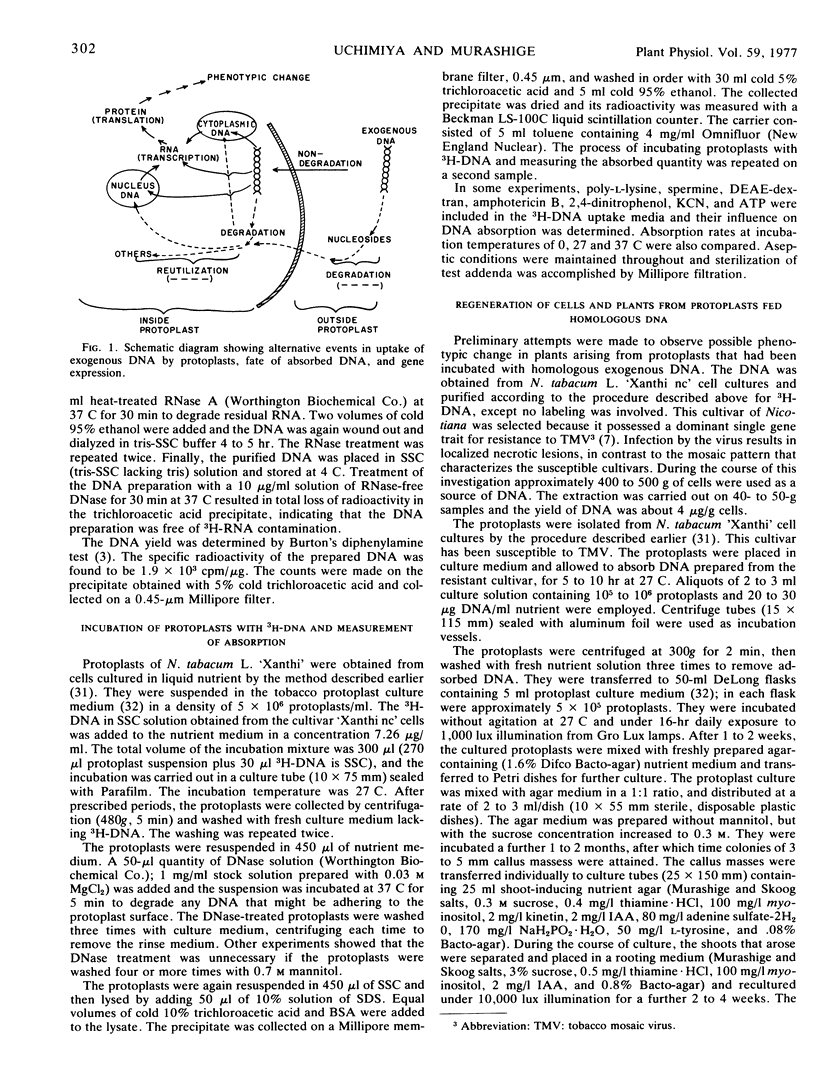
Abstract
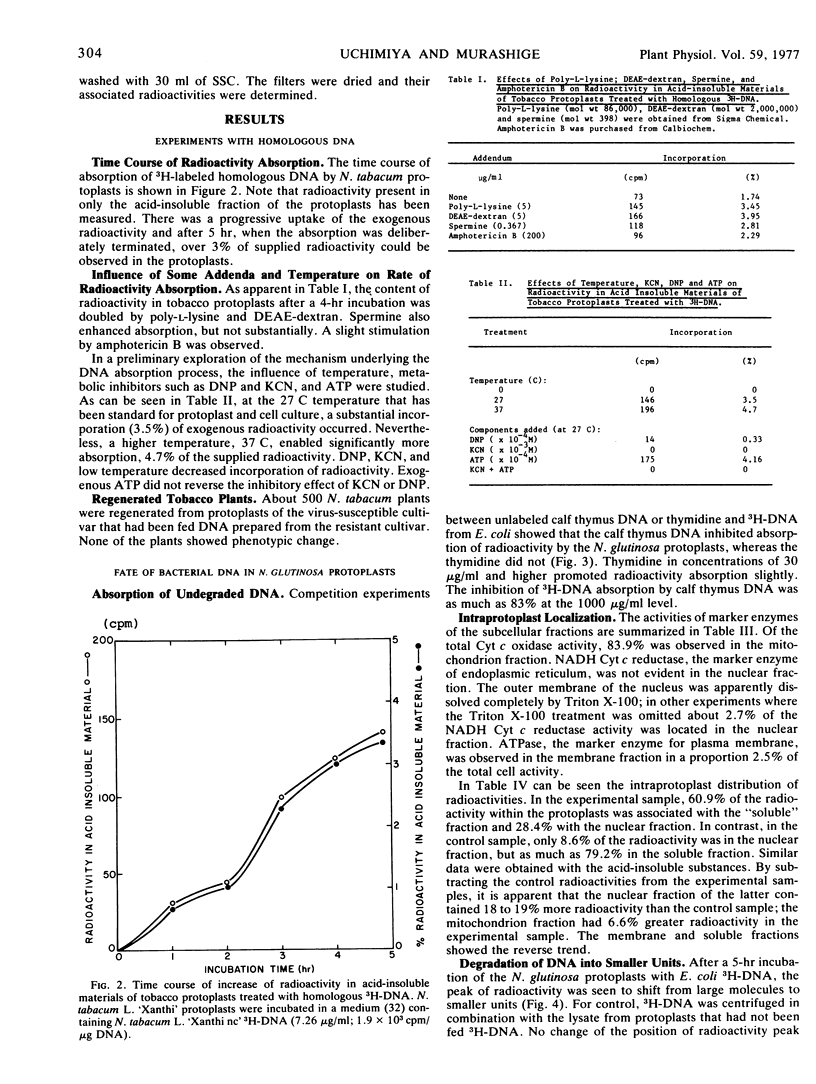
After a 5-hour incubation of protoplasts of Nicotiana tabacum L. `Xanthi' with 3H-DNA (7.26 μg/ml) from N. tabacum L. `Xanthi nc' 3.5% of the initial radioactivity was found in acid-insoluble substances of the protoplasts. The addition of DEAE-dextran and poly-l-lysine to the incubation medium nearly doubled radioactivity adsorption. The absorption was inhibited by 2,4-dinitrophenol, KCN, and low temperature (0 C); this inhibition could not be reversed by exogenous ATP. About 500 tobacco plants established from protoplasts of a normally tobacco-mosaic virus-susceptible cultivar that had been allowed to absorb DNA prepared from a resistant cultivar did not show transfer of the virus-resistant gene.
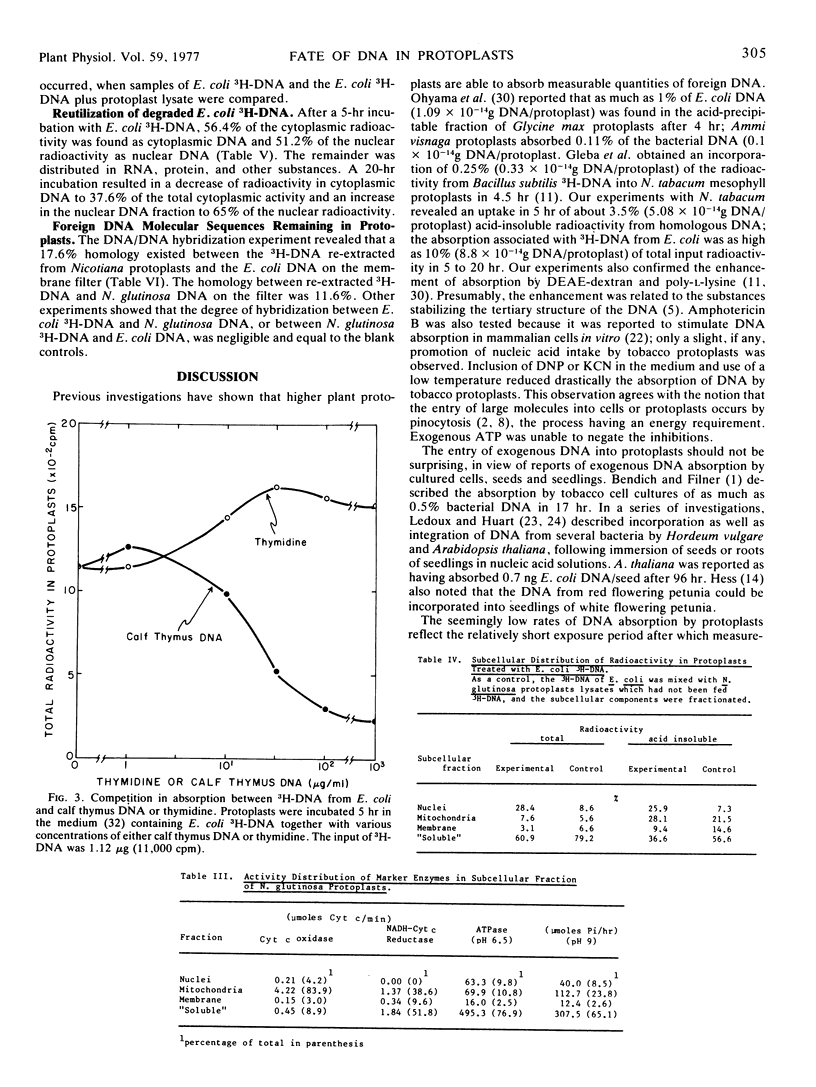
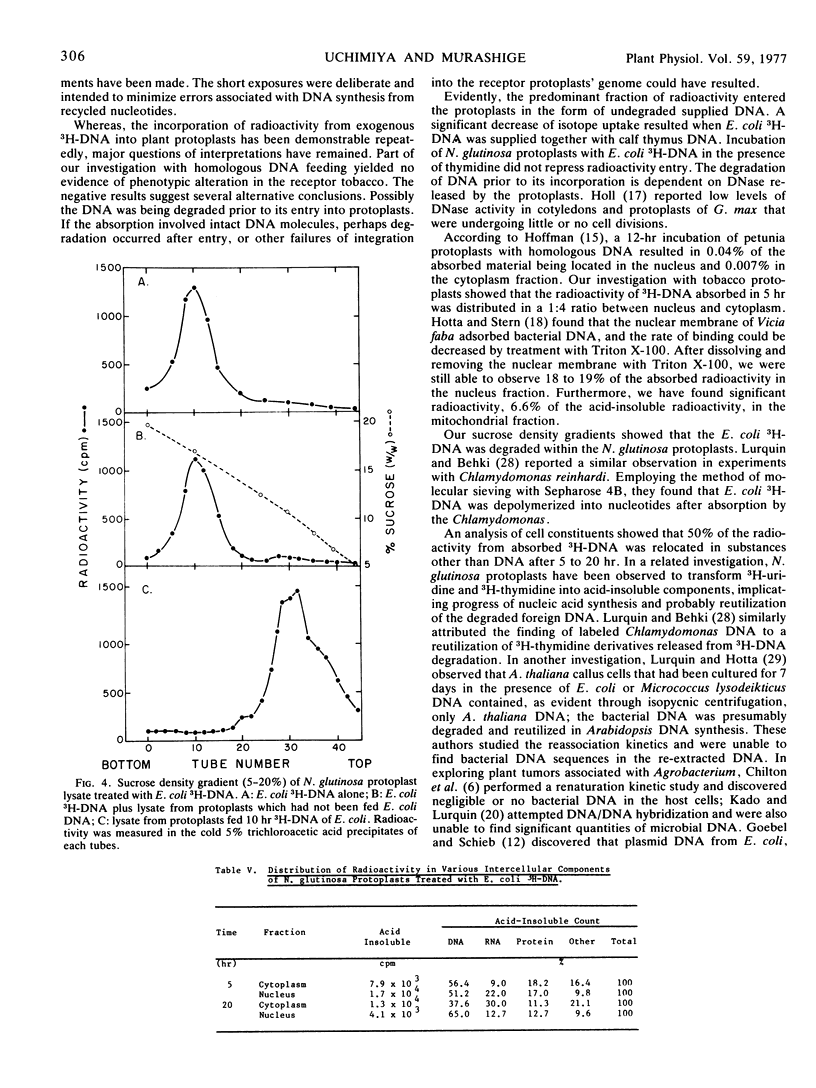
A detailed analysis was performed of the disposition of exogenous DNA in plant protoplasts, by employing Escherichia coli3H-DNA and Nicotiana glutinosa protoplasts. In 5 to 20 hours, about 10% of the 3H-DNA entered the protoplasts. Competition experiments between the 3H-DNA and unlabeled DNA or thymidine showed that the entry occurred as undegraded 3H-DNA. Examination of intraprotoplast fractions revealed that 60 to 80% of the absorbed radioactivity resided in the “soluble” fraction of the cytoplasm and 20% in the nuclear fraction. The mitochondrion fraction also contained measurable radioactivity. Sizing on sucrose density gradients showed that the bulk of the absorbed E. coli DNA had been