than six cycles of TMZ maintenance, intrinsically inverted in correlation to the number of TMZ cycles (supplemental online Table 2). Gross total resection at initial surgery was performed in 21/32 (66%) patients in group B and 35/43 (81%) patients in group C. In group C, furthermore, patients were younger (15/26 below the age of 50) than in group B (6/32) (supplemental online Table 2). For an overview, supplemental online Figure 1 demonstrates the risk profiles and courses of therapy of each individual patient of the patients’ cohort (n = 107). After completing TMZ maintenance therapy in groups B and C, at the end of the observation period, 11/75 patients continued treatment at another center, 14/75 patients had missing data in the records, 19/75 patients had no tumor progression so far (6 patients in group B and 13 patients in group C), and 31/75 patients had tumor progression. The response rates after first progression on TMZ rechallenge (n = 21) or lomustin (n = 10) were 47% (7/15) for patients who initially received 6 cycles of TMZ (group B) and 13% (2/16) for patients who initially received >6 cycles of TMZ (group C).
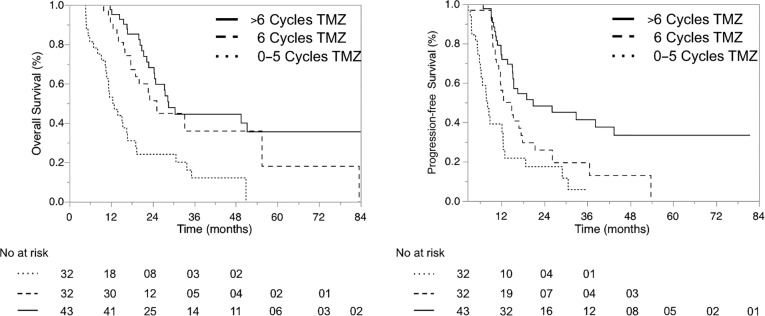
The median time to progression was 8.1 months (95% CI 6.1–12.4) in group A (up to 5 cycles of TMZ), 13.7 months (95% CI 10.6–17.5) in group B (6 cycles of TMZ), and 20.9 months (95% CI 15.2–43.5) in group C (>6 cycles of TMZ). OS was 12.7 months (95% CI 10.3–16.8) in group A, 25.2 months (95% CI 17.7–55.5) in group B, and 28.6 months (95% CI 24.4–open) in group C. Thus, the pure assessment of median OS suggests a benefit for patients who received more than six cycles. OS rates at 2 years were 24% in group A, 51% in group B, and 68% in group C (Fig. 2 and Table 1).
Figure 2.
Progression‐free and overall survival. Kaplan‐Meier plots that demonstrate overall survival and progression‐free survival of patients who received up to five cycles of TMZ (group A, dotted line), patients who received exactly six cycles of TMZ (group B, dashed line), and patients who received more than six cycles of TMZ (group C, full line).
Abbreviations: TMZ, temozolomide.
Table 1. Progression‐free and overall survival times.
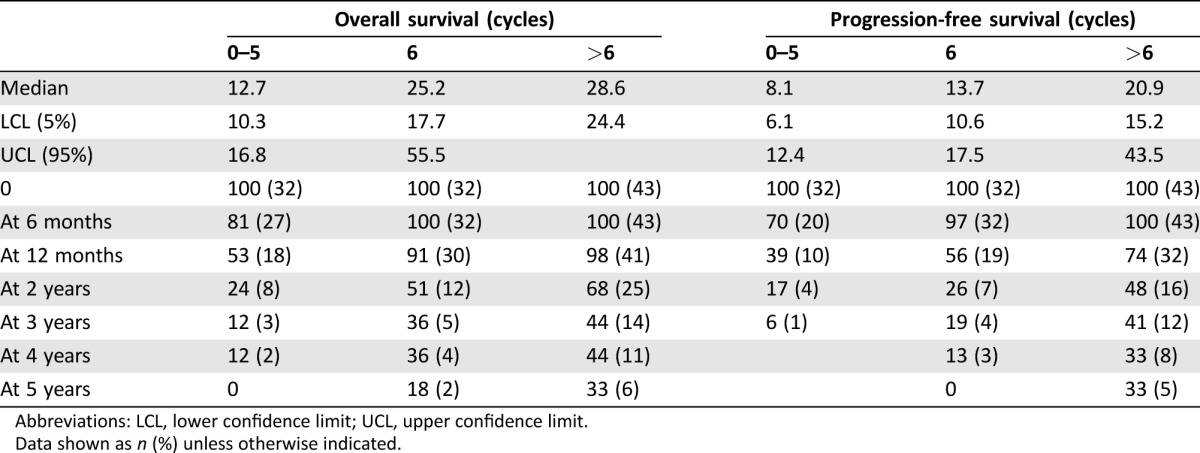
Abbreviations: LCL, lower confidence limit; UCL, upper confidence limit.
