This study aimed to identify prognostic factors of patients with hepatocellular carcinoma without portal vein tumor thrombosis and to establish prognostic nomograms for recurrence‐free survival and overall survival. The accuracy of these nomograms for predicting individual prognosis is compared with the accuracy of established clinical prognostic models to ascertain whether the nomograms are an accurate instrument of prognosis
Keywords: Nomogram, Hepatocellular carcinoma, Prognosis, Portal vein tumor thrombosis, Resection
Abstract
Background.
The prognosis of patients with hepatocellular carcinoma (HCC) without portal vein tumor thrombosis (PVTT) after curative resection is at variance. We identified the risk factors of poor postoperative prognosis and consequently developed prognostic nomograms generating individual risk of death and recurrence for this subgroup of patients with HCC.
Methods.
The risk factors were identified and nomograms were developed based on a retrospective study of 734 patients in the primary cohort who underwent curative resection for HCC from 2010 to 2012. The predictive accuracy and discriminative ability of the nomograms were determined by concordance index (C‐index) and calibration curve and compared with traditional staging systems of HCC. The results were validated in an independent cohort of 349 patients operated at the same institution in 2007.
Results.
All of the independent factors for survival in multivariate analysis in the primary cohort were selected into the nomograms. The calibration curve for probability of survival showed good agreement between prediction by nomograms and actual observation. The C‐indices of the nomograms for predicting overall survival and recurrence‐free survival were 0.755 (95% confidence interval [CI], 0.752–0.758) and 0.665 (95% CI, 0.662–0.668), respectively, which were statistically higher than the C‐indices of other HCC prognostic models. The results were further confirmed in the validation cohort.
Conclusion.
The proposed nomograms resulted in more accurate prognostic prediction for patients with HCC without PVTT after curative resection. The Oncologist 2017;22:561–569
Implications for Practice.
Hepatocellular carcinoma (HCC) poses a great therapeutic challenge due to the poor prognosis in patients underwent surgical resection. The portal vein tumor thrombosis (PVTT) as a robust risk factor for survival has been routinely integrated to staging systems. Nonetheless, the prognosis stratification for patients without PVTT was neglected to some extent. Herein, independent risk factors of OS and RFS in HCC patients without PVTT were reconfirmed. A predictive nomogram was constructed on these risk factors and was demonstrated to be a more accurate predictive model in HCC patients without PVTT, compared with the traditional staging systems.
摘要
背景. 业内对于无PVTT的HCC患者行根治性切除术后的预后仍有争议。我们已识别出这一HCC患者亚组术后预后不良的风险因素, 并据此开发了可生成各种死亡和复发风险的预后列线图。
方法. 对2010‐2012年间行根治性切除术治疗HCC的734例患者(主要队列)进行了一项回顾性研究, 在此基础之上识别风险因素并开发列线图。通过一致性指数(C指数)和校准曲线确定列线图的预测准确度和判别能力, 并与传统HCC分期系统进行比较。在一个独立队列, 即于2007年在同一机构接受手术的349例患者中验证所得结果。
结果. 将主要队列多变量分析中生存期的所有独立风险因素纳入列线图。在生存概率校准曲线中, 列线图预测值与实际观测值的吻合度较好。列线图预测总生存期和无复发生存期时的C指数分别为0.755[95%置信区间(CI):0.752‐0.758]和0.665(95% CI:0662‐0.668), 显著高于其他HCC预后模型的C指数。该结果在验证队列中得到了进一步确认。
结论. 本研究构建的列线图可以更准确地预测无PVTT的HCC患者行根治性切除术后的预后。The Oncologist 2017;22:561–569
对临床实践的提示:肝细胞癌(HCC)患者行切除手术后的预后较差, 从而给治疗带来了严峻挑战。门静脉癌栓(PVTT)是生存期的稳健风险因素之一, 已在常规实践中将其纳入分期系统。尽管如此, 我们仍在一定程度上忽视了无PVTT患者的预后分层。因此, 本研究在无PVTT的HCC患者中再次确认了总生存期(OS)和无复发生存期(RFS)的独立风险因素。针对上述风险因素构建了预测列线图, 结果证明在无PVTT的HCC患者中, 以该列线图作为预测模型时的准确度高于传统分期系统。
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the second leading cause of cancer‐related mortality worldwide [1]. Despite curative resection, the long‐term prognosis of HCC is still poor, with an extremely high tumor recurrence rate that exceeds 60% at 5 years even in patients with small tumors [2]. Fortunately, some highly selected patients may benefit from a prognosis predictive model and therapeutic assignment [3], [4].
Thus, identification of prognostic markers of HCC has long been of interest. According to a systematic review [5], portal vein tumor thrombosis (PVTT) was indicated to be one of the most robust predictors of survival. Accumulating investigations have been conducted referring to prognostic factors of HCC with PVTT following variable treatment modalities, including resection, transarterial chemoembolization, radiotherapy, and conservative management [6], [7], [8]. However, the prognostic factors associated with prognosis of HCC without PVTT, a subgroup lacking this robust indicator, remain to be elucidated.
In an attempt to stratify expected survival outcomes for HCC patients, a number of staging systems have also been developed for classification and prognostication of HCC, including the Barcelona Clinic Liver Cancer (BCLC), Okuda score, Cancer of the Liver Italian Program (CLIP), Chinese University Prognostic Index, and Japan Integrated Staging Score [9], [10], [11], [12], [13]. Unfortunately, their criteria vary greatly, are predominantly derived from patients with metastatic and locally advanced disease, often with impaired liver function, and only serve to classify patients into various groups with varying outcomes but do not predict individualized outcomes [14], [15].
