Abstract
Background
Colonic fermentation of dietary-fibre to short-chain-fatty-acids (SCFA) influences appetite hormone secretion in animals, but SCFA production is excessive in obese animals. This suggests there may be resistance to the effect of SCFA on appetite-hormones in obesity.
Objectives
to determine the effects of inulin (IN) and resistant-starch (RS) on postprandial SCFA, and gut hormone (GLP-1, PYY, and ghrelin) responses in healthy overweight/obese (OWO) vs lean (LN) humans.
Methods
Overnight fasted participants (13 OWO, 12 LN) consumed 300mL water containing 75g glucose (GLU) as Control, or 75g glucose plus 24g IN, or 28.2g RS using a randomized, single-blind, cross-over design. Blood for appetite-hormones and SCFA was collected at intervals over 6h. A standard lunch was served 4h after the test drink.
Results
Relative to GLU, IN, but not RS, significantly increased SCFA AUC from 4–6h (AUC4-6). Neither IN nor RS affected GLP-1 or PYY-AUC4-6. Although neither IN nor RS reduced ghrelin-AUC4-6 compared to GLU, ghrelin at 6h after IN was significantly lower than that after GLU (p<0.05). After IN, relative to GLU, the changes in SCFA-AUC4-6 were negatively related to the changes in ghrelin-AUC4-6 (p=0.017). SCFA and hormone responses did not differ significantly between LN and OWO.
Conclusions
Acute increases in colonic SCFA do not affect GLP-1 or PYY responses in LN or OWO subjects, but may reduce ghrelin. The results do not support the hypothesis that SCFA acutely stimulate PYY and GLP-1 secretion; however, a longer adaptation to increased colonic fermentation or a larger sample size may yield different results.
Keywords: Dietary-Fibre, Short-chain fatty acids, gut hormone, GLP 1, PYY, ghrelin, obese, microbiota
INTRODUCTION
High intakes of dietary fibre are associated with reduced weight gain (1,2). It has been suggested that this effect may be mediated by the fermentation of dietary fibre by colonic bacteria into the short-chain fatty acids (SCFA) acetate, propionate and butyrate (3). In vitro and animal studies have shown that the enteroendocrine L-cells, which are more numerous in the colon than the distal small intestine, are stimulated by SCFA via SCFA receptors (4,5) to secrete anorectic hormones such as peptide-tyrosine-tyrosine (PYY) and glucagon-like peptide (GLP)-1. This has been demonstrated directly via SCFA administration, or indirectly via dietary supplementation with fermentable dietary fibre (6,7). Animal studies have also suggested that serum concentrations of the hunger hormone ghrelin is reduced by colonic fermentation, though the underlying mechanism has not been clarified (8,9).
Some human studies have shown that dietary fibre influence PYY, GLP-1 and ghrelin secretion. However, the results are inconsistent regarding which specific hormone is affected (10–14), and whether the effects are mediated by SCFA, or by other effects of the fibre.
Two fermentable fibres of particular interest in this respect are resistant starch (RS) and inulin (IN). Inulin and RS are fermented at different rates and yield different SCFA profiles during fermentation (15,16), and, therefore, may have different effects on gut hormones.
The profile of colonic SCFA also depends on the nature of the colonic microbiota. Compared to lean animals, obese animals have a colonic microbiota that is more efficient in fermenting SCFA from a given diet (17) and is characterised by increased relative abundance of the phylum Firmicutes and a decrease in Bacteroidetes (18,19). Obese humans have been shown to have higher faecal SCFA concentrations than lean subjects but studies are limited and the differences in phylum abundances inconsistent (20–23). If the findings from studies in mice are true, excess SCFA production may promote obesity by contributing extra calories to diet. On the other hand, if colonic SCFA increase satiety by stimulating gut hormone secretion, obesity may be promoted by reduced SCFA production or by resistance to the effect of SCFA on appetite hormones.
Therefore, our objective was to compare the acute effects of RS and IN consumption on postprandial serum SCFA, PYY, GLP-1 and ghrelin responses in overweight and obese (OWO) vs lean (LN) individuals. We hypothesized that: 1) RS and IN would elicit different postprandial SCFA, PYY, GLP-1 and ghrelin responses; and 2) that OWO subjects would have higher SCFA responses than LN but be less sensitive to the effects of colonic SCFA on postprandial gut hormones.
METHODS
Participants
Male and non-pregnant, non-lactating females aged 18–65 years with body mass index (BMI) ≥20 and ≤353kg/m2 were recruited from a pool of participants previously involved in similar studies. Participants were excluded for any of the following reasons: presence of diabetes, cardiovascular, bowel, kidney or liver disease; use of medications which affect blood glucose or insulin sensitivity (such as diuretics); any use of antibiotics, laxatives, pre/probiotics or other drugs known to influence gastrointestinal function in the 3 months before the study; smoking; following any unusual dietary practices (such as weight loss diet, Atkins diet, vegan diet); abnormal plasma blood glucose (≥7.0 mmol/L); or anemia. Eligible participants were then divided prospectively into two groups based on their BMI; 12 participants in the LN group (BMI<25) and 13 participants in the OWO group (BMI≥25). All tests were conducted at the Glycemic Index Laboratories, Toronto. Ethical approval for the study was obtained from the Research Ethics Board, University of Toronto. Participants gave written informed consent to participate in the study.
Phase 1
Participants completed questionnaires related to demographics, medical history, drug use, bowel habit and physical activity. They were given instructions on how to record their dietary intake and asked to keep a 3-day diet record. Participants were also given a faecal collection kit which consisted of the Fisher brand commode specimen collection system (Fisher Scientific, Ottawa, ON) and plastic bags. On the third day of the diet record or the day after, participants collected a faecal sample. The completed 3-day diet record and the plastic bag containing the faecal sample was immediately placed on dry ice, and brought to the lab within 24h of being collected. The frozen faecal samples were stored at −20ºC until they were processed.
