Abstract
Superoxide dismutase was purified from pea (Pisum sativum L., cv. Wando) seeds and corn (Zea mays L., cv. Michigan 500) seedlings. The purified pea enzyme eluting as a single peak from gel exclusion chromatography columns contained the three electrophoretically distinct bands of superoxide dismutase characterizing the crude extract. The purified corn enzyme eluted as the same peak as the pea enzyme, and contained five of the seven active bands found in the crude extract. The similar molecular weights and the cyanide sensitivities of these bands indicated that they are probably isozymes of a cupro-zinc superoxide dismutase. One of the remaining corn bands was shown to be a peroxidase.
Superoxide dismutase accounted for 1.6 to 2.4% of the water-soluble protein in seedlings of corn, peas, and oats (Avena sativa L., cv. Au Sable). The superoxide dismutase activity per plant and per milligram water-soluble protein considerably increased during germination of oats and during greening and hook opening of peas.
Full text
PDF
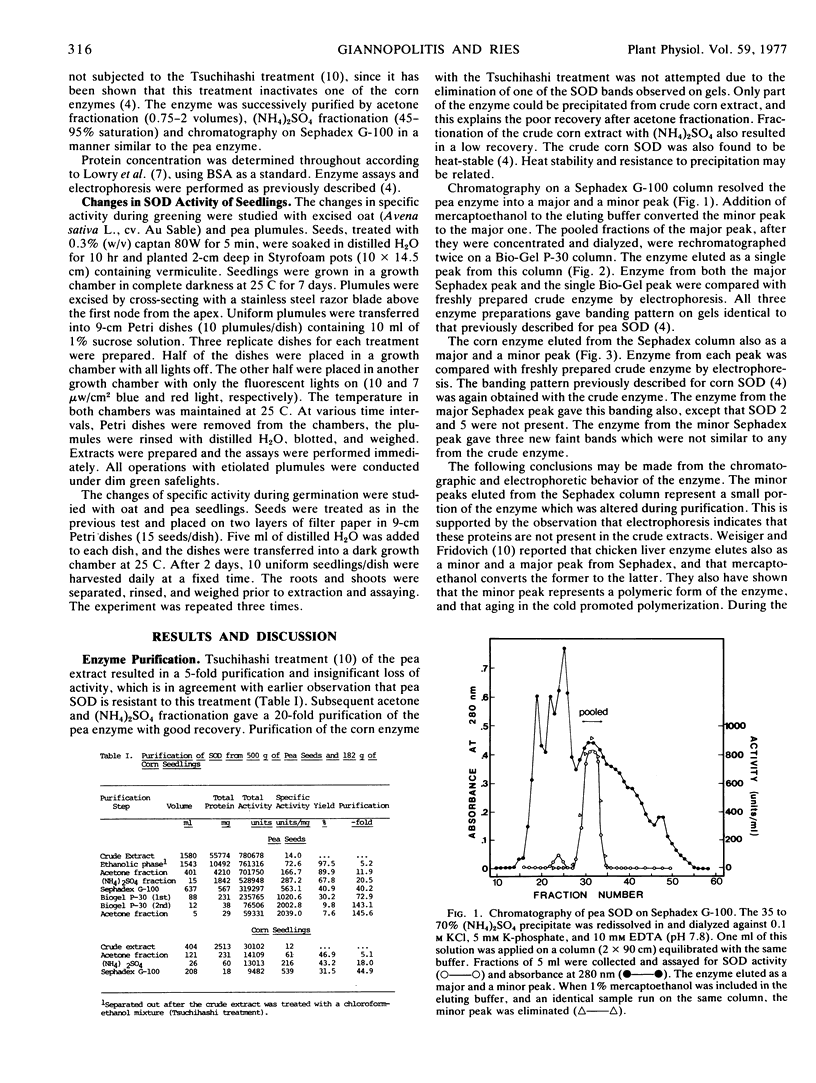

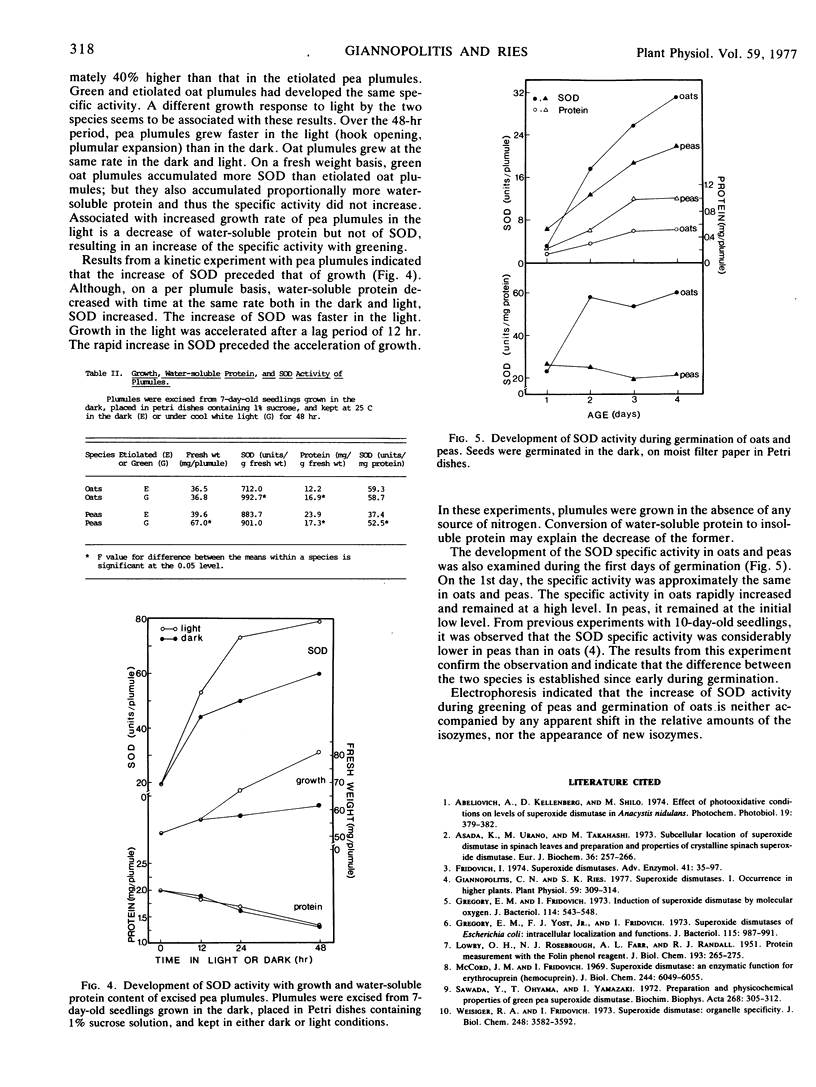
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeliovich A., Kellenberg D., Shilo M. Effect of photooxidative conditions on levels of superoxide dismutase in Anacystis nidulans. Photochem Photobiol. 1974 May;19(5):379–382. doi: 10.1111/j.1751-1097.1974.tb06526.x. [DOI] [PubMed] [Google Scholar]
- Asada K., Urano M., Takahashi M. Subcellular location of superoxide dismutase in spinach leaves and preparation and properties of crystalline spinach superoxide dismutase. Eur J Biochem. 1973 Jul 2;36(1):257–266. doi: 10.1111/j.1432-1033.1973.tb02908.x. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):35–97. doi: 10.1002/9780470122860.ch2. [DOI] [PubMed] [Google Scholar]
- Giannopolitis C. N., Ries S. K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977 Feb;59(2):309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Induction of superoxide dismutase by molecular oxygen. J Bacteriol. 1973 May;114(2):543–548. doi: 10.1128/jb.114.2.543-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Yost F. J., Jr, Fridovich I. Superoxide dismutases of Escherichia coli: intracellular localization and functions. J Bacteriol. 1973 Sep;115(3):987–991. doi: 10.1128/jb.115.3.987-991.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Sawada Y., Oyama T., Yamazaki I. Preparation and physicochemical properties of green pea superoxide dismutase. Biochim Biophys Acta. 1972 May 12;268(2):305–312. doi: 10.1016/0005-2744(72)90325-7. [DOI] [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Superoxide dismutase. Organelle specificity. J Biol Chem. 1973 May 25;248(10):3582–3592. [PubMed] [Google Scholar]


