This study aimed to determine the lowest safe starting doses observed in previously published phase I, II, and III adult oncology clinical protocols to help avoid excessive toxicity when dosing of de novo three‐drug combinations involving a targeted agent in clinical trials and practice.
Keywords: Targeted therapy, Cytotoxic chemotherapy, Maximum tolerated dose, Recommended phase II dose, Precision medicine
Abstract
Background.
Combining targeted and cytotoxic agents has the potential to improve efficacy and attenuate resistance for metastatic cancer. Information regarding safe starting doses for clinical trials of novel three‐drug combinations is lacking.
Materials and Methods.
Published phase I–III adult oncology clinical trials of three‐drug combinations involving a targeted agent were identified by PubMed search (January 1, 2010 to December 31, 2013). A dose percentage was calculated to compare the dose used in combination to the single agent recommended dose: (U.S. Food and Drug Administration‐approved/recommended phase II dose/maximum tolerated dose). The additive dose percentage was the sum of the dose percentages for each drug in the combination.
Results.
A total of 37,763 subjects and 243 drug combinations were included. Only 28% of studies could give each of the three agents at 100%. For combinations involving two targeted agents and a cytotoxic agent, the lowest starting additive dose percentage was 133%, which increased to 250% if two antibodies were included. For combinations of one targeted agent and two cytotoxic agents, the lowest additive safe dose percentage was 137%. When both cytotoxic agents were held at 100%, as occurred in 56% of studies (which generally used cytotoxic doublets with known combination safety dosing), the lowest safe dose percentage was 225% (providing that a histone deacetylase inhibitor was not the targeted agent).
Conclusion.
These findings serve as a safe starting point for dosing novel three‐drug combinations involving a targeted agent in clinical trials and practice. The Oncologist 2017;22:576–584
Implications for Practice.
Targeted and cytotoxic drug combinations can improve efficacy and overcome resistance. More knowledge of safe starting doses would facilitate use of combinations in clinical trials and practice. Analysis of 37,763 subjects (243 combinations) showed three drugs could be safely administered, but less than 30% of combinations could include all three drugs at full dose. Dose reductions to 45% of the dose of each single agent may be required. Combinations involving two antibodies required fewer dose reductions, and the use of established cytotoxic doublets made initial dose assignment easier.
Introduction
Combination therapy involving cytotoxic and targeted agents represents a potential approach to improve efficacy and overcome resistance in metastatic cancer. In recent years, better outcomes have been seen with the incorporation of targeted agents such as bevacizumab, rituximab, and trastuzumab into standard cytotoxic chemotherapy regimens [1], [2], [3], [4]. Most malignancies are characterized by multiple genetic alterations, and biologic heterogeneity can exist within the same histologic type or even within the individual [5], [6], [7], [8], [9], [10], [11]. Prior studies have suggested that directing therapy to match specific molecular alterations will lead to better outcomes [12], [13], [14], with additional large multi‐center studies ongoing [15]. Thus it is rational to believe that it would be beneficial to target multiple pathways concurrently with a therapy optimized to the unique biology of each individual cancer.
There are several algorithms that are the basis for deciding initial doses in phase I trials. As an example, one approach utilizes one tenth of the LD10 in mice (the dose that results in 10% lethality) as a starting dose. However, if toxicology studies in nonrodent species show significant toxicity and/or limited lethality, one sixth to one third of the lowest dose that results in toxicity (toxic dose low (TDL)) is used as the starting dose [16]. These guidelines have been applied to cytotoxic chemotherapy; however appropriate rules and models to use for first‐in‐human studies of molecularly targeted agents are less clear [17]. While many drugs used in combination therapy have previously been tested in clinical trials as single agents and/or have reached approval, the initial starting doses for targeted and cytotoxic agents in a novel combination therapy trial are also unclear. The current study aimed to determine the lowest safe starting doses observed in previously published phase I, II, and III adult oncology clinical protocols in order to help avoid excessive toxicity when initially administering de novo three drug combinations involving a targeted agent in clinical trials and practice.
Materials and Methods
Identification of Studies
Three‐drug combinations of targeted and cytotoxic agents in which at least one targeted agent was included were identified by a PubMed search restricted to clinical trials with the search terms “cancer,” “phase,” “combination,” or “combined.” The studies were limited to adult Phase I, II, and III oncology clinical trials published between January 1, 2010 and December 31, 2013. Cytotoxic agents are chemical substances that kill rapidly dividing cells but have the potential to harm rapidly dividing healthy tissue. In contrast, a targeted agent is a molecule designed to impact cancer cell signals that differ in expression or function between normal and tumor tissue. These agents are often cytostatic and can include antibodies targeting a specific protein or small molecule inhibitors with a low IC50 for a specific protein (concentration producing 50% enzyme inhibition). Studies of pediatric patients, exclusively elderly patients, organ dysfunction patients, hormonal therapy, immunotherapy, and radiation were excluded from the analysis (Fig. 1).
Figure 1.
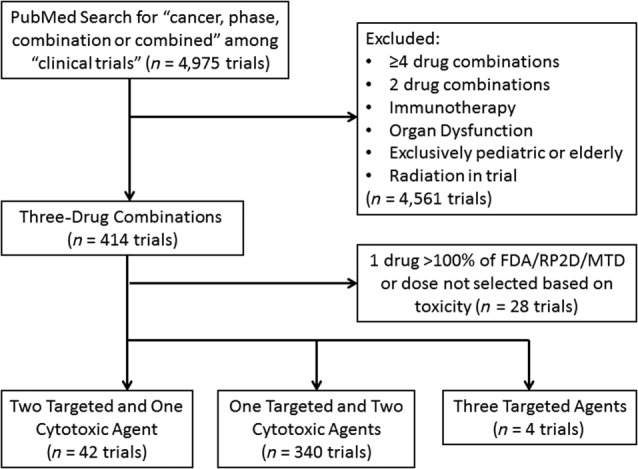
Consort diagram for three‐drug combinations. Articles identified from the PubMed search were screened to identify three‐drug combinations of cytotoxic and targeted agents that included at least one targeted agent. Studies including immunotherapy, radiation, organ dysfunction (renal and liver failure), pediatric patients, exclusively elderly patients, or those administering one drug of the combination at greater than the FDA‐approved/RP2D/MTD dose were excluded from the analysis.