While other predictive models assign prognosis based on risk groups, nomograms provide a more individualized prediction of outcome based on a combination of variables [16]. Currently, one nomogram (Memorial Sloan‐Kettering Cancer Center [MSKCC]) based on a small sample in the U.S. has been proposed to predict survival, and another has been proposed to predict pulmonary metastases, but neither has been externally validated; in addition, the MSKCC includes patients who, according to current guidelines, are generally not ideal candidates for hepatic resection (i.e., extrahepatic) along with some who didn't meet the criteria of R0 resection [14], [15].
The purpose of this study was to identify prognostic factors of patients with HCC without PVTT. Furthermore, we aim to establish and independently validate prognostic nomograms for recurrence‐free survival (RFS) and overall survival (OS) via integrating the clinicopathologic variables associated with HCC outcome from a large HCC cohort of patients without PVTT who underwent curative resection. In addition, we also wish to compare the accuracy of these nomograms for predicting individual prognosis with that obtained from the established clinical prognostic models to ascertain whether our nomograms are an accurate instrument of prognosis.
Materials and Methods
Patient Selection
Two independent cohorts of patients with HCC without PVTT following curative resection were enrolled in this study. The training and validation cohorts were randomly collected from patients with HCC who underwent curative hepatectomy in Zhongshan Hospital during the 3‐year period from 2010 to 2012 (n = 734) and in 2007 (n = 349), respectively. All the patients had survived for at least 30 days postoperatively. The inclusion and exclusion criteria for the patients analyzed in both training and validation cohort are as follows: (a) without any preoperative anticancer treatments that could introduce any bias; (b) exact diagnosis of pathologically proven HCC; (c) complete removal of the tumor without residual cancer, defined as a complete resection of all tumor lesions and the cut surface being free of cancer by histological examination (R0 resection); (d) with complete clinicopathologic and follow‐up data; (e) none of the patients were suffering from a recurrence of HCC or from any other concomitant malignancy; (f) without any macroscopic invasion to portal vein or metastasis to distant sites. All patients provided informed consent to participate in the study, and the study protocol was approved by the Clinical Research Ethic Committee of Zhongshan hospital.
Conventional clinicopathologic variables comprising age, gender, hepatitis B virus surface antigen (HBsAg), liver cirrhosis (LC), α‐fetoprotein (AFP), alanine aminotransferase (ALT), γ‐glutamyl transferase (GGT), tumor size, number, microvascular invasion, encapsulation, differentiation, BCLC stage, and CLIP score were recorded and detailed in Table 1.
Table 1. Basal clinicopathologic characteristics in training and validation cohort.
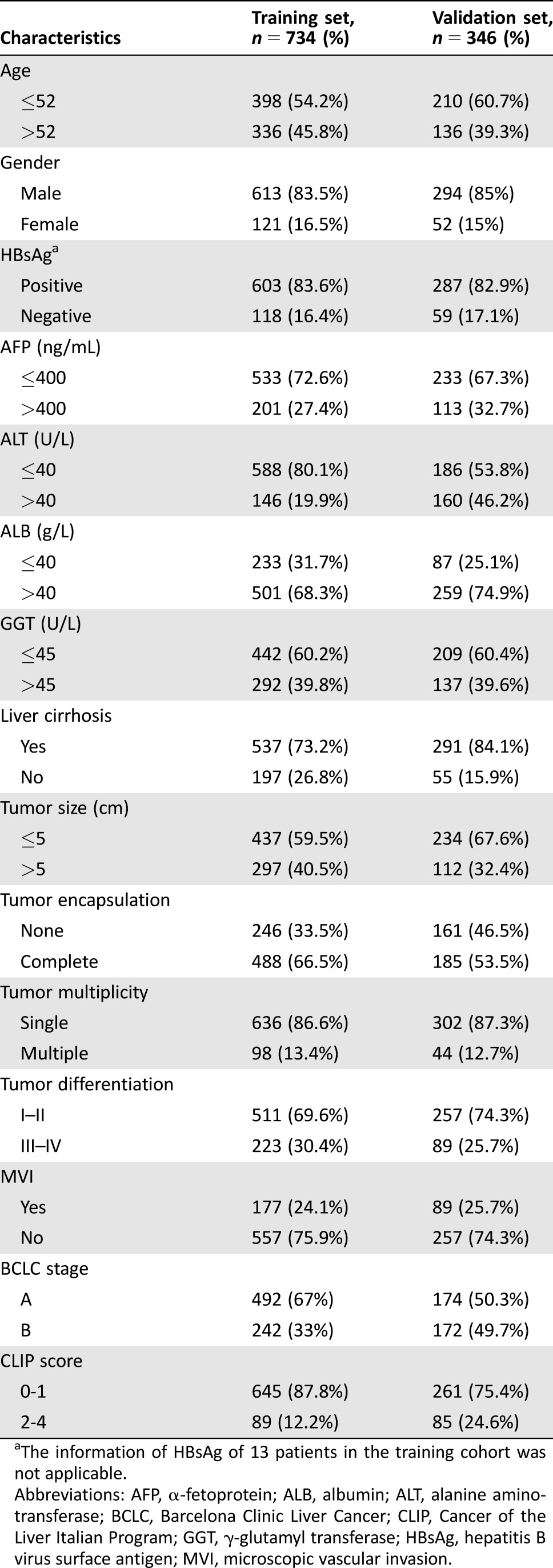
The information of HBsAg of 13 patients in the training cohort was not applicable.
Abbreviations: AFP, α‐fetoprotein; ALB, albumin; ALT, alanine aminotransferase; BCLC, Barcelona Clinic Liver Cancer; CLIP, Cancer of the Liver Italian Program; GGT, γ‐glutamyl transferase; HBsAg, hepatitis B virus surface antigen; MVI, microscopic vascular invasion.
