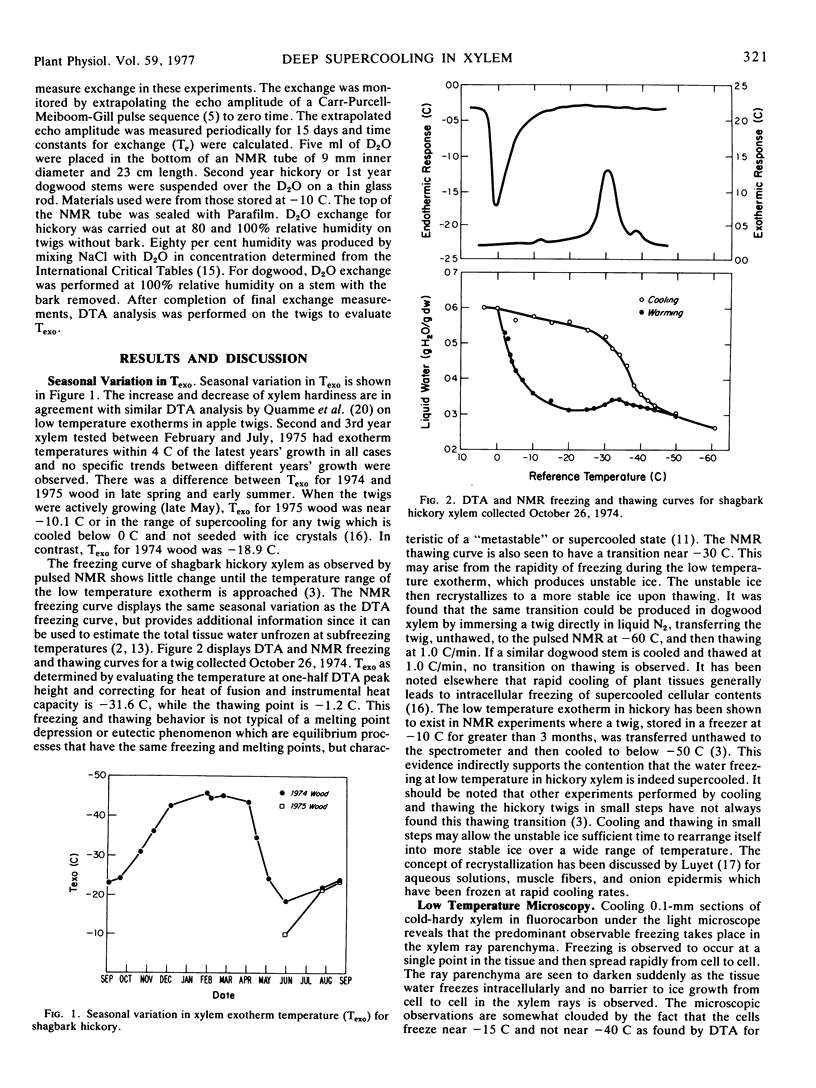
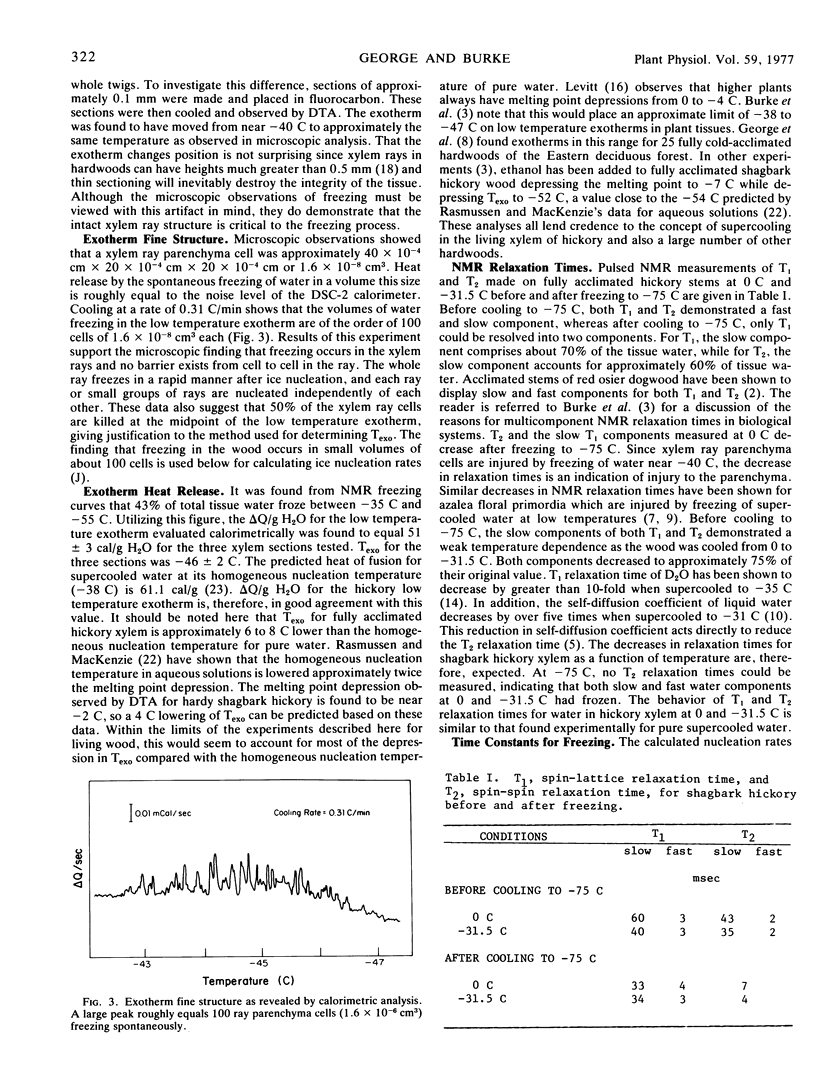
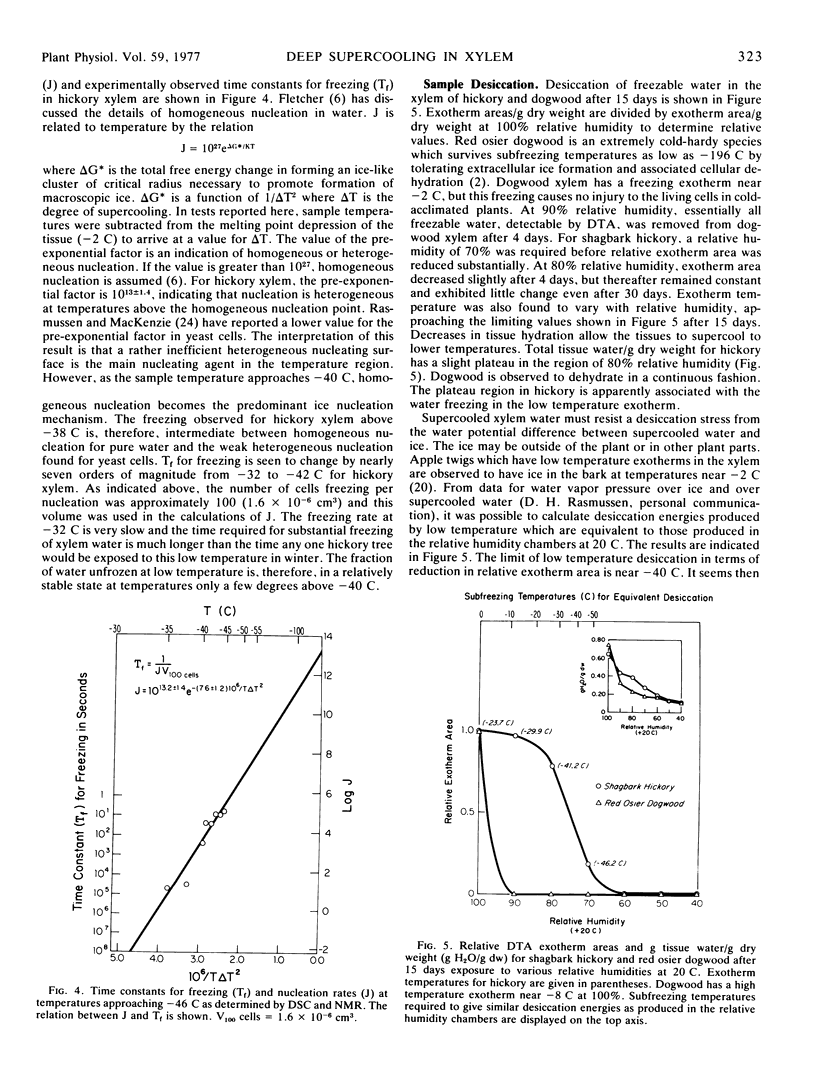
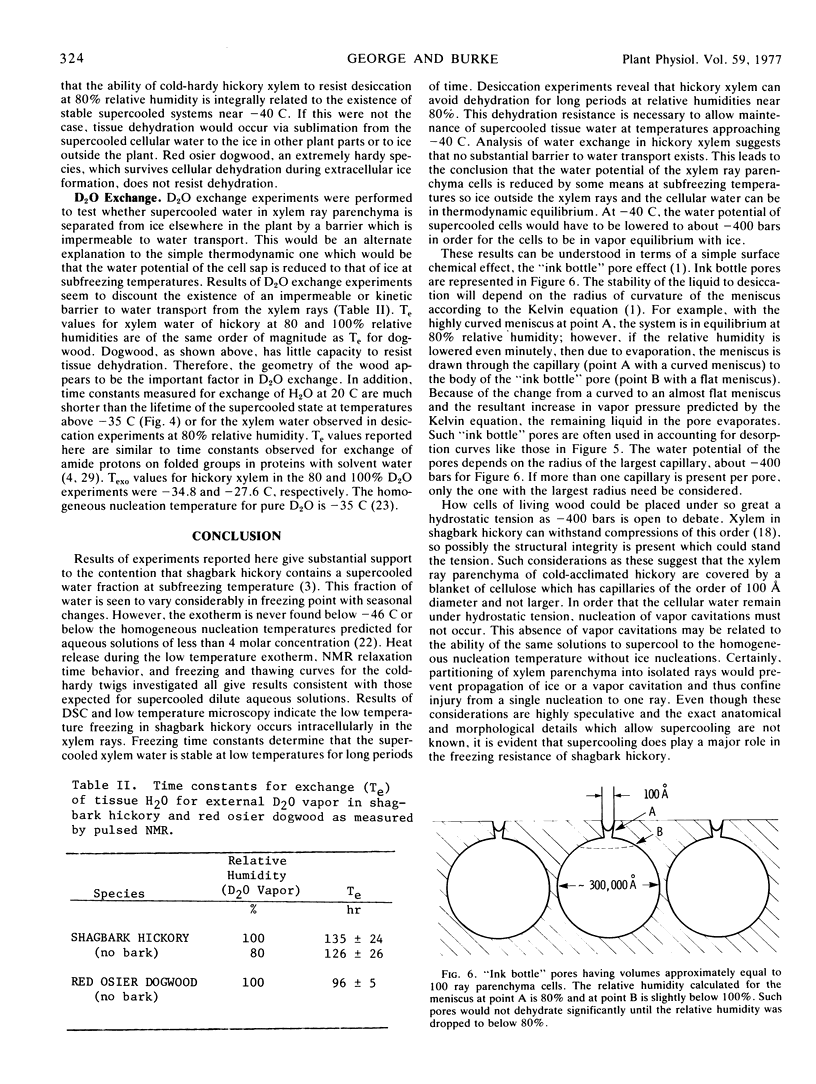
Abstract
Differential thermal analysis, differential scanning calorimetry, pulsed nuclear magnetic resonance spectroscopy, and low temperature microscopy are utilized to investigate low temperature freezing points or exotherms which occur near −40 C in the xylem of cold-acclimated shagbark hickory (Carya ovata L.). Experiments using these methods demonstrate that the low temperature exotherm results from the freezing of cellular water in a manner predicted for supercooled dilute aqueous solutions. Heat release on freezing, nuclear magnetic resonance relaxation times, and freezing and thawing curves for hickory twigs all point to a supercooled fraction in the xylem at subfreezing temperatures. Calorimetric and low temperature microscopic analyses indicate that freezing occurs intracellularly in the xylem ray parenchyma. The supercooled fraction is found to be extremely stable, even