Data Analysis and Definition of Additive Dose Percentage
Each publication was reviewed to determine the drugs used in combination, cancer evaluated, number of subjects, dose of drug, dose‐limiting toxicities, grade 3 or 4 toxicities, and, for phase I studies, the recommended phase II dose (RP2D) or maximum tolerated dose (MTD). Each unique combination was considered a separate study for trials that tested more than one drug combination. The “dose percentage” was defined as the safe dose of drug compared to the single agent recommended dose: (safe dose of drug in combination/dose of drug as a single agent at U.S. Food and Drug Administration (FDA)‐approved dose or RP2D or MTD) X 100. An FDA‐approved dose was used for approved drugs, and RP2D was given priority over MTD for non‐FDA‐approved drugs. Studies with dose percentages greater than 100% were excluded from the analysis. The dose percentage for each drug was added together to give the “additive dose percentage.” The maximum additive dose percentage was 300% (100% of each drug). If a study administered the drugs at lower than an established safe dose for the three‐drug combination or if a dose was not chosen based on toxicity, the combination was not considered in determining the lowest additive dose (and the study was not considered in our analysis). For agents with a range of standard doses and schedules, the average weekly dose for each dosing schedule was calculated to determine the range of average weekly doses. A study dose falling within the standard dose range was considered to be a dose percentage of 100%, whereas a dose falling below the range was compared to the lowest average weekly dose to determine the dose percentage.
Results
Two Cytotoxic Agents and One Targeted Agent
Three hundred forty studies were identified that combined a targeted agent with two cytotoxic agents (205 drug combinations; 34,835 subjects; supplemental online Appendix references 1–297; Table 1; supplemental online Table 1). The median (range) for the combined dose (additive dose percentage) was 267% (137%–300%) of the additive FDA/RP2D/MTD dose. In only 28% of the total 340 studies (59 drug combinations; n = 11,235 total subjects) could all three drugs be given at 100% of the FDA/RP2D/MTD dose.
Table 1. Three‐drug combinations (one targeted agent with two cytotoxic agents) reported over 4 yearsa.
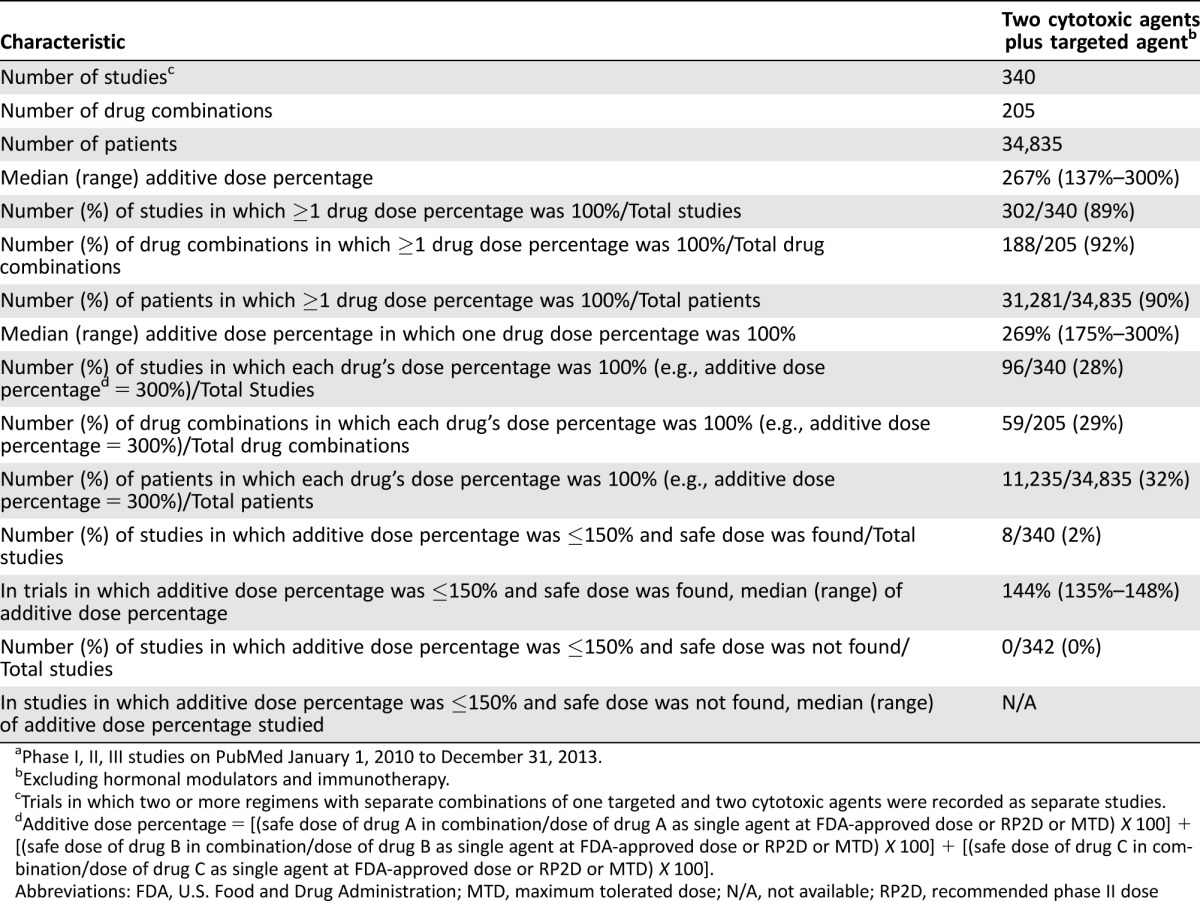
Phase I, II, III studies on PubMed January 1, 2010 to December 31, 2013.
Excluding hormonal modulators and immunotherapy.
Trials in which two or more regimens with separate combinations of one targeted and two cytotoxic agents were recorded as separate studies.
Additive dose percentage = [(safe dose of drug A in combination/dose of drug A as single agent at FDA‐approved dose or RP2D or MTD) X 100] + [(safe dose of drug B in combination/dose of drug B as single agent at FDA‐approved dose or RP2D or MTD) X 100] + [(safe dose of drug C in combination/dose of drug C as single agent at FDA‐approved dose or RP2D or MTD) X 100].
Abbreviations: FDA, U.S. Food and Drug Administration; MTD, maximum tolerated dose; N/A, not available; RP2D, recommended phase II dose
Two Cytotoxics and One Targeted Agent with at Least One Drug at 100% Dose Percentage of the FDA/RP2D/MTD Dose
Three hundred two studies (188 drug combinations; 31,281 subjects) were published in which one of the agents was administered at full dose (100% dose percentage) (supplemental online Appendix references 1–269; Table 2). These included 73 phase I studies (n = 1,798 subjects), 220 Phase II or III studies (n = 29,059 subjects), and 9 phase I/II combined studies (n = 424 subjects). (Only a subset of subjects were treated at the FDA/RP2D/MTD dose in phase I studies.) For studies in which a safe dose was found, 34% of studies (62 out of 180 studies) including an antibody as the targeted agent could administer all three drugs at 100% as compared to 28% (34 out of 122 studies) in which the targeted agent was a small molecule. If one drug was set at 100%, then in 32% of studies overall (96/302), all three drugs could be administered at full dose.