depolymerized. Of the incorporated radioactivity, 15% was accountable as DNA, exogenous as well as resynthesized, and 15% as RNA, protein, and other cell constituents. DNA/DNA hybridization test indicated that 17.6% of the re-extractable 3H-DNA retained homology with the E. coli DNA; this was equivalent to 2.6% of the absorbed radioactivity. Resynthesized receptor protoplast DNA was represented by a fraction at least 1.7% of the total absorbed radioactivity. The amount of bacterial DNA remaining in protoplasts suggests that each protoplast retained 2.3 × 10−15g donor DNA, or approximately half of the E. coli genome.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava P. M., Shanmugam G. Uptake of nonviral nucleic acids by mammalian cells. Prog Nucleic Acid Res Mol Biol. 1971;11:103–192. doi: 10.1016/s0079-6603(08)60327-x. [DOI] [PubMed] [Google Scholar]
- Carlson P. S. The use of protoplasts for genetic research. Proc Natl Acad Sci U S A. 1973 Feb;70(2):598–602. doi: 10.1073/pnas.70.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll D. Optical properties of deoxyribonucleic acid--polylysine complexes. Biochemistry. 1972 Feb 1;11(3):421–426. doi: 10.1021/bi00753a019. [DOI] [PubMed] [Google Scholar]
- Chilton M. D., Currier T. C., Farrand S. K., Bendich A. J., Gordon M. P., Nester E. W. Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3672–3676. doi: 10.1073/pnas.71.9.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen R. E., Cameron D. R. INHERITANCE IN Nicotiana Tabacum. XXVIII. THE CYTOGENETICS OF INTROGRESSION. Proc Natl Acad Sci U S A. 1957 Oct 15;43(10):908–913. doi: 10.1073/pnas.43.10.