Phase 2
A week after completing phase 1, participants began phase 2; a cross-over randomized controlled-trial, in which participants came to the laboratory on 3 separate occasions, separated by a 1-week washout. On each study day, subjects arrived at 8:00am after fasting for 12h; after warming their forearms with a heating pad for 2–3min, a cannula was inserted into a forearm vein and kept clear with periodic saline flushes. After a fasting blood sample was drawn, participants consumed a test drink within 5min, and further blood samples were drawn at 0.5, 1, 1.5, 2, 3 and 4h after the start of the test drink. Immediately after the 4-h blood sample, a standard lunch was provided. Participants ate the lunch within 15min, and further blood samples were drawn at 4.5, 5, 5.5 and 6h. Participants remained seated and awake for the duration of the study.
Test drinks
Each subject consumed all 3 treatments in random order. The sequence of the test meals were randomly assigned to the subjects by the study coordinator (by using Random Integer Subject Generator; http://www.random.org). The test drinks consisted of 75g glucose (GLU) or 75g glucose+24g Oliggo-Fiber Instant Inulin or 75g glucose+28g resistant starch (RS) dissolved in 300mL water on the morning of the study (for more details see 2016EJCN0594R; The acute effects of inulin and resistant-starch on postprandial serum short-chain fatty acids and second-meal glycaemic response in lean and overweight humans; Rahat-Rozenbloom, S., Fernandes, J., Cheng, J., Gloor, GB., Wolever, TMS; Eur J Clin Nutr under review). The dose of 24g was based on a previous study from our lab (10).
Standard lunch
The standard lunch consisted of a cheese and tomato sandwich, a drink of apple juice (200mL), a bottle of water (500mL), and two chocolate cookies (for more details see supplementary information of 2016EJCN0594R; The acute effects of inulin and resistant-starch on postprandial serum short-chain fatty acids and second-meal glycaemic response in lean and overweight humans; Rahat-Rozenbloom, S., Fernandes, J., Cheng, J., Gloor, GB., Wolever, TMS; Eur J Clin Nutr under review).
Biochemistry
The faecal sample was weighed and homogenized in a 400 series masticator (IUL Instruments, S.A., Barcelona, Spain). Aliquots of faeces were then transferred to individual vials for determination of pH and SCFA. Faecal pH was measured using a pH meter, and faecal SCFA were analyzed by gas chromatography as previously described (34). DNA extraction and Ion Torrent V6 16S-rRNA gene sequencing were performed as described elsewhere (35). Compositional analysis of the data using Principle Component Biplots was done as described (36).
Methods and results for glucose, insulin, c-peptide and FFA are reported elsewhere (2016EJCN0594R; The acute effects of inulin and resistant-starch on postprandial serum short-chain fatty acids and second-meal glycaemic response in lean and overweight humans; Rahat-Rozenbloom, S., Fernandes, J., Cheng, J., Gloor, GB., Wolever, TMS; Eur J Clin Nutr under review). Blood for SCFA was taken into plain tubes, allowed to clot at room temperature for 30min, centrifuged at 3000rpm for 15min at 4°C, and the serum aliquoted and stored at −70°C before analysis by gas chromatography after microfiltration and vacuum distillation as previously described (37). Blood for PYY and ghrelin was drawn into tubes containing spray-coated silica and a polymer gel (BD Canada Inc., Oakville, Ont.), and 1 mL of blood for ghrelin analysis was immediately transferred and mixed into plain tubes containing 10 10μl AEBSF(4-(2-Aminoethyl)-Benzene Sulfonyl Fluoride, Hidrochloride, Amresco). Blood for GLP-1 active and total was drawn into P700 Blood Collection System for Plasma GLP-1 Preservation (BD Canada Inc., Oakville, Ont.) and was immediately centrifuged. All other tubes were left to clot for 30 min before centrifuge and the serum was removed and stored at −70 °c prior to analysis. Serum for the analysis of ghrelin was additionally treated with HCl before freezing. Plasma total and active GLP-1 were measured using GLP-1 (Active 7–36) ELISA kit (ALPCO, NH, USA Catalog #43-GP1HU-E01) (intra- and inter-assay CV <6%, <6%; analytical sensitivity 0.05 pmol/L). Serum ghrelin was measured by ELISA (DRG Human Ghrelin (active) EIA-4710; DRG, NJ, USA) (intra- and inter-assay CV <6% and <6%, respectively; analytical sensitivity 0.05 pmol/L). Serum PYY was measured by ELISA (Alpco, Catalog #48-PYYHU-E01.1) (intra- and inter-assay CV <5.1% and <6.6%, respectively; analytical sensitivity 0.082 ng/mL).
Statistical analysis
The study was powered to detect changes in postprandial serum acetate (see supplementary information of 2016EJCN0594R; The acute effects of inulin and resistant-starch on postprandial serum short-chain fatty acids and second-meal glycaemic response in lean and overweight humans; Rahat-Rozenbloom, S., Fernandes, J., Cheng, J., Gloor, GB., Wolever, TMS; Eur J Clin Nutr under review) since we had no information on hormones on which to base a power analysis. Results suggest that differences in hormones, if any, are small and a larger number of subjects would be needed to detect them.