Starting with One Cytotoxic at Full Dose.
At least one of the cytotoxic agents was given at 100% of the FDA/RP2D/MTD dose in 292 studies (177 combinations; n = 30,924 subjects). The median (range) additive dose percentage was 275% (175%–300%). The lowest safe additive dose percentage was 175% (combination of gemcitabine, with carboplatin and iniparib [100%, 38%, and 37%, respectively]; supplemental online Appendix reference 228).
Starting with Two Cytotoxics at Full Dose
Both cytotoxic agents were given at 100% of the FDA/RP2D/MTD dose in 190 studies (117 combinations; n = 22,454 subjects). In general, in these studies, the combination of two cytotoxics had previously been shown to be tolerated at 100% of the full dose for each. The median (range) additive dose percentage was 300% (225%–300%). The lowest safe additive dose percentage was 225%, which was given for the following: (a) everolimus with etoposide and cisplatin (supplemental online Appendix reference 123), (b) pazopanib with paclitaxel and carboplatin (supplemental online Appendix reference 169), and (c) sorafenib with idarubicin and cytarabine (supplemental online Appendix reference 170); however, a safe dose was not found for vorinostat with carboplatin and paclitaxel (25%, 100%, 100%, respectively; additive 225%; (supplemental online Appendix reference 297). Thus, the lowest safe additive dose percentage required when both cytotoxics were held at 100% was 225%, providing that a histone deacetylase inhibitor was not the targeted agent (Table 2).
Table 2. Summary of three drugs (one targeted and two cytotoxics) in combinationa.
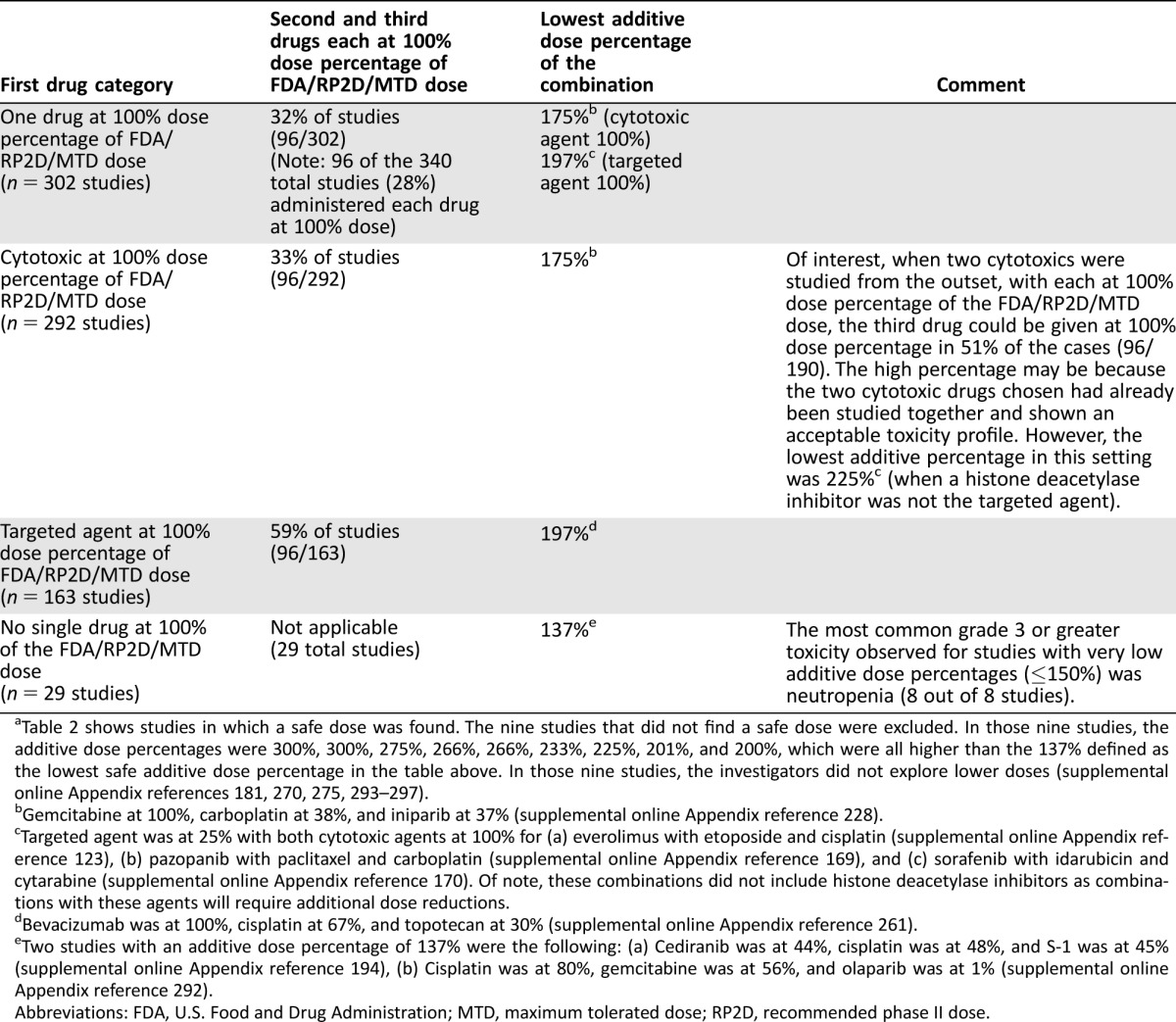
Table 2 shows studies in which a safe dose was found. The nine studies that did not find a safe dose were excluded. In those nine studies, the additive dose percentages were 300%, 300%, 275%, 266%, 266%, 233%, 225%, 201%, and 200%, which were all higher than the 137% defined as the lowest safe additive dose percentage in the table above. In those nine studies, the investigators did not explore lower doses (supplemental online Appendix references 181, 270, 275, 293–297).
Gemcitabine at 100%, carboplatin at 38%, and iniparib at 37% (supplemental online Appendix reference 228).
Targeted agent was at 25% with both cytotoxic agents at 100% for (a) everolimus with etoposide and cisplatin (supplemental online Appendix reference 123), (b) pazopanib with paclitaxel and carboplatin (supplemental online Appendix reference 169), and (c) sorafenib with idarubicin and cytarabine (supplemental online Appendix reference 170). Of note, these combinations did not include histone deacetylase inhibitors as combinations with these agents will require additional dose reductions.
Bevacizumab was at 100%, cisplatin at 67%, and topotecan at 30% (supplemental online Appendix reference 261).