Follow‐Up Procedure
The follow‐up procedure was described in our previous study [17]. In brief, all patients were monitored prospectively by serum AFP, liver function test, abdomen ultrasonography, and chest x‐ray every 1 to 6 months, according to the postoperative time. For patients with test results suggestive of recurrence, computed tomography and/or magnetic resonance imaging was used to verify whether intrahepatic recurrence and/or distal metastasis had occurred. A diagnosis of recurrence was based on typical imaging appearance in computed tomography and/or magnetic resonance imaging scan with or without an elevated AFP level. OS was defined as the interval between surgery and time of either death or last observation taken. RFS was defined as the interval between surgery and time of recurrence. Once evidence of recurrence was confirmed, RFS was calculated as the time when recurrence was first suspected. The median follow‐up time was 39 months (range, 2–60 months) in the training cohort and 43 months (range, 1.5–63 months) in the validation cohort, respectively.
Statistical Analysis
Risk factors were identified via SPSS 16.0 (SPSS, Chicago, IL). Chi‐square test or Fisher's exact test was used to compare results. Categorical variables were grouped based on clinical findings (definition of groups is shown in Table 1), and decisions on the groups were made before modeling. Continuous variables were compared using the Student's t test or Mann‐Whitney U test for variables with an abnormal distribution. Survival curves were depicted using the Kaplan‐Meier method and compared using the log‐rank test. Cox regression analysis was used for multivariate analyses. Independent prognostic factors were identified through backward selection in a Cox regression model. Variables significantly related to survival in the univariate Cox models (p < .05) were subsequently included in the multivariate model.
A nomogram was established based on the results of multivariate analysis and by using the package of rms in R version 2.14.1 (http://www.r-project.org/). The performance of the nomogram was measured by concordance index (C‐index) and assessed by comparing nomogram‐predicted survival probability versus observed Kaplan‐Meier estimates. Bootstraps with 1,000 resample were used for these activities. Comparisons between the nomogram and other staging systems were performed with the rcorrp.cens package in Hmisc in R and were evaluated by the C‐index. The larger the C‐index, the more accurate was the prognostic prediction. During the external validation of the nomogram, the total points of each patient in the validation cohort were calculated according to the formulated nomogram, then the total points as a factor in this cohort was analyzed via Cox regression; finally, the C‐index and calibration curve were derived on the basis of the regression analysis. The decision curve analysis (DCA) [18] was applied to evaluate the clinical usefulness of the nomograms for the prediction of prognosis.
Results
Clinicopathological Characteristics
The detailed characteristics of patients in the primary and validation cohorts were presented in Table 1; the two independent sets had an overall similar tumor burden. Most patients were men (83.5%), were positive for HBsAg (83.6%), and had a single tumor nodule at the time of resection in primary cohort (86.6%).
Identification of Prognostic Factors in Patients with HCC Without PVTT
The median follow‐up time was 39 months (range, 2–60 months), the median RFS was 28 months (range, 1–60 months), and the postoperative 1‐, 3‐, and 4‐year OS and RFS rates were 95.8%, 58.7%, 44.7% and 81.5%, 45.5%, and 41%, respectively.
Univariate analysis revealed that elevated AFP (p < .001), GGT (p < .001), lager tumor size (p < .001), multiple tumor number (p < .001), presence of microscopic vascular invasion (MVI; p < .001), absence of tumor encapsulation (p = .009), and poor cancer cell differentiation (p < .001) were identified as significant prognostic factors of OS. In multivariate analysis, AFP (hazard ratio [HR], 1.282; 95% confidence interval [CI], 1.119–1.468; p < .001), GGT (HR, 1.854; 95% CI, 1.264–2.719; p = .002), tumor size (HR, 1.108; 95% CI, 1.060–1.159; p < .001), tumor numbers (HR, 1.247; 95% CI, 1.023–1.521; p = .029), and presence of MVI (HR, 2.299; 95% CI, 1.570–3.367; p < .001) remained significant independent predictors of OS (Table 2).
Table 2. Independent prognostic factors predicting OS and RFS in training cohort.
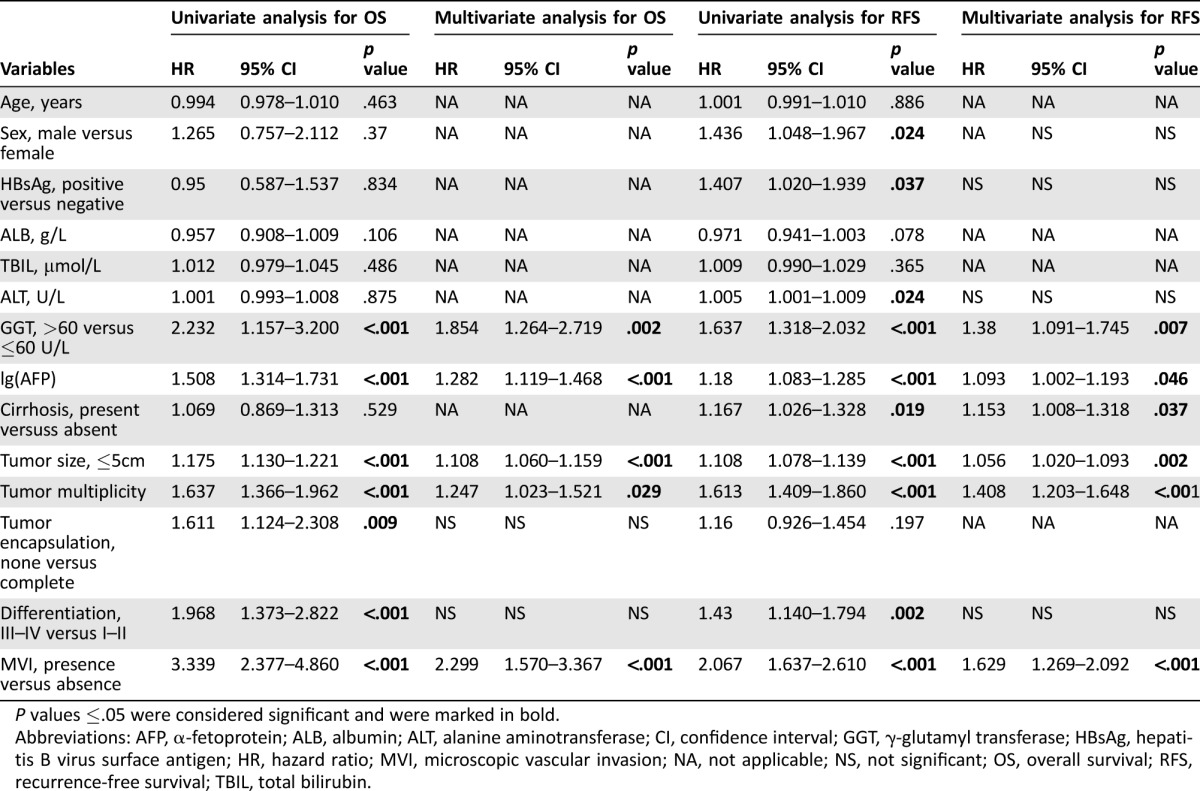
P values ≤.05 were considered significant and were marked in bold.