Primary results are reported as means ±SEM. For GLP-1 total and active and for PYY, net incremental areas under the curve (iAUC; subtracting area below the baseline) over 0–4 h were calculated using the trapezoid rule with the fasting concentration as the baseline. For ghrelin, iAUC from the nadir to 4h (iAUCmin4) was calculated using the minimum concentration achieved over the first 4h as the baseline. For SCFA, iAUC from the nadir to 6h (iAUCmin6) was calculated using the minimum concentration achieved over the first 4h as the baseline. For all variables, total areas under the curve (tAUC) were calculated over the 4–6h period. We used iAUC from 0–4 hours for the hormones because tAUC includes the AUC due to the fasting concentration which is not affected by the treatment. Thus, iAUC is a better measure of how the treatment affects the response of the hormone. However, from 4–6h we used total AUC because a standard meal was consumed at 4hr but the concentration before the meal (at 4h) could have been affected by the treatment, so we need tAUC to take that into account. For SCFA responses we calculated AUC over the entire 6h because the fermentation starts to occur before lunch and continues after it; tAUC reflects the mean concentration over the 6hr period whereas iAUC from the minimum value indicates how much the SCFA increased from its minimum baseline.
To investigate a possible relationship between serum SCFA and gut hormone responses the difference in response between IN and Control and between RS and Control were calculated, and the differences in SCFA were correlated with the differences in gut hormones tAUC from 0–4h and 4–6h. Two-group t-tests (two sided) were performed to analyze differences in baseline data between the groups.
Differences were considered statistically significant if p < =0.05. Statistical analyses were conducted using IBM SPSS Statistics version 22 (SPSS Inc., Chicago, IL, USA) and Stata 13.0 (College Station, TX, USA).
RESULTS
Twenty-five (25) subjects participated in the study; the lean group (LN) included 7 males and 5 females with mean±SEM age 33±4 yr and BMI 23.2±0.4 kg/m2; the overweight or obese group (OWO) included 5 males and 8 females aged 46±4 yr and BMI 31.5±1.0 kg/m2. There were no significant differences between LN and OWO in fecal SCFA and fasting glucose, but OWO had significantly higher fasting insulin, total and LDL-cholesterol and triglycerides than LN (Table 1 of companion manuscript: 2016EJCN0594R; The acute effects of inulin and resistant-starch on postprandial serum short-chain fatty acids and second-meal glycaemic response in lean and overweight humans; Rahat-Rozenbloom, S., Fernandes, J., Cheng, J., Gloor, GB., Wolever, TMS; Eur J Clin Nutr under review).
Table 1.
GLP1 (Active and Total), PYY and Ghrelin responses elicited by the test meals
| Hormone | Variable | Glucose | Inulin | P value a | Resistant starch | P value b |
|---|---|---|---|---|---|---|
| GLP-1 active (pmol×h/L) | iAUC04 | 3.07 ± 0.68 | 2.47 ± 1.17 | 0.574 | 3.82 ± 0.72 | 0.297 |
| tAUC46 | 11.3 ± 3.07 | 10.8 ± 3.05 | 0.102 | 10.7 ± 2.95 | 0.085 | |
|
| ||||||
| GLP-1 total (pmol×h/L) | iAUC04 | 6.45 ± 1.3 | 6.61 ± 1.4 | 0.893 | 7.82 ± 1.5 | 0.286 |
| tAUC46 | 16.3 ± 3.86 | 15.6 ± 3.86 | 0.099 | 16.2 ± 3.9 | 0.793 | |
|
| ||||||
| PYY (pmol×h/L) | iAUC04 | 1.06 ± 2.00 | 5.69 ± 1.96 | 0.003 | 3.94 ± 1.79 | 0.069 |
| tAUC46 | 42.4 ± 7.5 | 42.1 ± 7.5 | 0.837 | 43.3 ± 7.6 | 0.579 | |
|
| ||||||
| Ghrelin (pmol×h/L) | iAUCmin4 | 453 ± 84 | 379 ± 58 | 0.068 | 383 ± 64 | 0.088 |
| tAUC46 | 1062 ± 178 | 984 ± 164 | 0.099 | 1009 ± 155 | 0.294 | |
Values are mean±SEM for n=25. GLU, glucose drink; iAUC, net incremental area under the curve; tAUC, total area under the curve; iAUCmin4, from nadir to 4 hrs.
P value for the difference between glucose treatment and inulin treatment;
P value for the difference between glucose treatment and resistant starch treatment
Serum SCFA
In the entire group of n=25 serum acetate, propionate and butyrate responses were significantly higher after IN compared to GLU. However, the differences between RS and GLU were not significant, and, in addition, serum SCFA responses did not differ significantly in OWO vs LN subjects (Table 1 of companion manuscript: 2016EJCN0594R; The acute effects of inulin and resistant-starch on postprandial serum short-chain fatty acids and second-meal glycaemic response in lean and overweight humans; Rahat-Rozenbloom, S., Fernandes, J., Cheng, J., Gloor, GB., Wolever, TMS; Eur J Clin Nutr under review).