at temperatures only slightly above the homogeneous nucleation temperature for water (−38 C). Xylem water is also observed to be resistant to dehydration when exposed to 80% relative humidity at 20 C. D2O exchange experiments find that only a weak kinetic barrier to water transport exists in the xylem rays of shagbark hickory.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke M. J. Nuclear magnetic resonance of water in cold acclimating red osier dogwood stem. Plant Physiol. 1974 Sep;54(3):392–398. doi: 10.1104/pp.54.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L. M., Bloomfield V. A., Woodward C. K. Hydrogen-tritium exchange kinetics of soybean trypsin inhibitor (Kunitz). Solvent accessibility in the folded conformation. Biochemistry. 1975 Jul 29;14(15):3413–3419. doi: 10.1021/bi00686a019. [DOI] [PubMed] [Google Scholar]
- George M. F., Burke M. J. Supercooling in overwintering azalea flower buds: additional freezing parameters. Plant Physiol. 1977 Feb;59(2):326–328. doi: 10.1104/pp.59.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George M. F., Burke M. J., Weiser C. J. Supercooling in overwintering azalea flower buds. Plant Physiol. 1974 Jul;54(1):29–35. doi: 10.1104/pp.54.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusta L. V. Determination of unfrozen water in winter cereals at subfreezing temperatures. Plant Physiol. 1975 Nov;56(5):707–709. doi: 10.1104/pp.56.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen D. H., Macaulay M. N., MacKenzie A. P. Supercooling and nucleation of ice in single cells. Cryobiology. 1975 Aug;12(4):328–339. doi: 10.1016/0011-2240(75)90006-1. [DOI] [PubMed] [Google Scholar]
- Weiser C. J. Cold Resistance and Injury in Woody Plants: Knowledge of hardy plant adaptations to freezing stress may help us to reduce winter damage. Science. 1970 Sep 25;169(3952):1269–1278. doi: 10.1126/science.169.3952.1269. [DOI] [PubMed] [Google Scholar]
- Woodward C. K., Ellis L. M., Rosenberg A. The solvent dependence of hydrogen exchange kinetics of folded proteins. J Biol Chem. 1975 Jan 25;250(2):440–444. [PubMed] [Google Scholar]