Abbreviations: AFP, α‐fetoprotein; ALB, albumin; ALT, alanine aminotransferase; CI, confidence interval; GGT, γ‐glutamyl transferase; HBsAg, hepatitis B virus surface antigen; HR, hazard ratio; MVI, microscopic vascular invasion; NA, not applicable; NS, not significant; OS, overall survival; RFS, recurrence‐free survival; TBIL, total bilirubin.
In univariate analysis, sex (p = .024), HBsAg (p = .037), elevated ALT (p = .024), raised AFP (p < .001), LC (p = .019), GGT (p < .001), larger tumor size (p < .001), multiple tumor number (p < .001), presence of MVI (p < .001), and poor cancer cell differentiation (p < .001) were identified as significant predictors of RFS. In multivariate analysis, GGT (HR, 1.38; 95% CI, 1.091–1.745; p = .007), raised AFP (HR, 1.093; 95% CI, 1.002–1.193; p = .046), LC (HR, 1.153; 95% CI, 1.008–1.318; p = .037), tumor size (HR, 1.056; 95% CI, 1.020–1.093; p = .002), tumor multiplicity (HR, 1.408; 95% CI, 1.203–1.648; p < .001), and presence of MVI (HR, 1.629; 95% CI, 1.269–2.092; p < .001) remained significant independent predictors of RFS (Table 2).
Construction of Nomograms for OS and RFS
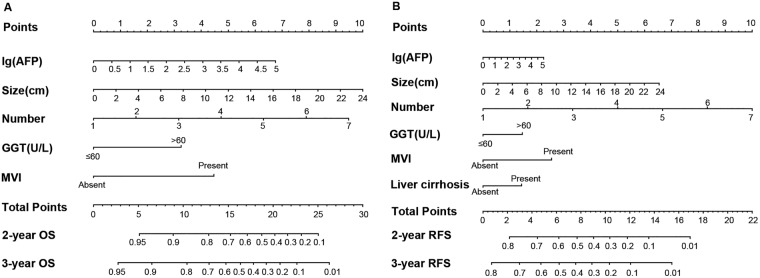
The prognostic nomogram that integrated all the independent prognostic factors for OS derived from the training cohort is shown in Figure 1A. The C‐index for OS prediction in training cohort was 0.755 (95% CI, 0.752–0.758). The calibration plot for the probability of survival at 2 and 3 years after surgery showed optimal consistency between the prediction by nomogram and actual observation (Fig. 2A and 2B).
Figure 1.
Hepatocellular carcinoma OS (A) and RFS (B) nomograms. (To use the nomogram, the value of an individual patient is located on each variable axis, and a line is drawn upward to determine the number of points received for each variable value. The sum of these numbers is located on the total point axis, and a line is drawn downward to the survival axes to determine the likelihood of 2‐ or 3‐year survival).
Abbreviations: AFP, α‐fetoprotein; GGT, γ‐glutamyl transferase; MVI, microscopic vascular invasion; OS, overall survival; RFS, recurrence‐free survival.
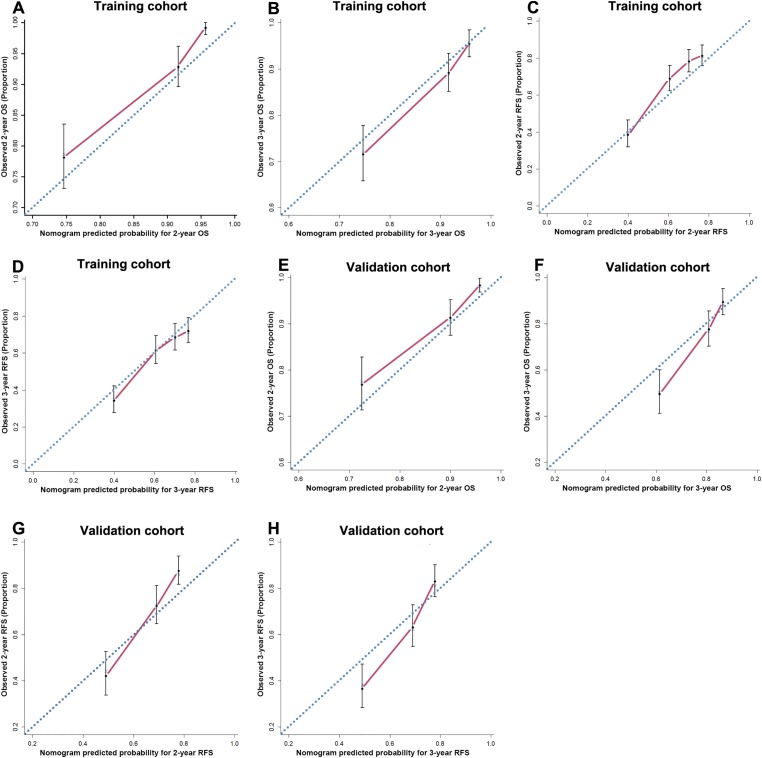
Figure 2.