Serum GLP-1 Total and Active
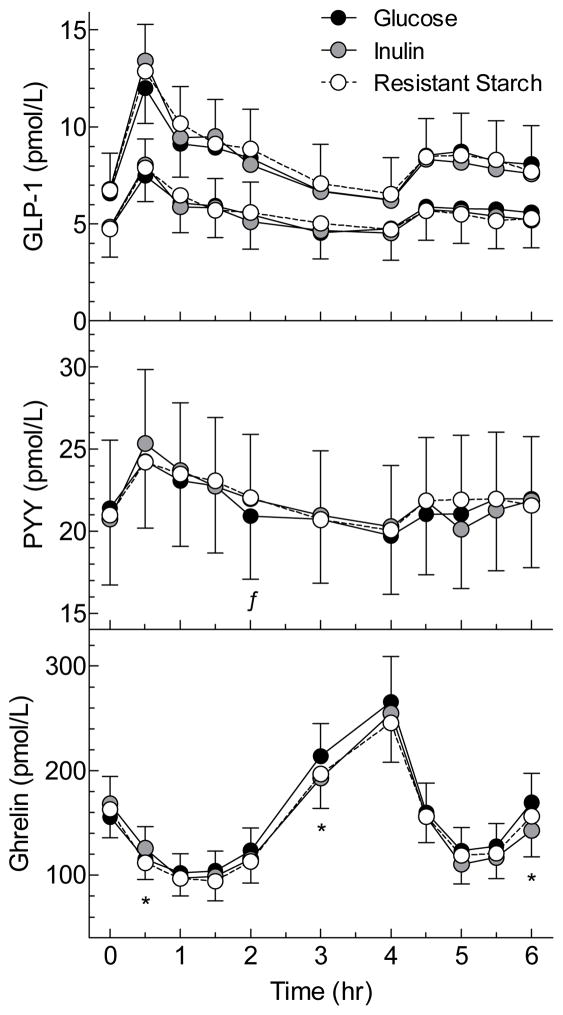
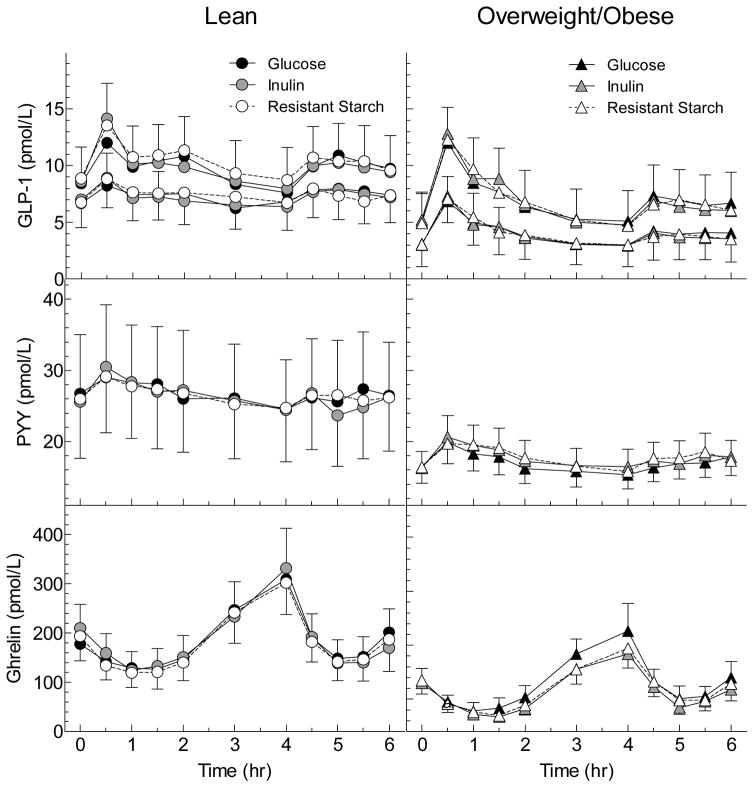
There were no differences among treatments in serum GLP-1 total and active responses (Table 1, Figure 1). Relative to the GLU, changes in serum GLP-1 did not correlate with changes in serum SCFA from 0–4hr or from 4–6hr after either IN or RS (Supplementary Figure 1, Figure 3). Serum GLP-1 active response between 0–2h was significantly greater in the OWO group compared to the LN group (Figure 2, Table 2).
Figure 1. Serum gut hormone responses elicited by the test meals.
Values are means±SEM for n=25 subjects. For GLP-1, the upper curves are total GLP-1 and the bottom curves are active GLP-1.
* Mean for Inulin significantly different from Glucose by related samples Wilcoxon signed rank test (p<0.05).
ƒMean for RS significantly different from Glucose by related samples Wilcoxon signed rank test (p<0.05).
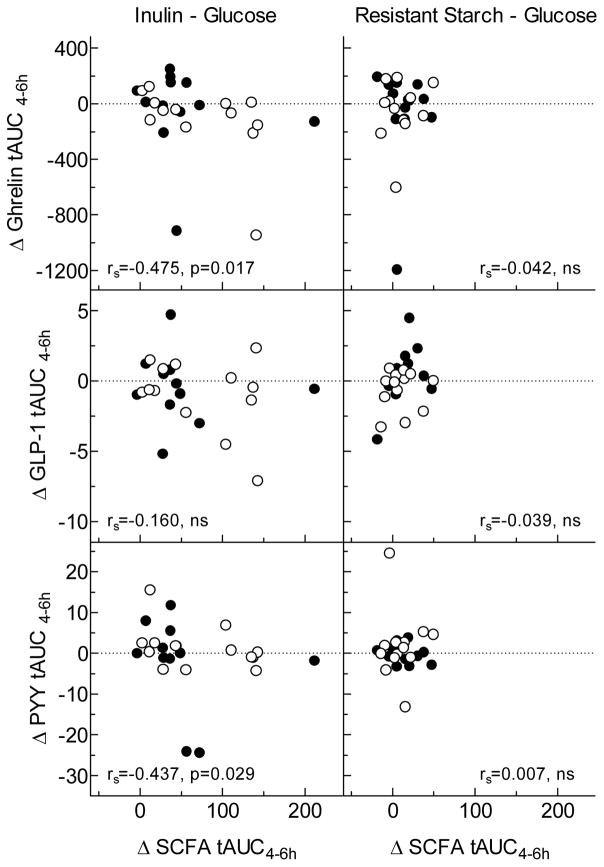
Figure 3. Relationships between changes in gut hormone responses and changes in SCFA responses between 4 and 6 hours.