Two studies with an additive dose percentage of 137% were the following: (a) Cediranib was at 44%, cisplatin was at 48%, and S‐1 was at 45% (supplemental online Appendix reference 194), (b) Cisplatin was at 80%, gemcitabine was at 56%, and olaparib was at 1% (supplemental online Appendix reference 292).
Abbreviations: FDA, U.S. Food and Drug Administration; MTD, maximum tolerated dose; RP2D, recommended phase II dose.
Starting with the Targeted Agent at Full Dose
The targeted agent was given at 100% of the FDA/RP2D/MTD dose for 163 studies (106 combinations; n = 16,052 subjects). The median (range) additive dose percentage was 300% (197%–300%). The lowest safe additive dose percentage was 197% (combination of bevacizumab with cisplatin and topotecan [100%, 67%, and 30%, respectively]; supplemental online Appendix reference 261; Table 2).
Two Cytotoxics and One Targeted Agent with Additive Dose Percentage ≤150%
Eight studies were published in which the additive dose percentage was 150% or less. These studies included the following (individual dose percentages [additive dose percentage]): rituximab with fludarabine and mitoxantrone (25%, 60%, 63% [148%]; supplemental online Appendix references 287 and 288), gefitinib with paclitaxel and irinotecan (33%, 91%, 24% [148%]; supplemental online Appendix reference 289), alemtuzumab with fludarabine and cyclophosphamide (17%, 60%, 69% [146%]; supplemental online Appendix reference 290), rituximab with cyclophosphamide and pentostatin (33%, 86%, 25% [144%]; supplemental online Appendix reference 281), rituximab with fludarabine and cyclophosphamide (33%, 60%, 45% [138%]; supplemental online Appendix reference 291), olaparib with cisplatin and gemcitabine (1%, 80%, 56% [137%]; supplemental online Appendix reference 292), cediranib with cisplatin, and S‐1 (oral fluorouracil; 44%, 48%, 45% [137%]; supplemental online Appendix reference 194). The lowest safe additive dose percentage was 137% as was seen for cediranib with cisplatin and S‐1 (oral fluorouracil), and olaparib with cisplatin and gemcitabine.
Combinations of Two Cytotoxics and One Targeted Agent in Which the Safety of the Combination Dose Was Unacceptable
There were nine studies published in which the additive dose was >150% and no safe dose was found and included (individual dose percentages [additive dose percentage]): cediranib with carboplatin and paclitaxel (100%, 100%, 100% [300%]; supplemental online Appendix reference 293); bevacizumab with epirubicin and docetaxel (100%, 100%, 100% [300%]; supplemental online Appendix reference 294); sunitinib with cisplatin and gemcitabine (75%, 100%, 100% [275%] supplemental online Appendix reference 270); bevacizumab with docetaxel and capecitabine (100%, 100%, 66% [266%]; supplemental online Appendix references 181 and 295); vandetanib with cisplatin and gemcitabine (33%, 100%, 100% [233%]; supplemental online Appendix reference 296); vorinostat with carboplatin and paclitaxel (25%, 100%, 100% [225%]; supplementary online Appendix 297); alemtuzumab with fludarabine and cyclophosphamide (25%, 96%, 80% [201%]; supplemental online Appendix reference 275); and vandetanib with cisplatin and vinorelbine (33%, 100%, 67% [200%]; supplemental online Appendix reference 296).
These studies did not attempt lower doses. All studies that lowered the additive dose to ≤150% found a safe dose.
Two Targeted and One Cytotoxic Agent
Forty‐two studies combined two targeted agents with a cytotoxic agent (2,824 subjects; 34 drug combinations; supplemental online Appendix references 78, 157, and 298–337; Table 3; supplemental online Table 1). The median (range) for the combined dose was 263% (133%–300%) of the additive FDA/RP2D/MTD dose. For studies in which an acceptable dose was found, combinations of two antibodies as the targeted agents had a median (range) additive dose percentage of 300% (250%–300%; n = 10) as compared to 255% (133%–300%; n = 28) when a small molecule was included as at least one of the targeted agents.
Table 3. Three‐drug combinations (two targeted agents with one cytotoxic agent) reported over 4 yearsa.
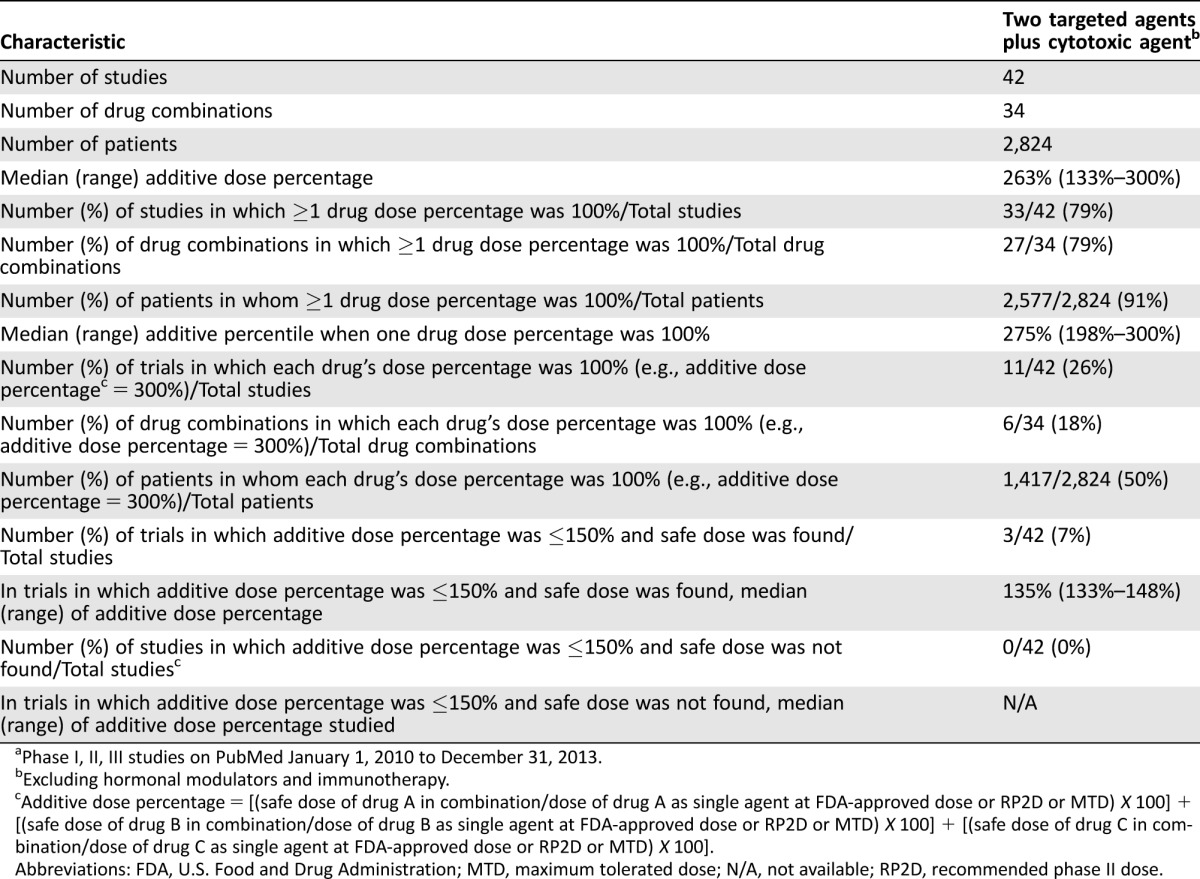
Phase I, II, III studies on PubMed January 1, 2010 to December 31, 2013.