Data shown as n (%) unless otherwise indicated.
The RR of the unadjusted Cox regression for death of groups B and C in comparison with group A were 0.38 (95% CI 0.20–0.68, p = .001) and 0.26 (95% CI 0.14–0.46, p < .0001), respectively. After adjustment for potential confounders (age, KPS, EOR, and MGMT status), Cox regression still demonstrated a considerable adjusted RR of 0.36 (95% CI 0.19–0.68, p = .002) and 0.28 (95% CI 0.14–0.54, p = .0002), respectively. The direct comparison of group C with group B, however, did not reveal any significant risk reduction for death (RR 0.77, 95% CI 0.39–1.55, p = .46) but did for progression (RR 0.52, 95% CI 0.28–0.94, p = .03; Table 2).
Table 2. Cox‐regression analyses of progression‐free and overall survival.
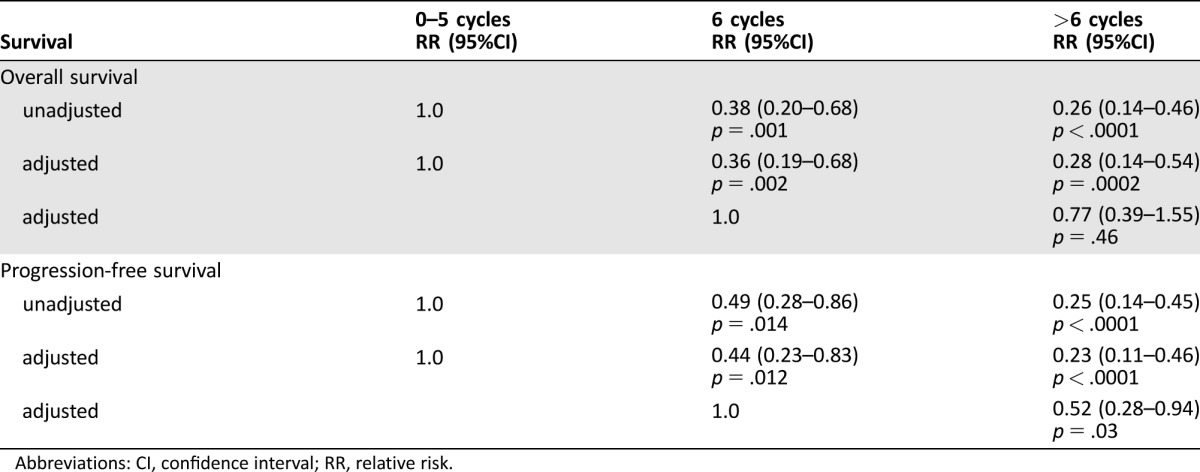
Abbreviations: CI, confidence interval; RR, relative risk.
Cox regression confirmed the prognostic roles of MGMT gene promoter methylation (RR 0.44, 95% CI 0.26–0.75, p = .002), EOR (RR 0.49, 95% CI 0.28–0.87, p = .015), and age at diagnosis (RR 0.40, 95% CI 0.19–0.78, p = .006) as significant predictors for OS in our patient cohort, whereas KPS at diagnosis had no significant effect on OS (Table 3). Similar relationships were also identified for PFS (Table 3). Adjusted Kaplan‐Meier estimation accounting for unequal distribution of covariates demonstrated OS and PFS (supplemental online Fig. 2).
Table 3. Cox regression of prognostic covariates of overall and progression free survival.