Values for hormones are differences in total AUC from 4–6h between Glucose and Inulin (left) and Glucose and Resistant Starch (right). Values for SCFA are differences in total AUC from 4–6h between Glucose and Inulin (left) and Glucose and Resistant Starch (right). SCFA tAUCs are the sum of the tAUCs for acetate, propionate and butyrate. Closed circles are lean subjects and open circles are overweight/obese subjects. Spearman’s Closed circles are lean subjects and open circles are overweight/obese subjects. Spearman’s correlation coefficients and approximate p-values are shown in each panel.
Figure 2. Serum GLP1 (Active and Total), PYY and Ghrelin responses elicited by the test meals in Lean and overweight and obese groups.
Values are means±SEM for n=12 lean and n=13 overweight/obese subjects. For GLP-1, the upper curves are total GLP-1 and the bottom curves are active GLP-1.
Table 2.
Mean serum GLP-1 active and total, PYY and Ghrelin responses (means for the 3 test meals) in lean vs. overweight and obese participants
| Hormone (pmol×h/L) | Variable | Lean (n=12) | Overweight and Obese (n=13) | P value a |
|---|---|---|---|---|
| GLP-1 Active | iAUC04 | 1.8 ± 1.1 | 4.3 ± 0.9 | 0.053 |
| tAUC46 | 15.0 ± 4.5 | 7.5 ± 4.0 | 0.199 | |
|
| ||||
| GLP-1 Total | iAUC04 | 5.5 ± 1.6 | 8.2 ± 1.8 | 0.215 |
| tAUC46 | 1 ± 0.7 | 0.8 ± 0.5 | 0.836 | |
|
| ||||
| PYY | iAUC04 | 0.11 ± 0.10 | 0.2 ±0.1 | 0.595 |
| tAUC46 | 2.1 ± 0.6 | 1.4 ± 0.2 | 0.231 | |
|
| ||||
| Ghrelin | iAUCmin4 | 483 ± 122 | 333 ± 52 | 0.226 |
| tAUC46 | 1228 ± 300 | 825 ± 142 | 0.206 | |
Values are mean±SEM; iAUC, net incremental area under the curve; tAUC, total area under the curve.
P value for the difference between the LN group and OWO group.
Serum PYY
Incremental AUC during the 0–4h period for serum PYY response was greater after IN than GLU (p=0.003) and after RS compared to GLU (p=0.069) (Table 1, Figure 1). However, after the standard lunch, there were no differences in tAUC 4–6h between IN and GLU and between RS and GLU (Table 1, Figure 1). Relative to GLU, changes in PYY AUC after IN and RS were not related to changes in SCFA either from 0–4h or from 4–6h (Supplementary Figure 1, Figure 3), except for the difference in PYY tAUC from 4–6h between GLU and IN which was negatively related to the difference in SCFA tAUC (p=0.029); i.e. PYY was lower in those subjects with higher SCFA responses. There were no significant differences in PYY response between the LN and OWO group (Table 2, Figure 2).
1.1.1 Serum Ghrelin
Incremental serum ghrelin responses between the nadir and 4h, relative to GLU, were non-significantly lower after IN (p=0.068) and RS (p=0.088) (Table 1, Figure 1). Over the 4–6h period the total AUC for ghrelin did not differ significantly between IN and GLU nor between RS and GLU (Table 1, Figure 1). However, by Wilcoxon signed rank test, serum ghrelin at 6h was significantly lower after IN than GLU, uncorrected for multiple comparisons. In addition, the differences in ghrelin AUC between GLU and IN were negatively related to the differences in SCFA both between 0–4h (p=0.057) and 4–6h (Supplementary Figure 1, Figure 3). Ghrelin responses in LN did not differ significantly from those in OWO (Table 2, Figure 2).
DISCUSSION
Animal and cell-cultures studies suggest that colonic SCFA increase PYY and GLP-1 and decrease ghrelin secretion and, hence, may promote weight loss (6,7). However, our results do not support this, since despite evidence of large increases in colonic fermentation at 4–6h after IN, GLP-1 and PYY responses were not significantly different from the responses after GLU, although a reduction in ghrelin response was demonstrated.
The inability of IN to increase GLP-1 or PYY in the face of large increases in serum SCFA after lunch seems inconsistent with previous human studies. These studies have shown that acute elevation of circulating SCFA by administration of rectal or intravenous infusions of acetate (24), or by enrichment of colonic propionate (using inulin-propionate ester (IPE))(25), resulted in increased PYY and GLP-1 responses, but had no effect on ghrelin. However, the changes in serum acetate after rectal and IV infusions, and the changes in serum propionate after colonic propionate supplementation, respectively were approximately 4-, 15- and 5-times higher than the mean change elicited by oral IN in the present study. This suggests that the serum concentrations of SCFA achieved in our study were not high enough to affect gut hormones. Furthermore, it was recently shown that neither acute (26) nor chronic (25) enrichment of colonic propionate via dietary IPE supplementation, affected postprandial PYY and GLP-1, despite a reduced subjective postprandial appetite and a reduced weight gain at the end of the long-term study. Thus, the authors suggested that propionate may mediate satiety by other mechanisms.
Although we were unable to demonstrate a statistically significant effect of IN on gut hormones, our results are consistent with those of Tarini and Wolever (10) who showed that, compared to consuming 56g high-fructose corn-syrup (HFCS) alone, 56g HFCS plus 24g IN after an overnight fast significantly increased serum GLP-1 at 30min, and decreased serum ghrelin concentrations at 4–6h. In the current study, mean serum GLP-1 30min after IN was higher than after GLU, but the difference was not statistically significant. Moreover, although IN reduced ghrelin tAUC from 4–6h insignificantly (P=0.099), serum ghrelin 6hr after IN was significantly less than that after GLU (p=0.003, uncorrected for multiple comparisons), and the changes in serum ghrelin after IN at 4–6h were negatively related to the changes in SCFA (p=0.017). This, at least, is consistent with the suggestion that colonic fermentation reduces ghrelin secretion (9), though the mechanism is yet to be explored. Longer term consumption of IN has generally resulted in positive effects on at least one of the appetite hormones (27–29), however, very few studies assessed if these effects were SCFA-mediated (30, 31). Overall, there is a paucity of data in humans exploring whether SCFA modulate gut hormone responses, and which specific hormone is being influenced.