Excluding hormonal modulators and immunotherapy.
Additive dose percentage = [(safe dose of drug A in combination/dose of drug A as single agent at FDA‐approved dose or RP2D or MTD) X 100] + [(safe dose of drug B in combination/dose of drug B as single agent at FDA‐approved dose or RP2D or MTD) X 100] + [(safe dose of drug C in combination/dose of drug C as single agent at FDA‐approved dose or RP2D or MTD) X 100].
Abbreviations: FDA, U.S. Food and Drug Administration; MTD, maximum tolerated dose; N/A, not available; RP2D, recommended phase II dose.
Table 4. Summary of three drugs (two targeted and one cytotoxic) in combinationa.
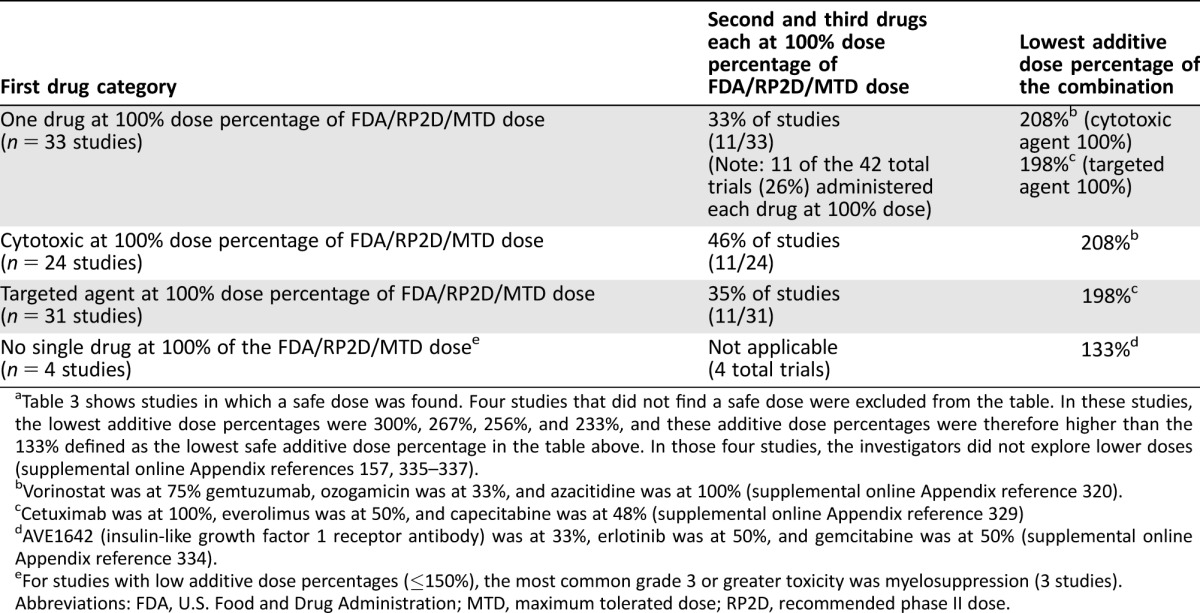
Table 3 shows studies in which a safe dose was found. Four studies that did not find a safe dose were excluded from the table. In these studies, the lowest additive dose percentages were 300%, 267%, 256%, and 233%, and these additive dose percentages were therefore higher than the 133% defined as the lowest safe additive dose percentage in the table above. In those four studies, the investigators did not explore lower doses (supplemental online Appendix references 157, 335–337).
Vorinostat was at 75% gemtuzumab, ozogamicin was at 33%, and azacitidine was at 100% (supplemental online Appendix reference 320).
Cetuximab was at 100%, everolimus was at 50%, and capecitabine was at 48% (supplemental online Appendix reference 329)
AVE1642 (insulin‐like growth factor 1 receptor antibody) was at 33%, erlotinib was at 50%, and gemcitabine was at 50% (supplemental online Appendix reference 334).
For studies with low additive dose percentages (≤150%), the most common grade 3 or greater toxicity was myelosuppression (3 studies).
Abbreviations: FDA, U.S. Food and Drug Administration; MTD, maximum tolerated dose; RP2D, recommended phase II dose.
Two Targeted and One Cytotoxic Agent and One Drug at 100% Dose Percentage of the FDA/RP2D/MTD Dose
Thirty‐three studies (including 27 drug combinations) were published (n = 2,577 subjects) in which one of the three agents in the combination were administered at full dose (100% of the FDA/RP2D/MTD dose; supplemental online Appendix references 78, 298–329; Table 2). These included 13 phase I studies (n = 448 subjects), 16 Phase II or III studies (n = 1,947 subjects), and 4 phase I/II combined studies (n = 182 patients). In total, in 11 studies (26% of the 42 studies), all three drugs were administered at 100% of the FDA/RP2D/MTD dose (n = 6 drug combinations; 1,417 subjects). All 11 studies included an antibody as one of the targeted agents. For studies in which an acceptable dose was found, combinations that included two antibodies were associated with administration of all three drugs at 100% of the FDA/RP2D/MTD dose in 60% of studies (n = 6 out of 10 studies; 3 out of 7 drug combinations with all three drugs at 100%), as compared to 18% (n = 5 of 28 studies; 3 out of 24 combinations) when a small molecule was included in the combination.
Starting with the Cytotoxic at Full Dose
Twenty‐four studies (including 18 drug combinations) were published (n = 2,193 subjects) in which the cytotoxic agent was given at 100% of the FDA/RP2D/MTD dose with a median (range) additive dose percentage of 272% (208%–300%). The lowest safe dose was determined to be 208% for vorinostat, gemtuzumab ozogamicin, and azacitidine (75%, 33%, 100%, respectively; supplemental online Appendix reference 320; Table 2).