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Doy C. H., Gresshoff P. M., Rolfe B. G. Biological and molecular evidence for the transgenosis of genes from bacteria to plant cells. Proc Natl Acad Sci U S A. 1973 Mar;70(3):723–726. doi: 10.1073/pnas.70.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeba Iu Iu, Khasanov M. M., Sliusarenko A. G., Butenko R. G., Vinetskii Iu P. Proniknovenie 3H-DNK Bacillus subtilis v izolirovannye protoplasty kletox tabaka Nicotiana tabacum L. Dokl Akad Nauk SSSR. 1974;219(6):1478–1481. [PubMed] [Google Scholar]
- Goebel W., Schiess W. The fate of a bacterial plasmid in mammalian cells. Mol Gen Genet. 1975 Jun 19;138(3):213–223. doi: 10.1007/BF00269348. [DOI] [PubMed] [Google Scholar]
- Hamilton R. H., Künsch U., Temperli A. Simple rapid procedures for isolation of tobacco leaf nuclei. Anal Biochem. 1972 Sep;49(1):48–57. doi: 10.1016/0003-2697(72)90241-2. [DOI] [PubMed] [Google Scholar]
- Johnson C. B., Grierson D., Smith H. Expression of lambda plac5 DNA in cultured cells of a higher plant. Nat New Biol. 1973 Jul 25;244(134):105–107. doi: 10.1038/newbio244105a0. [DOI] [PubMed] [Google Scholar]
- Kleinhofs A., Eden F. C., Chilton M. D., Bendich A. J. On the question of the integration of exogenous bacterial DNA into plant DNA. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2748–2752. doi: 10.1073/pnas.72.7.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B. V., Medoff G., Kobayashi G., Schlessinger D. Uptake of Escherichia coli DNA into HeLa cells enhanced by amphotericin B. Nature. 