Surprisingly, IN, and to a lesser extent RS, increased PYY and decreased ghrelin between 0–4h. However, these increases were too early to be affected by colonic fermentation. We believe these early effects of RS are due to the presence of a larger than expected amount of available carbohydrate in the RS ingredient used, since the reduction in serum FFA after RS was significantly prolonged compared to that after GLU (2016EJCN0594R; The acute effects of inulin and resistant-starch on postprandial serum short-chain fatty acids and second-meal glycaemic response in lean and overweight humans; Rahat-Rozenbloom, S., Fernandes, J., Cheng, J., Gloor, GB., Wolever, TMS; Eur J Clin Nutr under review) and both PYY and ghrelin have been shown to respond proportionally to the caloric load and macronutrient content (32,33).
Previous acute studies have demonstrated either no effect of RS on postprandial gut hormones (34), or a reduced postprandial GLP-1 response following breakfast meal (35,36), but no effect after lunch (36). However, in these studies SCFA responses were not measured. Longer term studies that measured postprandial SCFA did not make the picture clearer, as the effects of RS on both fasting and postprandial SCFA and hormone responses vary markedly among the studies (37–39). In summary, though animal research has shown consistent increases in gut hormones following RS fermentation (6,40,41), human data are inconsistent. More acute and chronic RS feeding studies are required to determine whether gut hormones are affected by colonic fermentation.
Some of the inconsistencies seen above in human studies could be explained by the large variations in study designs; such as the different fibre types used (i.e., oligofructose vs. inulin) (8), the different doses, the different time points that are being measured and the different duration of the studies. The latter could be especially important for two reasons. First, IN (and potentially RS) is a prebiotic that promotes gradual changes in gut microbiota composition faecal SCFA over time (42). Second, SCFA have been shown to increase the number of L-cells in mouse intestinal epithelium, and as a result increase GLP-1 and PYY secretion (43); since these are time-related effects, no alteration in microbiota or L-cells, and hence no increase in GLP-1 or PYY would be expected after an acute increase in colonic fermentation such as the present study. However, consistent with this concept, we found that long-term (1 year) increased dietary fibre intake eventually resulted in an increased postprandial GLP-1 responses in hyperinsulinaemic humans (49). In addition, within- and between-individual variability in gut hormone concentrations may mask the effect of SCFA on gut hormones; therefore, a larger sample size may be needed.
Based on the results of animals studies, we hypothesized that OWO individuals would have higher SCFA responses than LN but would be less sensitive to the effects of colonic SCFA on gut hormones. However, this was not confirmed in our study, as there were no differences between the groups in serum SCFA and hormone responses. The similar relative abundances of Firmicutes and Bacteroidetes, and the similar dietary intakes in our LN and OWO groups, together with the observation that no differences in faecal SCFA concentrations (2016EJCN0594R; The acute effects of inulin and resistant-starch on postprandial serum short-chain fatty acids and second-meal glycaemic response in lean and overweight humans; Rahat-Rozenbloom, S., Fernandes, J., Cheng, J., Gloor, GB., Wolever, TMS; Eur J Clin Nutr under review), strengthen our assumption that SCFA production did not differ in our LN and OWO groups. The results of other human studies are inconsistent in this respect, as reviewed elsewhere (44,45). Interestingly, recent in vitro fermentation studies using faecal inocula from lean vs. obese donors showed that 4 different types of RS produced similar SCFA concentrations in LN and OWO inocula (46), while other types of fibre showed either increased or decreased SCFA production in OWO compared to LN (47). Therefore, further research is needed to fully elucidate whether the SCFA production and metabolism differ in lean and obese humans.
It is important to note that circulating SCFA may not adequately reflect colonic SCFA production, as they are the net result of endogenous and exogenous production, absorption, hepatic extraction of the colonic SCFA (48). However, the comparison of the IN and the RS treatments to the GLU treatment, allowed us to control, at least in part, for the endogenous SCFA production.
We conclude that acute increases in colonic fermentation do not affect GLP-1 or PYY responses, but may reduce ghrelin. A longer adaptation to increased colonic fermentation may affect gut hormones differently. The similarity of SCFA responses in LN and OWO subjects does not support the hypothesis that colonic SCFA production is increased in obesity; however, increased SCFA production may not occur in all overweight/obese subjects and serum SCFA responses may not necessarily reflect colonic SCFA production.
Supplementary Material
Acknowledgments
We are thankful to Kervan Rivera-Rufner for analyzing serum and faecal SCFA. Supported by grant no. 486906 from the Canadian Institutes of Health Research (CIHR), Institute of Nutrition, Metabolism and Diabetes.
Footnotes
Supplementary information is available at European Journal of Clinical Nutrition’s website.