Starting with One Targeted Agent at Full Dose
Thirty‐one studies (including 25 drug combinations) were published (n = 2,467 subjects) in which the targeted agent was given at 100% of the FDA/RP2D/MTD dose with a median (range) additive dose percentage of 275% (198%–300%). The lowest safe additive dose was 198% for the combination of cetuximab with everolimus and capecitabine (100%, 50%, and 48% dose percentage, respectively; supplemental online Appendix reference 329; Table 2).
No Single Drug at 100% of the FDA/RP2D/MTD Dose
There were five studies (n = 5 drug combinations) in which no single drug was at 100% of the FDA/RP2D/MTD dose due to toxicity of higher doses (supplemental online Appendix references 330–334). Three of these studies had an additive dose percentage of less than 150% and included the following therapies (individual dose percentages [additive dose percentage]): rituximab with alvocidib and cyclophosphamide (70%, 25%, 53% [148%]; supplemental online Appendix reference 332), rituximab with lenalidomide and bendamustine (25%, 40%, 70% [135%]; supplemental online Appendix reference 333), and AVE1642 (insulin‐like growth factor 1 receptor antibody) with erlotinib and gemcitabine (33%, 50%, 50% [133%]; supplemental online Appendix reference 334; Table 2).
Combinations in Which the Safety of the Combination Dose Was Unacceptable
There were four studies published in which the additive dose was >150% and the studies did not find an acceptable dose (individual dose percentages [additive dose percentage]): trastuzumab, bevacizumab, and vinorelbine (100%, 100%, 100% [300%]; supplemental online Appendix reference 335), cediranib, cetuximab, and irinotecan (67%, 100%, 100% [267%]; supplemental online Appendix reference 157), lapatinib, trastuzumab, and paclitaxel (56%, 100%, 100% [256%]; supplemental online Appendix reference 336), and sunitinib, bevacizumab, and paclitaxel (56%, 100%, 77% [233%]; supplemental online Appendix reference 337). Lower doses were not attempted.
Three Targeted Agents
Four studies assessed three targeted agents in combination therapy and their additive dose percentages were 201%, 300%, 269%, and 183%. All studies were phase I clinical trials and encompassed a total of 104 subjects. The targeted agents and their dose percentages included the following (individual dose percentages [additive dose percentage]): bevacizumab with panitumumab and everolimus (100%, 80%, 21% [201%]; supplemental online Appendix reference 338), bevacizumab with cextuximab and erlotinib (100%, 100%, 100% [300%]; supplemental online Appendix reference 339), and bevacizumab with trastuzumab and lapatinib (100%, 100%, 69% [269%]; supplemental online Appendix reference 340). The combination of bevacizumab with panobinostat and everolimus did not identify a safe dose (100%, 50%, 33% [183%]; supplemental online Appendix reference 341); however, lower doses were not attempted.
Discussion
The molecular heterogeneity of metastatic malignancy necessitates the use of multiple agents to optimize efficacy and minimize resistance. While significant responses have been seen with single agent targeted therapy [10], [18], [19], metastatic cancers generally develop resistance within a few months, as they mostly harbor multiple genomic alterations [20], [21], [22], [23]. Prior combination therapy efforts have focused on cytotoxic agents; however, with the advent of molecular profiling and development of targeted agents to block specific pathways driving cell proliferation and survival, there is great potential for improved therapeutic outcomes by incorporating antibodies and small molecule inhibitors in therapeutic regimens. The addition of the monoclonal antibodies bevacizumab to colon cancer therapy regimens, rituximab to non‐Hodgkin's lymphoma regimens, and trastuzumab to breast cancer chemotherapy combinations has led to improved outcomes with minimal excess toxicity [1], [2], [3], [4]. For some cancers (childhood leukemia, Hodgkin disease) and for non‐cancer illnesses such as acquired immunodeficiency syndrome, combination therapy has proven to be curative or to result in long‐term disease control. However, determining safe starting doses for novel therapeutic anticancer combinations in clinical trials can be challenging.
The aim of the current study was to determine the lowest safe starting doses previously observed for three‐drug combinations involving a targeted agent in order to help avoid excessive toxicity with drug combinations for phase I clinical trials. We evaluated 386 previously published phase I–III clinical trials (n = 37,763 subjects). In our prior study of two‐drug combinations involving a targeted and cytotoxic agent (n = 24,326 patients) [24], we found that more significant dose reductions were required to achieve safe doses as compared to combinations of two targeted agents (n = 8,568 patients assessed) [25]. Only 38% of studies were able to administer both the targeted and cytotoxic drug at 100% dose percentage, while 51% of two targeted agents could be administered at full dose. One would assume that, with three‐drug combinations, two targeted agents and a cytotoxic agent would be better tolerated than a targeted agent combined with two cytotoxic agents. However, in 28% of studies with a targeted agent combined with two cytotoxic agents, all three drugs could be given at 100% dose percentage (for each drug) as compared to 26% for two targeted agents and a cytotoxic agent. The lowest safe dose percentages were similar between the two groups (137% vs. 133%).
We found that both cytotoxic agents were given at 100% of the FDA‐approved dose/RP2D/MTD dose in 56% of studies for combinations involving a targeted agent with two cytotoxic agents. The lowest safe dose percentage for the targeted agent was 25% for these studies (providing no histone deacetylase inhibitor was used as the targeted agent—with the latter, the lowest safe dose percentage was not defined). Given the toxicity associated with cytotoxic agents, many three‐drug combination studies presumably added a targeted agent onto a known, well‐tolerated two‐drug cytotoxic chemotherapy combination. This strategy may provide the best chance of minimizing toxicity for a novel three‐drug combination involving two cytotoxic agents.
For combinations of two targeted agents with a cytotoxic agent, two antibody combinations were significantly less toxic and could be given at higher doses. The lowest additive dose percentage was 250%. This is similar to what was observed previously for two‐drug combinations: inclusion of a small molecule inhibitor necessitated greater dose reductions for toxicity than when two antibodies were given. Indeed, two small molecule inhibitors required the greatest dose reduction, a small molecule inhibitor and an antibody was next, and the best tolerated was two antibodies [24], [25]. In contrast, for combinations of one targeted agent with two cytotoxic agents, the lowest additive dose percentages and number of combinations that could be given at 100% were similar regardless of whether a small molecule or antibody was used in the combination.
Studies of three targeted agent combinations were few in the literature; only four studies were found during the time period (years 2010–2013) delineated for this analysis (supplemental online Appendix references 338–341). In these triplet targeted agent studies, the additive dose percentage tolerable ranged between 201% and 300% in the three studies in which a safe dose was defined; it was 183% in the fourth study, and this dose was too high (but the investigators did not explore lower doses). Additional information and studies will be needed to determine the factors affecting safe dosing of three targeted agents. Our prior studies, as well as more recent papers on two‐drug combinations of targeted therapies [25], [26], found that combinations involving overlapping drug targets, the use of mTOR inhibitors (especially in the presence of other survival pathway inhibitors such as MEK inhibitors), and combinations of small molecule inhibitors would require dose reduction in de novo combinations.