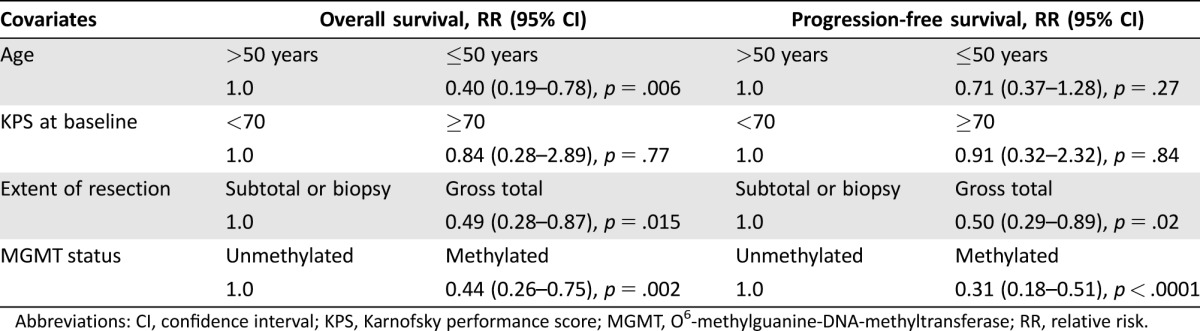
Abbreviations: CI, confidence interval; KPS, Karnofsky performance score; MGMT, O6‐methylguanine‐DNA‐methyltransferase; RR, relative risk.
Discussion
Improvement in OS in subgroups of GBM patients is an urgent necessity. We investigated the impact of prolonged TMZ maintenance therapy on OS and PFS in a retrospective single‐center cohort of 107 patients (Fig. 1). OS and PFS demonstrated a positive correlation with the grouped numbers of TMZ cycles (groups A to C). In the direct comparison of patients who received 6 cycles of TMZ with patients who received more than 6 cycles (median 12 cycles) of TMZ, OS and PFS were increased. In addition to the grouped numbers of TMZ cycles, MGMT status, EOR, and age were confirmed to be significant covariates for survival in our cohort. Nevertheless, multivariate Cox regression and adjusted survival curve analysis did not suggest a benefit in hazard rates for prolonged TMZ maintenance therapy on OS (p = .46) but did on PFS (p = .03).
Limitations of the Retrospective Study Design
We are aware that retrospective studies have intrinsic limitations, mainly due to limited patient numbers, lack of standardized documentation, or adherence to guidelines; for example, there was no homogenous protocol for follow‐up parameters, and some patients received more than the recommended six cycles of TMZ maintenance. Some patients received radiation therapy with concomitant and adjuvant TMZ close to home and were only seen as outpatients every 3 months. The lack of complete follow‐up parameters is a common issue in retrospective studies and might produce biases due to patient selection in some studies, for example, increased MGMT methylation frequency in 74% of patients (>6 cycles of TMZ) in a study by Roldan Urgoiti et al. [12] or gross total resection in 95% of patients in a study by Barbagallo et al. [10]. For further details about cited studies, see supplemental online Table 3. In randomized controlled studies in which other covariates (e.g., age, MGMT status, EOR) are balanced by inclusion criteria, randomization, and stratification, univariate survival analyses, for example, Kaplan‐Meier, are appropriate methods to demonstrate the impact of a specific therapy. In contrast, in our retrospective study, univariate survival curve analysis was distorted because of imbalances in age and EOR between the groups that received six cycles of TMZ compared with more than six cycles of TMZ (supplemental online Table 2). Therefore, we performed a multivariate Cox regression to account for the unequal distributions of other covariates, in particular age and EOR, and adjusted survival curves by inverse probability weights (supplemental online Fig. 2).
The observed increased PFS in group C (20.9 months) compared with group B (13.7 months) is likely to be higher due to the intrinsic design of the study, to either stop (group B) or continue (group C) TMZ after completion of 6 TMZ maintenance cycles without evidence of progression. In contrast, the response rate of TMZ/lomustin after rechallenge at first progression was 47% in group B compared with 13% in group C. This might, at least partially, explain the missing translation of increased PFS to OS, and it outlines the importance of assessing OS even in retrospective studies.