Clinical Trials registration number (at www.ClinicalTrials.gov): NCT02562014
Conflict of interest: The authors declared no conflict of interest
References
- 1.Liu S, Willett WC, Manson JE, Hu FB, Rosner B, Colditz G. Relation between changes in intakes of dietary fiber and grain products and changes in weight and development of obesity among middle-aged women. Am J Clin Nutr. 2003;78(5):920–927. doi: 10.1093/ajcn/78.5.920. [DOI] [PubMed] [Google Scholar]
- 2.Howarth NC, Saltzman E, Roberts SB. Dietary fiber and weight regulation. Nutr Rev. 2001;59(5):129–139. doi: 10.1111/j.1753-4887.2001.tb07001.x. [DOI] [PubMed] [Google Scholar]
- 3.Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015;7(4):2839–2849. doi: 10.3390/nu7042839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nature Communications. 2013 May;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61(2):364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou J, Martin RJ, Tulley RT, Raggio AM, McCutcheon KL, Shen L, et al. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. American Journal of Physiology - Endocrinology and Metabolism. 2008;295(5):E1160–E1166. doi: 10.1152/ajpendo.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keenan MJ, Zhou J, McCutcheon KL, Raggio AM, Bateman HG, Todd E, et al. Effects of resistant starch, a non-digestible fermentable fiber, on reducing body fat. Obesity. 2006;14(9):1523–1534. doi: 10.1038/oby.2006.176. [DOI] [PubMed] [Google Scholar]
- 8.Delzenne NM, Cani PD, Daubioul C, Neyrinck AM. Impact of inulin and oligofructose on gastrointestinal peptides. Br J Nutr. 2005;93(SUPP):S157–S161. doi: 10.1079/bjn20041342. [DOI] [PubMed] [Google Scholar]
- 9.Cani PD, Dewever C, Delzenne NM. Inulin-type fructans modulate gastrointestinal peptides involved in appetite regulation (glucagon-like peptide-1 and ghrelin) in rats. Br J Nutr. 2004;92(3):521–526. doi: 10.1079/bjn20041225. [DOI] [PubMed] [Google Scholar]
- 10.Tarini J, Wolever TMS. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Applied Physiology, Nutrition and Metabolism. 2010;35(1):9–16. doi: 10.1139/H09-119. [DOI] [PubMed] [Google Scholar]
- 11.Nilsson A, Johansson E, Ekström L, Björck I. Effects of a Brown Beans Evening Meal on Metabolic Risk Markers and Appetite Regulating Hormones at a Subsequent Standardized Breakfast: A Randomized Cross-Over Study. PLoS ONE. 2013;8(4) doi: 10.1371/journal.pone.0059985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cani PD, Lecourt E, Dewulf EM, Sohet FM, Pachikian BD, Naslain D, et al. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr. 2009;90(5):1236–1243. doi: 10.3945/ajcn.2009.28095. [DOI] [PubMed] [Google Scholar]
- 13.Johansson EV, Nilsson AC, Östman EM, Björck IME. Effects of indigestible carbohydrates in barley on glucose metabolism, appetite and voluntary food intake over 16 h in healthy adults. Nutr J. 2013;12(1) doi: 10.1186/1475-2891-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klosterbuer AS, Thomas W, Slavin JL. Resistant starch and pullulan reduce postprandial glucose, insulin, and GLP-1, but have no effect on satiety in healthy humans. J Agric Food Chem. 2012;60(48):11928–11934. doi: 10.1021/jf303083r. [DOI] [PubMed] [Google Scholar]
- 15.Slavin J, Stewart M, Timm D, Grabitske H, Hospattankar A. Fermentation patterns and short chain fatty acid profiles of wheat dextrin and other functional fibres. Dietary Fibre: New Frontiers for Food and Health. 2010:177–191. [Google Scholar]
- 16.Jenkins DJA, Vuksan V, Kendall CWC, Würsch P, Jeffcoat R, Waring S, et al. Physiological effects of resistant starches on fecal bulk, short chain fatty acids, blood lipids and glycemic index. J Am Coll Nutr. 1998;17(6):609–616. doi: 10.1080/07315724.1998.10718810. [DOI] [PubMed] [Google Scholar]
- 17.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 18.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 20.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:6150. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TMS, Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes. 2014;4(JUNE) doi: 10.1038/nutd.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahat-Rozenbloom S, Fernandes J, Gloor GB, Wolever TMS. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans. Int J Obes. 2014;38(12):1525–1531. doi: 10.1038/ijo.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ismail NA, Ragab SH, ElBaky AA, Shoeib ARS, Alhosary Y, Fekry D. Frequency of Firmicutes and Bacteroidetes in gut microbiota in obese and normal weight Egyptian children and adults. Arch Med Sci. 2011;7(3):501–507. doi: 10.5114/aoms.2011.23418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freeland KR, Wolever TMS. Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-alpha. Br J Nutr. 2010 Feb 14;103(3):460–466. doi: 10.1017/S0007114509991863. [DOI] [PubMed] [Google Scholar]
- 25.Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SEK, et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64(11):1744–1754. doi: 10.1136/gutjnl-2014-307913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byrne CS, Chambers ES, Alhabeeb H, Chhina N, Morrison DJ, Preston T, et al. Increased colonic propionate reduces anticipatory reward responses in the human striatum to high-energy foods. Am J Clin Nutr. 2016;104:5–14. doi: 10.3945/ajcn.115.126706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verhoef SPM, Meyer D, Westerterp KR. Effects of oligofructose on appetite profile, glucagon-like peptide 1 and peptide YY3-36 concentrations and energy intake. Br J Nutr. 2011;106(11):1757–1762. doi: 10.1017/S0007114511002194. [DOI] [PubMed] [Google Scholar]