The calibration curve for predicting OS of patients at 2 years (A) and 3 years (B), RFS at 2 years (C) and 3 (D) years in the training cohort; predicting patient OS at 2 years (E) and 3 years (F) and predicting patient RFS at 2 years (G) and 3 years (H) in the validation cohort. Nomogram‐predicted probability of survival is plotted on the x‐axis; actual survival is plotted on the y‐axis.
Abbreviations: OS, overall survival; RFS, recurrence‐free survival.
The prognostic nomogram that combined all the independent prognostic factors for RFS derived from the training cohort is shown in Figure 1B. The C‐index for RFS prediction in training cohort was 0.665 (95% CI, 0.662–0.668). The calibration plot for the probability of RFS at 2 and 3 years after surgery showed optimal consistency between the prediction by nomogram and actual observation (Fig. 2C and 2D).
Validation of the Nomogram
In the validation cohort, the median follow‐up time was 43 months (range 1.5–63 months); the median time to recurrence was 35.8 months (range 1–63 months). The postoperative 1‐, 3‐, and 5‐year OS and RFS rates were 88.4%, 61.1%, 51.2% and 81.5%, 54.3%, and 46.4%, respectively.
The C‐index of the constructed nomogram for predicting OS was 0.7 (95% CI, 0.697–0.703), and a calibration curve fit well between prediction and observation in the probability of both 2‐year and 3‐year OS (Fig. 2E and 2F). The C‐index of the proposed nomogram for predicting RFS was 0.658 (95% CI, 0.655–0.661), and a calibration curve showed good agreement between prediction and observation in the probability of both 2‐year and 3‐year RFS (Fig. 2G and 2H).
Comparative Performance of Staging Systems
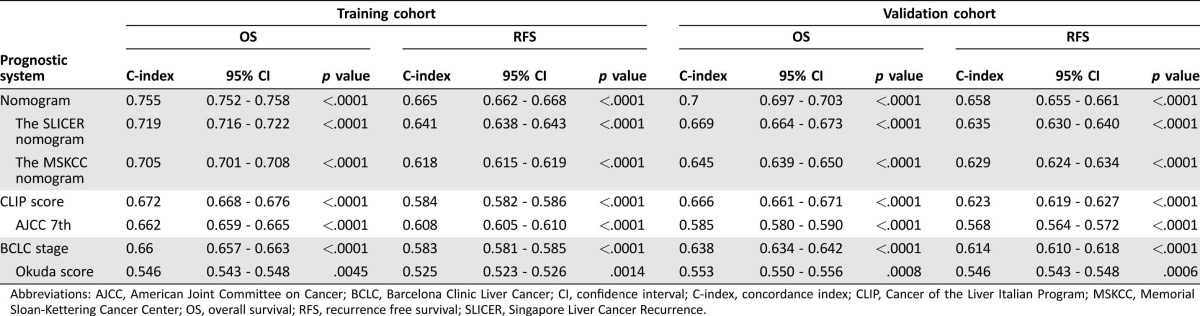
We compared the accuracy of our nomograms with the routinely clinically used prognostic models of BCLC stage, CLIP score, Okuda Stage, and AJCC seventh edition together with two other nomograms, MSKCC and Singapore Liver Cancer Recurrence (SLICER) [19], to ascertain whether our nomograms are feasible prognostic models. The discriminatory capacity of each prognostic system was compared by means of Harrell's C‐index, as shown in Table 3. The discriminatory capacity of the first ranked prognostic model in the primary cohort was the nomogram with a C‐index of 0.755 (95% CI; 0.752–0.758) for OS and 0.665 (95% CI, 0.662–0.668) for RFS. The nomogram consistently ranked the first in terms of discriminatory accuracy in the validation cohort with C‐index of 0.7 (95% CI, 0.697–0.703) for OS and 0.658 (95% CI, 0.655–0.661) for RFS.
Table 3. Ranking of staging systems via the C‐index.
Abbreviations: AJCC, American Joint Committee on Cancer; BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; C‐index, concordance index; CLIP, Cancer of the Liver Italian Program; MSKCC, Memorial Sloan‐Kettering Cancer Center; OS, overall survival; RFS, recurrence free survival; SLICER, Singapore Liver Cancer Recurrence.
Clinical Usefulness of Nomograms as Evaluated by DCA
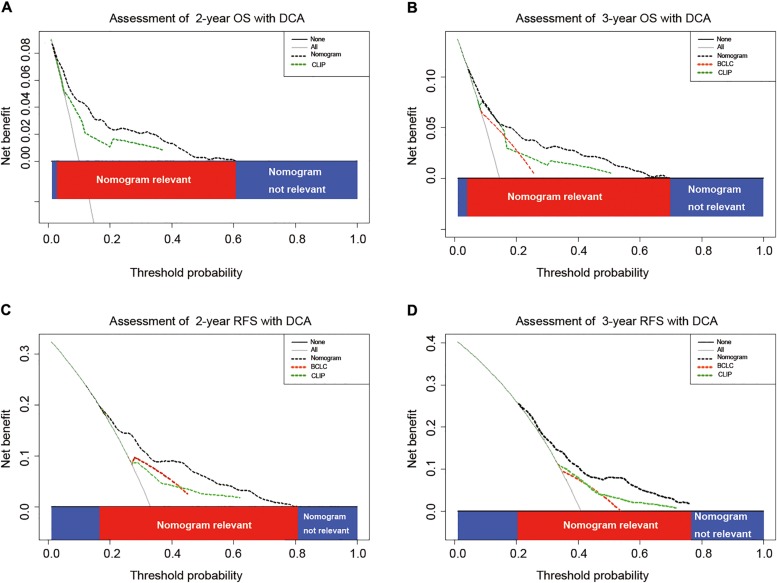
Given that the proposed nomograms demonstrated superior predictive capabilities relative to the BCLC stage and CLIP score in terms of C‐index, comparison of constructed nomograms with BCLC stage and CLIP score as predictor of prognosis on DCA was necessary to be performed to ascertain the clinical usefulness of the nomograms. On DCA, compared with BCLC stage and CLIP score, nomograms showed better net benefit with a wider range of threshold probability and improved performance for predicting 2‐ and 3‐year OS and RFS (Fig. 3A–3D). This further represents superior estimation of decision outcomes at higher threshold probability and net benefit levels.