Comparability of Results
In our study, the main clinical data were comparable with prospective trials [2], [3], [4], [5]. Yet, EOR and MGMT methylation status were different in our cohort. We had a higher amount of patients with complete resections and a higher percentage of patients with MGMT‐methylated GBMs in comparison with prospective trials [2], [3], [4], [5], suggesting a selection bias in our cohort. Forty‐four percent (75/169) of patients completed the full protocol of concomitant radio‐chemotherapy and at least 6 cycles of TMZ, which is a rate 20% above the European Organisation for Research and Treatment of Cancer (EORTC) National Cancer Institute of Canada Clinical Trials Group (NCIC) trial and other standard arms of prospective phase III studies in GBM (36%–37%) [2], [3], [4], [5]. Toxicity‐related discontinuation of standard therapy was observed in 11% (19/169) of patients, consistent with results of prospective trials (12%–16%) [2], [3], [4], [5]. TMZ therapy was stopped in 37% (62/169) of patients because of tumor progression during the first 6 TMZ maintenance cycles, which was lower than reported in prospective studies (47%–49%) [2], [5]. We excluded these 62 patients with early tumor progression from analyses, because these patients possibly represent a very unfavorable GBM subgroup regardless of the number of maintenance cycles or the well‐established prognostic factors like MGMT methylation status. We reasoned that the exclusion of this unfavorable subgroup of patients was necessary to avoid any underestimation of survival times in group A. Consequently, we included only patients in group A who stopped TMZ maintenance therapy because of toxicity or patient's wish. On physicians’ choice and patients’ requests, 43 patients received more than the recommended 6 cycles of TMZ maintenance therapy.
Comparability of Clinical Outcome Data
Our survival data regarding the dependency between the grouped numbers of TMZ cycles and OS are in agreement with other retrospective studies, with an OS of 25.2 months in patients who received 6 cycles (16.5–28.2 months) [10], [11], [12], [13] and 28.6 months in patients who received >6 cycles (20.4–30.0 months) [10], [11], [12], [13]. Nevertheless, Cox regression and adjusted survival curve analyses did not confirm a significant benefit of prolonged TMZ maintenance therapy in comparison with 6 cycles of TMZ in our patient cohort for OS (RR 0.77, 95% CI 0.39–1.55, p = .46) but did for PFS (RR 0.52, 95% CI 0.28–0.94, p = .03). We observed an unequal distribution of gross total resection (66% versus 81%) and age ≤50 years (19% versus 35%) in favor of patients who received more than 6 cycles of TMZ. As these features were considered in the Cox regression, possible distortions of the results caused by EOR or age have been avoided. We also used adjusted survival curves analyses to graphically depict the impact of the unequal distribution of age and EOR. Yet, we are aware that the power of this analysis is restricted because of the limited number of eligible patients (n = 102).
Taken together, we found individual increases in PFS and OS by prolongation of TMZ maintenance therapy based on univariate Kaplan‐Meier analyses, but only based on multivariate Cox regression for PFS, which is likely due to the study design but not for OS. Our data are further confirmed by the recently presented pooled analyses of four large phase III trials by Blumenthal et al. at the Annual Meeting of the Society of Neuro‐Oncology (Abstract number ATCT‐08) [16]. This secondary data analysis showed no increase of OS by increasing TMZ maintenance cycles beyond 6 (p = .99) but did show an increase of PFS (HR 0.77, p = .03).
Conclusion
Prolonged TMZ therapy seems to be well tolerated, with only minor side effects [17]. Yet, our data do not support a general extension of TMZ maintenance therapy beyond six cycles.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
This retrospective single‐center cohort study was not funded. Parts of this work were presented in abstract form at the Annual Meeting of the German Neurosurgical Society in Frankfurt, Germany on June 13, 2016.