- 28.Parnell JA, Reimer RA. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am J Clin Nutr. 2009;89(6):1751–1759. doi: 10.3945/ajcn.2009.27465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedersen C, Lefevre S, Peters V, Patterson M, Ghatei MA, Morgan LM, et al. Gut hormone release and appetite regulation in healthy non-obese participants following oligofructose intake. A dose-escalation study. Appetite. 2013;66:44–53. doi: 10.1016/j.appet.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Cani PD, Lecourt E, Dewulf EM, Sohet FM, Pachikian BD, Naslain D, et al. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr. 2009;90(5):1236–1243. doi: 10.3945/ajcn.2009.28095. [DOI] [PubMed] [Google Scholar]
- 31.Daud NM, Ismail NA, Thomas EL, Fitzpatrick JA, Bell JD, Swann JR, et al. The impact of oligofructose on stimulation of gut hormones, appetite regulation and adiposity. Obesity. 2014;22(6):1430–1438. doi: 10.1002/oby.20754. [DOI] [PubMed] [Google Scholar]
- 32.Field BCT, Chaudhri OB, Bloom SR. Bowels control brain: Gut hormones and obesity. Nat Rev Endocrionol. 2010;6(8):444–453. doi: 10.1038/nrendo.2010.93. [DOI] [PubMed] [Google Scholar]
- 33.Müller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, et al. Ghrelin. Mol Metab. 2015;4(6):437–460. doi: 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gentile CL, Ward E, Holst JJ, Astrup A, Ormsbee MJ, Connelly S, et al. Resistant starch and protein intake enhances fat oxidation and feelings of fullness in lean and overweight/obese women. Nutr J. 2015;14(1) doi: 10.1186/s12937-015-0104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raben A, Tagliabue A, Christensen NJ, Madsen J, Holst JJ, Astrup A. Resistant starch: The effect on postprandial glycemia, hormonal response, and satiety. Am J Clin Nutr. 1994;60(4):544–551. doi: 10.1093/ajcn/60.4.544. [DOI] [PubMed] [Google Scholar]
- 36.Bodinham CL, Al-Mana NM, Smith L, Robertson MD. Endogenous plasma glucagon-like peptide-1 following acute dietary fibre consumption. Br J Nutr. 2013;110(8):1429–1433. doi: 10.1017/S0007114513000731. [DOI] [PubMed] [Google Scholar]
- 37.Robertson MD, Currie JM, Morgan LM, Jewell DP, Frayn KN. Prior short-term consumption of resistant starch enhances postprandial insulin sensitivity in healthy subjects. Diabetologia. 2003;46(5):659–665. doi: 10.1007/s00125-003-1081-0. [DOI] [PubMed] [Google Scholar]
- 38.Nilsson AC, Johansson-Boll EV, Björck IME. Increased gut hormones and insulin sensitivity index following a 3-d intervention with a barley kernel-based product: A randomised cross-over study in healthy middle-Aged subjects. Br J Nutr. 2015;114(6):899–907. doi: 10.1017/S0007114515002524. [DOI] [PubMed] [Google Scholar]
- 39.Robertson MD, Bickerton AS, Dennis AL, Vidal H, Frayn KN. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr. 2005;82(3):559–567. doi: 10.1093/ajcn.82.3.559. [DOI] [PubMed] [Google Scholar]
- 40.Zhou J, Martin RJ, Raggio AM, Shen L, McCutcheon K, Keenan MJ. The importance of GLP-1 and PYY in resistant starch’s effect on body fat in mice. Mol Nutr Food Res. 2015;59(5):1000–1003. doi: 10.1002/mnfr.201400904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vidrine K, Ye J, Martin RJ, McCutcheon KL, Raggio AM, Pelkman C, et al. Resistant starch from high amylose maize (HAM-RS2) and Dietary butyrate reduce abdominal fat by a different apparent mechanism. Obesity. 2014;22(2):344–348. doi: 10.1002/oby.20501. [DOI] [PubMed] [Google Scholar]
- 42.Salazar N, Dewulf EM, Neyrinck AM, Bindels LB, Cani PD, Mahillon J, et al. Inulin-type fructans modulate intestinal Bifidobacterium species populations and decrease fecal short-chain fatty acids in obese women. Clin Nutr. 2015;34(3):501–507. doi: 10.1016/j.clnu.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Petersen N, Reimann F, Bartfeld S, Farin HF, Ringnalda FC, Vries RGJ, et al. Generation of l cells in mouse and human small intestine organoids. Diabetes. 2014;63(2):410–420. doi: 10.2337/db13-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conterno L, Fava F, Viola R, Tuohy KM. Obesity and the gut microbiota: Does up-regulating colonic fermentation protect against obesity and metabolic disease? Genes Nutr. 2011;6(3):241–260. doi: 10.1007/s12263-011-0230-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenbaum M, Knight R, Leibel RL. The gut microbiota in human energy homeostasis and obesity. Trends Endocrinol Metab. 2015;26(9):493–501. doi: 10.1016/j.tem.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li L, Jiang H, Kim H, Yum M, Campbell MR, Jane J, et al. Increased Butyrate Production During Long-Term Fermentation of In Vitro-Digested High Amylose Cornstarch Residues with Human Feces. J Food Sci. 2015;80(9):1997–2004. doi: 10.1111/1750-3841.12982. [DOI] [PubMed] [Google Scholar]
- 47.Aguirre M, Jonkers DMAE, Troost FJ, Roeselers G, Venema K. In vitro characterization of the impact of different substrates on metabolite production, energy extraction and composition of gut microbiota from lean and obese subjects. PLoS ONE. 2014;9(11) doi: 10.1371/journal.pone.0113864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernandes J, Vogt J, Wolever TMS. Kinetic model of acetate metabolism in healthy and hyperinsulinaemic humans. Eur J Clin Nutr. 2014 doi: 10.1038/ejcn.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freeland KR, Wilson C, Wolever TMS. Adaptation of colonic fermentation and glucagon-like peptide-1 secretion with increased wheat fibre intake for 1 year in hyperinsulinaemic human subjects. Br J Nutr. 2010;103:82–90. doi: 10.1017/S0007114509991462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.