Figure 3.
DCA. DCAs depict the clinical net benefit in pairwise comparisons between integrated nomogram, BCLC stage, and CLIP score. Nomograms are compared against the BCLC stage and CLIP score in terms of 2‐ and 3‐year OS (A, B) and 2‐ and 3‐year RFS (C, D). Dashed lines indicate the net benefit of nomogram in each of the curves across a range of threshold probabilities. The horizontal solid black line represents the assumption that no patients will experience the event, and the solid gray line represents the assumption that all patients will die or relapse. On DCA, nomogram showed superior net benefit compared to BCLC stage and CLIP score across a range of threshold probabilities.
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; CLIP, Cancer of the Liver Italian Program; DCA, decision curve analysis; RFS, recurrence‐free survival; OS, overall survival.
Discussion
Based on the two large independent cohorts of patients who underwent curative resection for HCC, novel and validated nomograms were developed for predicting individual probabilities of recurrence and cancer‐specific death at 2 and 3 years. The nomograms, integrating independent prognostic factors that reflect the preoperative clinical essentials and postoperative pathologic findings, are reliable and convenient graphic tools.
The advantage of the current study was that homogeneous early‐stage HCC patients without PVTT were enrolled for analysis, and all of them underwent curative resection. Thus, significant confounders on cancer outcome such as variation of tumor stage and therapeutic modalities were eliminated [20]; furthermore, postoperative outcome and prognostic factors in a subset without the influence of the powerful prognostic indicator PVTT can be elucidated. These nomograms possess C‐indices of 0.755 and 0.655 for OS and RFS, respectively; although not perfect, they are more accurate than traditional staging systems such as BCLC, CLIP, AJCC seventh edition and Okuda stage, which was in line with the fact that predictive accuracy of nomograms has been superior compared with the traditional staging systems in other cancer populations [16], [21].
Early HCC without PVTT is generally allocated for surgical resection; nevertheless, tumor biology, hepatic function, and eventual prognosis are quite discrepant. A more precise staging system would give clinicians the ability to identify patients with statistically heterogeneous outcomes from within otherwise homogeneous clinical groups. Prognostic discrepancies within patients diagnosed with same BCLC stage, which can be identified by our nomogram, would help to stratify individual risk for recurrence and, thus, would also help to optimize postoperative follow‐up and treatment. In the present study, in the validation cohort, we first found 5‐year OS and RFS were 51.2% and 46.4%, respectively; although indicating a relatively favorable prognosis for patients without PVTT, the result remains not optimal. Given that there is no consensus on the follow‐up procedures for detection of recurrent HCC after resection [22], these nomograms enable surgical patients to be easily monitored on an individual basis and to be appropriately allocated for participation in clinical trials of postoperative adjuvant therapy (e.g., patients with total risk scores for recurrence of ≥10 in whom the probability of 1‐year RFS rate is ≤60% and 3‐year RFS rate is ≤20%).
PVTT was considered as a special type of metastasis with formation of tumor embolus in portal vein in HCC [23]. Liver function status was reported to impact long‐term prognosis in HCC with PVTT. The ascites, jaundice, hepatic encephalopathy, and liver failure may induced by the formation of PVTT and thus might account for, at least in part, the difference in prognostic factors between HCC with and without PVTT [24]. In the present paper, serum GGT and AFP level, tumor size and number, and MVI were identified as independent prognostic factors for both OS and RFS in multivariate analysis; in addition, LC was found to be an independent prognostic factor for RFS. Accordingly, most of these factors are tumor specific, different from those in PVTT‐present HCC to some extent [25].
These nomograms integrated the independent prognostic variables of HCC, including the pathological characteristics and the serum tumor biomarkers, thus making it accurate for prediction of survival. For the pathological variables, in addition to tumor size and number, reflecting the disease stage, MVI and LC were also included. Many studies have confirmed the presence of MVI to be associated with external hepatic metastasis [26], [27], [28]; thus, it is a predictor of poor prognosis after curative resection for HCC. On the contrary, only a few studies defined the role of underlying LC for patient prognosis after liver resection, although LC is the predominant preneoplastic condition for HCC [29] and was responsible for shortened RFS and OS of HCC patients following resection [30], [31].
For the serum tumor biomarkers, AFP is the most universally investigated marker in HCC prognosis stratification [32]. According to a recent systematic review of prognostic factors, AFP was one of the most robust predictors of death in patients with cirrhosis and HCC [5]. However, serum AFP level was elevated in 15%–58% of patients with chronic hepatitis and 11%–47% of patients with LC [33]. Thus, reevaluation of tumor biomarkers to find potential candidates to be included in the surveillance of HCC prognosis might be of great importance in clinical practice. Until now, various tumor markers such as GGT, AFP‐L3, and glypican‐3 have emerged as complement or substitute for AFP in the diagnosis and prognosis of HCC [32], [34].