Author Contributions
Conception/Design: Marco Skardelly, Marcos Soares Tatagiba, Ghazaleh Tabatabai
Collection and/or assembly of data: Marco Skardelly, Elena Dangel, Julia Gohde, Susan Noell, Felix Behling, Christian Borchers, Marilin Koch, Jens Schittenhelm, Sotirios Bisdas, Frank Paulsen, Rainer Ritz
Data analysis and interpretation: Marco Skardelly, Guilherme Lepski, Jens Schittenhelm, Sotirios Bisdas, Aline Naumann, Daniel Zips, Ulrike von Hehn, Ghazaleh Tabatabai
Manuscript writing: Marco Skardelly, Elena Dangel, Julia Gohde, Susan Noell, Felix Behling, Guilherme Lepski, Christian Borchers, Marilin Koch, Jens Schittenhelm, Sotirios Bisdas, Aline Naumann, Frank Paulsen, Daniel Zips, Ulrike von Hehn, Rainer Ritz, Marcos Soares Tatagiba, Ghazaleh Tabatabai
Final approval of manuscript: Marco Skardelly, Elena Dangel, Julia Gohde, Susan Noell, Felix Behling, Guilherme Lepski, Christian Borchers, Marilin Koch, Jens Schittenhelm, Sotirios Bisdas, Aline Naumann, Frank Paulsen, Daniel Zips, Ulrike von Hehn, Rainer Ritz, Marcos Soares Tatagiba, Ghazaleh Tabatabai
Disclosures
Ghazaleh Tabatabai: Bristol‐Myers Squibb (C/A), Medac Travel Grant (H), Roche Diagnostics (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Ostrom QT, Gittleman H, Fulop J et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol 2015;17 Suppl 4:iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–996. [DOI] [PubMed] [Google Scholar]
- 3. Gilbert MR, Wang M, Aldape KD et al. Dose‐dense temozolomide for newly diagnosed glioblastoma: A randomized phase III clinical trial. J Clin Oncol 2013;31:4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chinot OL, Wick W, Mason W et al. Bevacizumab plus radiotherapy‐temozolomide for newly diagnosed glioblastoma. N Engl J Med 2014;370:709–722. [DOI] [PubMed] [Google Scholar]
- 5. Gilbert MR, Dignam JJ, Armstrong TS et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 2014;370:699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stupp R, Hegi ME, Gorlia T et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated mgmt promoter (CENTRIC EORTC 26071‐22072 study): A multicentre, randomised, open‐label, phase 3 trial. Lancet Oncol 2014;15:1100–1108. [DOI] [PubMed] [Google Scholar]
- 7. Stupp R, Taillibert S, Kanner AA et al. Maintenance therapy with tumor‐treating fields plus temozolomide vs temozolomide alone for glioblastoma. JAMA 2015;314:2535–2543. [DOI] [PubMed] [Google Scholar]
- 8. Gorlia T, van den Bent MJ, Hegi ME, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: Prognostic factor analysis of EORTC and NCIC trial 26981‐22981/CE.3. Lancet Oncol 2008;9:29–38. [DOI] [PubMed] [Google Scholar]
- 9. Hegi ME, Liu L, Herman JG et al. Correlation of O6‐methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol 2008;26:4189–4199. [DOI] [PubMed] [Google Scholar]
- 10. Barbagallo GM, Paratore S, Caltabiano R et al. Long‐term therapy with temozolomide is a feasible option for newly diagnosed glioblastoma: A single‐institution experience with as many as 101 temozolomide cycles. Neurosurg Focus 2014;37:E4. [DOI] [PubMed] [Google Scholar]
- 11. Darlix A, Baumann C, Lorgis V et al. Prolonged administration of adjuvant temozolomide improves survival in adult patients with glioblastoma. Anticancer Res 2013;33:3467–3474. [PubMed] [Google Scholar]
- 12. Roldán Urgoiti GB, Singh AD, Easaw JC. Extended adjuvant temozolomide for treatment of newly diagnosed glioblastoma multiforme. J Neurooncol 2012;108:173–177. [DOI] [PubMed] [Google Scholar]
- 13. Seiz M, Krafft U, Freyschlag CF et al. Long‐term adjuvant administration of temozolomide in patients with glioblastoma multiforme: Experience of a single institution. J Cancer Res Clin Oncol 2010;136:1691–1695. [DOI] [PubMed] [Google Scholar]
- 14. Le Borgne F, Foucher Y. Ipwsurvival: Adjusted survival curves and corresponding log‐rank statistic. Available at http://www.r-project.org and http://www.divat.fr. Accessed: March 10, 2016.
- 15. Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed 2004;75:45–49. [DOI] [PubMed] [Google Scholar]
- 16. Blumenthal DT, Stupp R, Zhang P et al. The impact of extended adjuvant temozolomide in newly‐diagnosed glioblastoma: A secondary analysis of EORTC and NRG oncology/RTOG. Neurooncol 2015;17(suppl 5):v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hau P, Koch D, Hundsberger T et al. Safety and feasibility of long‐term temozolomide treatment in patients with high‐grade glioma. Neurology 2007;68:688–690. Accessed: 03/10/2016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.