Toxicity has been a significant concern in prior clinical trials of classic cytotoxic chemotherapy combinations; the focus of the current work was to determine the lowest safe starting doses necessary to avoid excessive toxicity for de novo combinations involving at least one targeted agent. The most significant dose reductions in the current study (additive dose percentage ≤150% [which was seen in 11 studies]) were likely due to overlapping toxicity between two cytotoxic agents or between the targeted agent (i.e., PARP inhibitor or lenalidomide) and a cytotoxic agent, as myelosuppression was the most commonly observed grade 3 or greater toxicity in all 11 studies. However, with over 300 anti‐cancer drugs approved by the FDA or in advanced clinical trials, there are approximately 4.5 million possible three‐drug combinations. It is therefore important to have some guidance for safe starting points for new drug combinations. Given that doses below the FDA‐approved/RP2D/MTD were required for over 70% of patients, it can be anticipated that reduced doses will be necessary to avoid toxicity in the majority of triplets. It is unclear if dose reductions of targeted agents will alter efficacy in the same manner that it does for cytotoxics [27], [28]. Regardless, since over 25% of patients were able to receive all three drugs at 100% of the single‐agent dose, inter‐patient and intra‐patient dose escalation of varying degrees should be possible for many combinations. In patients with strong driver mutations such as BCR‐ABL and BRCA, prioritizing dose escalation of the associated targeted agent can be considered to provide enhanced efficacy [29].
Standard of care for patients with multiple medical issues outside of oncology involves combining medications based on algorithms. Patients with advanced cancer are on a median of eight drugs for other health problems prior to starting treatment for malignancy [30]. Patients in the intensive care unit are on even greater numbers of concurrent medications, with prior studies showing an average of 10.5 to 14.6 prescriptions per patient [31], [32], [33]. Thus, it may be possible to use information such as that in the current study and an understanding of renal function, liver function, and drug metabolism to develop algorithms that will help guide safe starting doses for de novo combination therapy involving targeted agents for clinical practice in addition to clinical trials.
The current study is limited, as it was restricted to three‐drug combinations in adult patients with adequate renal or hepatic function. Thus, one cannot extrapolate the data to the dose adjustments that may be needed for elderly patients, children, or those with organ dysfunction. The pharmacology of drugs including the effects of drug‐drug interactions and pharmacogenomic variants in metabolism was not considered. (Some of this data is available through specific websites [https://www.drugs.com/interactions-check.php?drug_list=377-0,3126-0; http://reference.medscape.com/drug-interactionchecker].) Furthermore, clinical trials with significant toxicity may not have been published, leading to publication bias; thus, although the study represented a large number of subjects and drug combinations, it may not be representative of all drug combinations. Combinations involving two cytotoxic agents often used a known safe cytotoxic combination; thus, the study cannot fully assess de novo combinations of a targeted agent with two cytotoxic agents. The investigators of some of the studies may have held one or more drugs at a preconceived dosing level; therefore, higher doses may have been possible for some combinations. Given that the study included phase I, II, and III clinical trials with different objectives, the dataset was heterogeneous. The current study addresses safe starting doses but does not speak to escalation schemes, which will be important for optimization of therapy and have been discussed elsewhere [34], [35]. While the study represents a large number of drug combinations, the majority of combinations were not tested in multiple organ specific studies; hence, conclusions regarding the relationship between toxicity and organ type of cancer for the different combinations cannot be made. The study also did not address target engagement, which represents the minimal amount of drug required for a full effect and the biologic and pharmacologic basis for combining drugs.
Conclusion
All studies were able to find a safe dose if the additive dose percentage was sufficiently lowered or alternative dosing schedules were attempted. The current report suggests that, for adults with intact organ function, the lowest safe starting additive dose percentage for a targeted agent combined with two cytotoxic agents was 137%, while for a well‐established two drug cytotoxic combination given at full dose, the targeted agent could be safely given if doses were lowered to 25% of the single agent dose (provided that a histone deacetylase inhibitor was not included, in which case a safe dose has not yet been defined). For combinations of two targeted agents and a single cytotoxic agent involving a small molecular inhibitor, the lowest additive dose percentage was 133%, but increased to 250% with two antibodies. The results herein are similar to our previous findings with novel two‐drug combinations: histone deacetylase inhibitors or PARP inhibitors led to significant side effects when combined with cytotoxic chemotherapy, drugs with overlapping targets were more likely to need compromised doses, mTOR inhibitors tended to compromise the doses of other targeted agents, and a safe starting dose could be found for the vast majority of other combinations, usually by lowering the starting dose of each drug to no more than about half of the FDA‐approved dose/RP2D/MTD [24], [25]. Antibodies tended to compromise dosing the least. In approximately 28% of the studies of triplet combinations, all three drugs could be given at full doses and, in many others, doses above the lowest safe additive percentage could be administered. This is similar to the findings with doublet combinations, where more combinations could be given at full doses—in the case of dual targeted doublets, approximately 51% percent could be administered at full doses as compared to 38% for targeted‐cytotoxic doublets [24], [25]. Therefore, for never‐before‐attempted combinations, even if initial doses are conservative and use the most toxic triplets described herein to define the safe additive percentage that can be used as a starting point, interpatient or intrapatient dose escalation will often be feasible. These data should provide a framework for initial dosing of triple drug combinations that include at least one targeted agent.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
This study was funded in part by the Joan and Irwin Jacobs philanthropic fund and by National Cancer Institute grant P30 CA16672. Dr. Nikanjam received salary support from a National Institutes of Health grant (4T32HL066992 – Academic Training in Hematology) and a Tower Cancer Research Foundation Career Development Award.
Author Contributions
Conception and design: Mina Nikanjam, Sariah Liu, Razelle Kurzrock
Collection and/or assembly of data: Mina Nikanjam, Sariah Liu, Razelle Kurzrock
Data analysis and interpretation: Mina Nikanjam, Sariah Liu, Jincheng Yang, Razelle Kurzrock
Manuscript writing: Mina Nikanjam, Razelle Kurzrock
Final approval of manuscript: Mina Nikanjam, Sariah Liu, Jincheng Yang, Razelle Kurzrock
Disclosures
Razelle Kurzrock: Actuate Therapeutics, Xbiotech (C/A), Genentech, Merck Serono, Pfizer, Sequenom, Foundation Medicine, Guardant (RF), CureMatch (E, OI), Casdin Capital, NXT Health, Xbiotech, Health Advances (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Averbuch SD, Wolf MK, El‐Rayes BF et al. Clinical development issues. In: Prendergast GC, ed. Molecular Cancer Therapeutics: Strategies for Drug Discovery and Development. Hoboken, NJ: Wiley‐LISS, 2004:287–306.