Previous studies concerning GGT in HCC have indicated that raised expression of hepatic GGT may be closely associated with the development of HCC [35], [36], [37]. The expression of GGT provides tumor cells with an additional source of cysteine and cystine from the cleavage of extracellular glutathione and oxidized glutathione [38]. Furthermore, the GGT was significantly higher in HCC patients with larger tumors [39]. In addition, the survival of HCC patients decreased as their GGT levels rose, as did their tumor masses [37]. Consistent with the previous studies, the present study showed that elevated serum GGT, as a stable serum molecule from liver, served as an independent predictive factor for HCC RFS and OS.
Some limitations in the present study need to be considered. Firstly, because the dataset was obtained from a single institute with HCC in a hepatitis B virus‐endemic area, cross validation and further investigations of these nomograms in multicenter and prospective settings with more etiologic factors should be conducted in our future research. Secondly, these nomograms are clinically applicable for postoperative decision‐making rather than for preoperative decision‐making. The anticipated future utility of molecular or genomic pathological biomarkers would substantially enhance the usage of these nomograms. Thirdly, in the SLICER nomogram, margin distance was a risk factor; however, the patients enrolled in our retrospective study all underwent curative resection with margin distance over 1 cm, which might hamper the predictive performance of the SLICER nomogram in our cohort. It is noteworthy that MSKCC was generated from a small series of 184 patients and had evident divergence between observed and expected outcomes during calibration, possibly due to the low numbers involved. This nomogram performed less well in our dataset, possibly due to the different patient profile of HCC in the Asian population; moreover, our study only enrolled patients with negative resection margin and patients without macroscopic vascular invasion, which would also affect the performance of the MSKCC nomogram.
Conclusion
In conclusion, these data indicated that variables associated with poor OS and RFS in HCC without PVTT are all tumor related, to some extent, and different from those in PVTT‐present HCC [25]. Furthermore, a unique and consistent postoperative prognosis prediction nomogram was generated via integrating these independent prognostic factors. These nomograms possess much better accuracy of both OS and RFS prediction than traditional systems, including BCLC stage and CLIP scores.
Acknowledgements
This work was in part supported by National Key Sci‐Tech Special Project of China (Grant 2012ZX10002010‐001/002), the National Natural Science Foundation of China (Grant 81302102), and the Basic Research Programs of Science and Technology Commission Foundation of Shanghai (Grants 13JC1401800, XBR2013074, and 13CG04).
Contributed equally.
Footnotes
For Further Reading: Yingqiang Zhang, Wenzhe Fan, Yu Wang et al. Sorafenib With and Without Transarterial Chemoembolization for Advanced Hepatocellular Carcinoma With Main Portal Vein Tumor Thrombosis: A Retrospective Analysis. The Oncologist 2015;20:1417–1424.
Implications for Practice: For patients with advanced hepatocellular carcinoma (HCC) and main portal vein tumor thrombosis (MPVTT), no benefit was seen in this study in terms of disease control rate, time to progression, and overall survival for patients receiving sorafenib and transarterial chemoembolization compared with those receiving sorafenib monotherapy. Considering the patients' morbidity after combination therapy, monotherapy is appropriate for managing patients with advanced HCC and MPVTT.
Author Contributions
Conception/Design: Yi‐Peng Fu, Jian Zhou, Jia Fan, Shuang‐Jian Qiu
Provision of study material or patients: Yi‐Peng Fu, Jian Sun, Xiao‐Chun Ni
Collection and/or assembly of data: Yi‐Peng Fu, Jin‐Long Huang, Chu‐Yu Jing, Jian Sun, Xiao‐Chun Ni, Zhu‐Feng Lu, Ya Cao
Data analysis and interpretation: Yi‐Peng Fu, Yong Yi, Chu‐Yu Jing, Shuang‐Jian Qiu
Manuscript writing: Yi‐Peng Fu, Yong Yi, Shuang‐Jian Qiu
Final approval of manuscript: Yi‐Peng Fu, Jian Zhou, Jia Fan, Shuang‐Jian Qiu
Disclosures
The authors indicated no financial relationships.
References
- 1. Torre LA, Bray F, Siegel RL et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2. Poon RT, Fan ST, Lo CM et al. Long‐term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: Implications for a strategy of salvage transplantation. Ann Surg 2002;235:373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Teng F, Han QC, Ding GS et al. Validation of a criteria‐specific long‐term survival prediction model for hepatocellular carcinoma patients after liver transplantation. Sci Rep 2015;5:11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu SL, Chen J, Li H et al. Efficacy of hepatic resection for huge (≥10 cm) hepatocellular carcinoma: Good prognosis associated with the uninodular subtype. Int J Clin Exp Med 2015;8:20581–20588. [PMC free article] [PubMed] [Google Scholar]
- 5. Tandon P, Garcia‐Tsao G. Prognostic indicators in hepatocellular carcinoma: A systematic review of 72 studies. Liver Int 2009;29:502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fan J, Zhou J, Wu ZQ et al. Efficacy of different treatment strategies for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol 2005;11:1215–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luo J, Guo RP, Lai EC et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: A prospective comparative study. Ann Surg Oncol 2011;18:413–420. [DOI] [PubMed] [Google Scholar]
- 8. Peng ZW, Guo RP, Zhang YJ et al. Hepatic resection versus transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with portal vein tumor thrombus. Cancer 2012;118:4725–4736. [DOI] [PubMed] [Google Scholar]
- 9. Okuda K, Ohtsuki T, Obata H et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer 1985;56:918–928. [DOI] [PubMed] [Google Scholar]