1974 Jul 26;250(464):323–325. doi: 10.1038/250323a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ledoux L., Huart R. Fate of exogenous bacterial deoxyribonucleic acids in barley seedlings. J Mol Biol. 1969 Jul 28;43(2):243–262. doi: 10.1016/0022-2836(69)90265-4. [DOI] [PubMed] [Google Scholar]
- Ledoux L., Huart R., Jacobs M. DNA-mediated genetic correction of thiamineless Arabidopsis thaliana. Nature. 1974 May 3;249(452):17–21. doi: 10.1038/249017a0. [DOI] [PubMed] [Google Scholar]
- Ledoux L., Huart R., Jacobs M. Fate of exogenous DNA in Arabidopsis thaliana. Translocation and integration. Eur J Biochem. 1971 Nov 11;23(1):96–108. doi: 10.1111/j.1432-1033.1971.tb01596.x. [DOI] [PubMed] [Google Scholar]
- Leonard R. T., Hansen D., Hodges T. K. Membrane-bound Adenosine Triphosphatase Activities of Oat Roots. Plant Physiol. 1973 Apr;51(4):749–754. doi: 10.1104/pp.51.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurquin P. F., Behki R. M. Uptake of bacterial DNA by Chlamydomonas reinhardi. Mutat Res. 1975 Jul;29(1):35–51. doi: 10.1016/0027-5107(75)90019-6. [DOI] [PubMed] [Google Scholar]
- Uchimiya H., Murashige T. Evaluation of parameters in the isolation of viable protoplasts from cultured tobacco cells. Plant Physiol. 1974 Dec;54(6):936–944. doi: 10.1104/pp.54.6.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimiya H., Murashige T. Influence of the nutrient medium on the recovery of dividing cells from tobacco protoplasts. Plant Physiol. 1976 Mar;57(3):424–429. doi: 10.1104/pp.57.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]