- 2. Hurwitz H, Fehrenbacher L, Novotny W et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Eng J Med 2004;350:2335–2342. [DOI] [PubMed] [Google Scholar]
- 3. Sehn LH, Donaldson J, Chhanabhai M et al. Introduction of combined chop plus rituximab therapy dramatically improved outcome of diffuse large b‐cell lymphoma in British Columbia. J Clin Oncol 2005;23:5027–5033. [DOI] [PubMed] [Google Scholar]
- 4. Baselga J. Herceptin alone or in combination with chemotherapy in the treatment of HER2‐positive metastatic breast cancer: Pivotal trials. Oncology 2001;61(suppl 2):14–21. [DOI] [PubMed] [Google Scholar]
- 5. Kurzrock R, Giles FJ. Precision oncology for patients with advanced cancer: The challenges of malignant snowflakes. Cell Cycle 2015;14:2219–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gerlinger M, Rowan AJ, Horswell S et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012;366:883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kandoth C, McLellan MD, Vandin F et al. Mutational landscape and significance across 12 major cancer types. Nature 2013;502:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wheler JJ, Parker BA, Lee JJ et al. Unique molecular signatures as a hallmark of patients with metastatic breast cancer: Implications for current treatment paradigms. Oncotarget 2014;5:2349–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wheler J, Lee JJ, Kurzrock R. Unique molecular landscapes in cancer: Implications for individualized, curated drug combinations. Cancer Res 2014;74:7181–7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsimberidou AM, Iskander NG, Hong DS et al. Personalized medicine in a phase I clinical trials program: The MD Anderson Cancer Center initiative. Clin Cancer Res 2012;18:6373–6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morris LG, Chandramohan R, West L et al. The molecular landscape of recurrent and metastatic head and neck cancers: Insights from a precision oncology sequencing platform. JAMA Oncol: 2016. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wheler JJ, Janku F, Naing A et al. Cancer therapy directed by comprehensive genomic profiling: A single center study. Cancer Res 2016;76:3690–3701. [DOI] [PubMed] [Google Scholar]
- 13. Schwaederle M, Parker BA, Schwab RB et al. Precision oncology: The UC San Diego Moores Cancer Center PREDICT experience. Mol Cancer Ther 2016;15:743–752. [DOI] [PubMed] [Google Scholar]
- 14. Von Hoff DD, Stephenson JJ Jr, Rosen P et al. Pilot study using molecular profiling of patients' tumors to find potential targets and select treatments for their refractory cancers. J Clin Oncol 2010;28:4877–4883. [DOI] [PubMed] [Google Scholar]
- 15. Schmidt KT, Chau CH, Price DK et al. Precision oncology medicine: The clinical relevance of patient specific biomarkers used to optimize cancer treatment. J Clin Pharmacol 2016;56:1484–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for industry estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Available at http://www.Fda.Gov/downloads/drugs/…/guidances/ucm078932.Pdf. 2005. Accessed March 22, 2017.
- 17. Le Tourneau C, Stathis A, Vidal L et al. Choice of starting dose for molecularly targeted agents evaluated in first‐in‐human phase I cancer clinical trials. J Clin Oncol 2010;28:1401–1407. [DOI] [PubMed] [Google Scholar]
- 18. Shaw AT, Kim DW, Nakagawa K et al. Crizotinib versus chemotherapy in advanced ALK‐positive lung cancer. N Engl J Med 2013;368:2385–2394. [DOI] [PubMed] [Google Scholar]
- 19. Sosman JA, Kim KB, Schuchter L et al. Survival in BRAF V600‐mutant advanced melanoma treated with vemurafenib. N Engl J Med 2012;366:707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bean J, Brennan C, Shih JY et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA 2007;104:20932–20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flaherty KT, Infante JR, Daud A et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Eng J Med 2012;367:1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pao W, Miller VA, Politi KA et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2005;2:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yap TA, Omlin A, de Bono JS. Development of therapeutic combinations targeting major cancer signaling pathways. J Clin Oncol 2013;31:1592–1605. [DOI] [PubMed] [Google Scholar]
- 24. Nikanjam M, Liu S, Kurzrock R. Dosing targeted and cytotoxic two‐drug combinations: Lessons learned from analysis of 24,326 patients reported 2010 through 2013. Int J Cancer 2016;139:2135–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu S, Nikanjam M, Kurzrock R. Dosing de novo combinations of two targeted drugs: Towards a customized precision medicine approach to advanced cancers. Oncotarget 2016;7:11310–11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tolcher AW, Bendell JC, Papadopoulos KP et al. A phase IB trial of the oral MEK inhibitor trametinib (GSK1120212) in combination with everolimus in patients with advanced solid tumors. Ann Oncol 2015;26:58–64. [DOI] [PubMed] [Google Scholar]
- 27. Jain RK, Lee JJ, Hong D et al. Phase I oncology studies: Evidence that in the era of targeted therapies patients on lower doses do not fare worse. Clin Cancer Res 2010;16:1289–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gupta S, Hunsberger S, Boerner SA et al. Meta‐analysis of the relationship between dose and benefit in phase I targeted agent trials. J Natl Cancer Inst 2012;104:1860–1866. [DOI] [PubMed] [Google Scholar]
- 29. Torti D, Trusolino L. Oncogene addiction as a foundational rationale for targeted anti‐cancer therapy: Promises and perils. EMBO Mol Med 2011;3:623–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Borad MJ, Curtis KK, Babiker HM et al. The impact of concomitant medication use on patient eligibility for phase I cancer clinical trials. J Cancer 2012;3:345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alvim MM, Silva LA, Leite IC et al. Adverse events caused by potential drug‐drug interactions in an intensive care unit of a teaching hospital. Rev Bras Ter Intensiva 2015;27:353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pichala PT, Kumar BM, Zachariah S et al. An interventional study on intensive care unit drug therapy assessment in a rural district hospital in India. J Basic Clin Pharm 2013;4:64–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tavallaee M, Fahimi F, Kiani S. Drug‐use patterns in an intensive care unit of a hospital in Iran: An observational prospective study. Int J Pharm Pract 2010;18:370–376. [DOI] [PubMed] [Google Scholar]
- 34. Hamberg P, Verweij J. Phase I drug combination trial design: Walking the tightrope. J Clin Oncol 2009;27:4441–4443. [DOI] [PubMed] [Google Scholar]
- 35. Hamberg P, Ratain MJ, Lesaffre E et al. Dose‐escalation models for combination phase I trials in oncology. Eur J Cancer 2010;46:2870–2878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