- 10. Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin Liver Dis 1999;19:329–338. [DOI] [PubMed] [Google Scholar]
- 11.A new prognostic system for hepatocellular carcinoma: A retrospective study of 435 patients: The Cancer of the Liver Italian Program (CLIP) investigators . Hepatology 1998;28:751–755. [DOI] [PubMed] [Google Scholar]
- 12. Leung TW, Tang AM, Zee B et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: A study based on 926 patients. Cancer 2002;94:1760–1769. [DOI] [PubMed] [Google Scholar]
- 13. Kudo M, Chung H, Haji S et al. Validation of a new prognostic staging system for hepatocellular carcinoma: The JIS score compared with the CLIP score. Hepatology 2004;40:1396–1405. [DOI] [PubMed] [Google Scholar]
- 14. Cho CS, Gonen M, Shia J et al. A novel prognostic nomogram is more accurate than conventional staging systems for predicting survival after resection of hepatocellular carcinoma. J Am Coll Surg 2008;206:281–291. [DOI] [PubMed] [Google Scholar]
- 15. Li J, Liu Y, Yan Z et al. A nomogram predicting pulmonary metastasis of hepatocellular carcinoma following partial hepatectomy. Br J Cancer 2014;110:1110–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Y, Li J, Xia Y et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013;31:1188–1195. [DOI] [PubMed] [Google Scholar]
- 17. Sun HC, Zhang W, Qin LX et al. Positive serum hepatitis B e antigen is associated with higher risk of early recurrence and poorer survival in patients after curative resection of hepatitis B‐related hepatocellular carcinoma. J Hepatol 2007;47:684–690. [DOI] [PubMed] [Google Scholar]
- 18. Vickers AJ, Elkin EB. Decision curve analysis: A novel method for evaluating prediction models. Med Decis Making 2006;26:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ang SF, Ng ES, Li H et al. The Singapore Liver Cancer Recurrence (SLICER) Score for relapse prediction in patients with surgically resected hepatocellular carcinoma. PLoS One 2015;10:e0118658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shim JH, Jun MJ, Han S et al. Prognostic nomograms for prediction of recurrence and survival after curative liver resection for hepatocellular carcinoma. Ann Surg 2015;261:939–946. [DOI] [PubMed] [Google Scholar]
- 21. Bochner BH, Kattan MW, Vora KC. Postoperative nomogram predicting risk of recurrence after radical cystectomy for bladder cancer. J Clin Oncol 2006;24:3967–3972. [DOI] [PubMed] [Google Scholar]
- 22.European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL‐EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol 2012;56:908–943. [DOI] [PubMed] [Google Scholar]
- 23. Wang T, Hu HS, Feng YX et al. Characterisation of a novel cell line (CSQT‐2) with high metastatic activity derived from portal vein tumour thrombus of hepatocellular carcinoma. Br J Cancer 2010;102:1618–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol 2006;12:7561–7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou L, Rui JA, Wang SB et al. Clinicopathological predictors of poor survival and recurrence after curative resection in hepatocellular carcinoma without portal vein tumor thrombosis. Pathol Oncol Res 2015;21:131–138. [DOI] [PubMed] [Google Scholar]
- 26. Tung‐Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg 2000;232:10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jun L, Zhenlin Y, Renyan G et al. Independent factors and predictive score for extrahepatic metastasis of hepatocellular carcinoma following curative hepatectomy. The Oncologist 2012;17:963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fu YP, Yi Y, Cai XY et al. Overexpression of interleukin‐35 associates with hepatocellular carcinoma aggressiveness and recurrence after curative resection. Br J Cancer 2016;114:767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schütte K, Bornschein J, Malfertheiner P. Hepatocellular carcinoma–Epidemiological trends and risk factors. Dig Dis 2009;27:80–92. [DOI] [PubMed] [Google Scholar]
- 30. Wang CC, Iyer SG, Low JK et al. Perioperative factors affecting long‐term outcomes of 473 consecutive patients undergoing hepatectomy for hepatocellular carcinoma. Ann Surg Oncol 2009;16:1832–1842. [DOI] [PubMed] [Google Scholar]
- 31. Chen VL, Le AK, Kim NG et al. Effects of cirrhosis on short‐ and long‐term survival of patients with hepatitis B‐related hepatocellular carcinoma. Clin Gastroenterol Hepatol 2016;14:887–895.e1. [DOI] [PubMed] [Google Scholar]
- 32. Rich N, Singal AG. Hepatocellular carcinoma tumour markers: Current role and expectations. Best Pract Res Clin Gastroenterol 2014;28:843–853. [DOI] [PubMed] [Google Scholar]
- 33. Johnson PJ. The role of serum alpha‐fetoprotein estimation in the diagnosis and management of hepatocellular carcinoma. Clin Liver Dis 2001;5:145–159. [DOI] [PubMed] [Google Scholar]
- 34. Singhal A, Jayaraman M, Dhanasekaran DN et al. Molecular and serum markers in hepatocellular carcinoma: Predictive tools for prognosis and recurrence. Crit Rev Oncol Hematol 2012;82:116–140. [DOI] [PubMed] [Google Scholar]
- 35. Tsutsumi M, Sakamuro D, Takada A et al. Detection of a unique gamma‐glutamyl transpeptidase messenger RNA species closely related to the development of hepatocellular carcinoma in humans: A new candidate for early diagnosis of hepatocellular carcinoma. Hepatology 1996;23:1093–1097. [DOI] [PubMed] [Google Scholar]
- 36. Jeng KS, Sheen IS, Tsai YC. Gamma‐glutamyl transpeptidase messenger RNA may serve as a diagnostic aid in hepatocellular carcinoma. Br J Surg 2001;88:986–987. [DOI] [PubMed] [Google Scholar]
- 37. Carr BI, Pancoska P, Branch RA. Significance of increased serum GGTP levels in HCC patients. Hepatogastroenterology 2010;57:869–874. [PubMed] [Google Scholar]
- 38. Hanigan MH. Gamma‐glutamyl transpeptidase: Redox regulation and drug resistance. Adv Cancer Res 2014;122:103–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carr BI, Guerra V. Hepatocellular carcinoma size: Platelets, gamma‐glutamyl transpeptidase, and alkaline phosphatase. Oncology 2013;85:153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]