Abstract
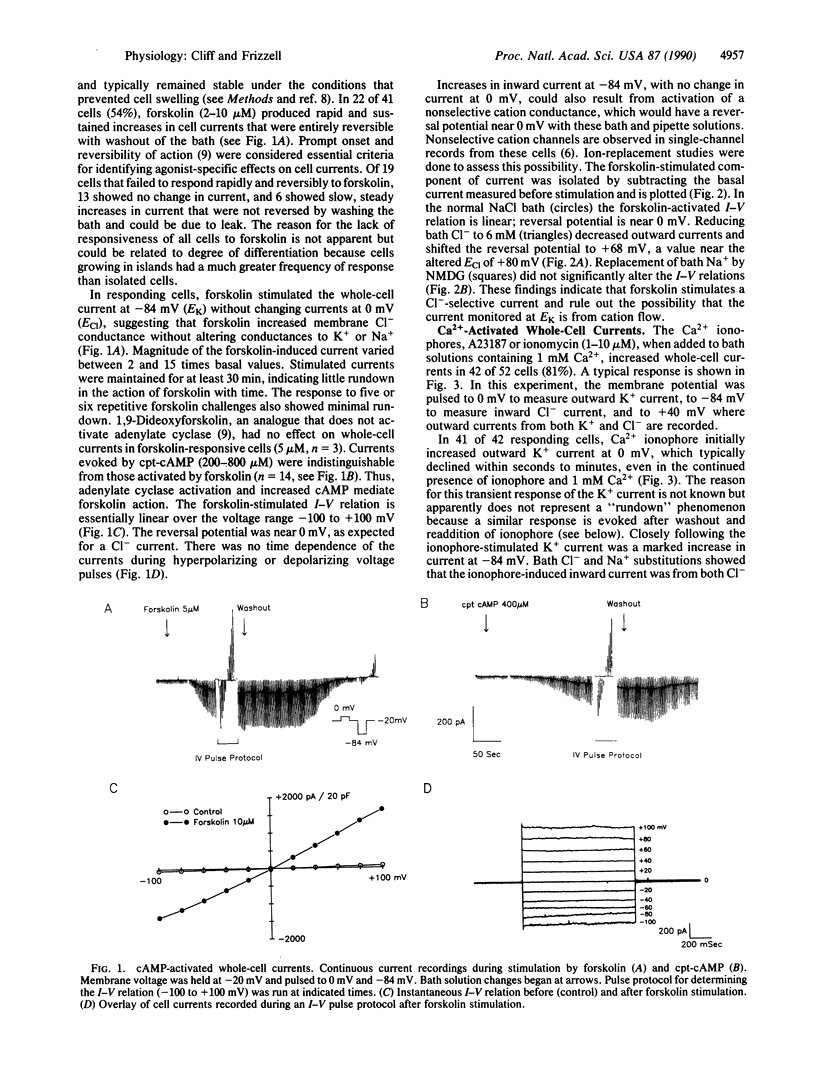
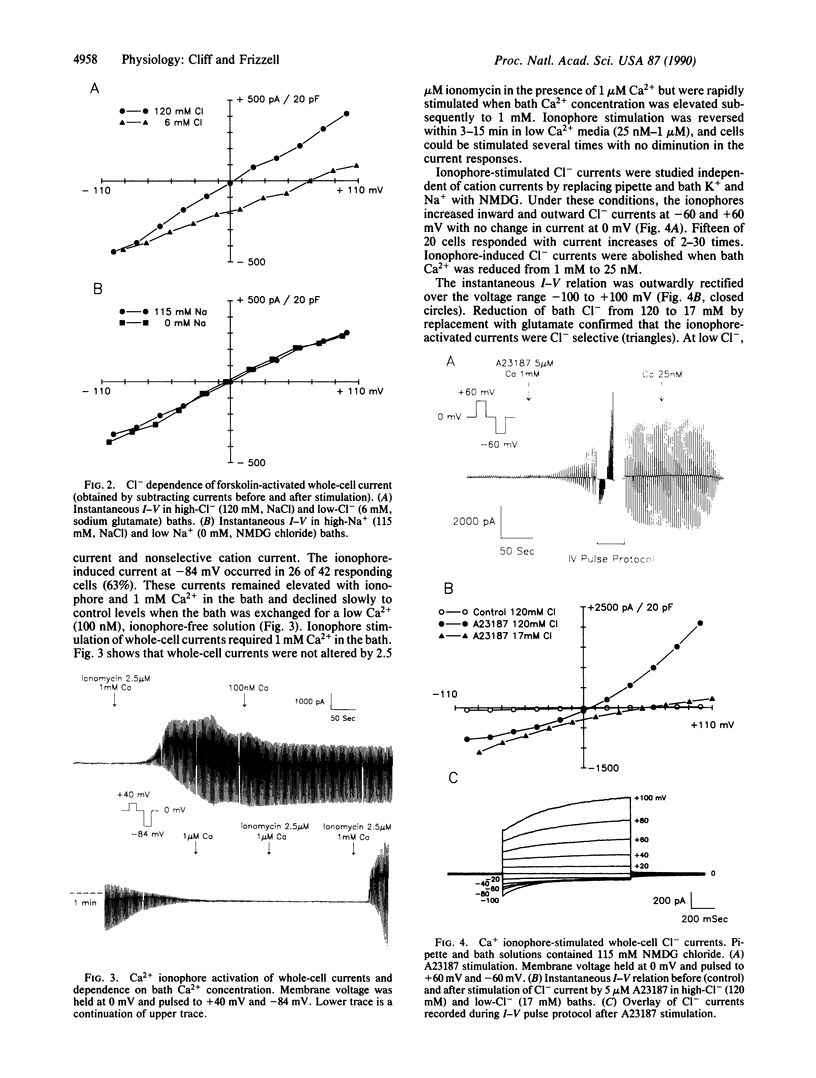
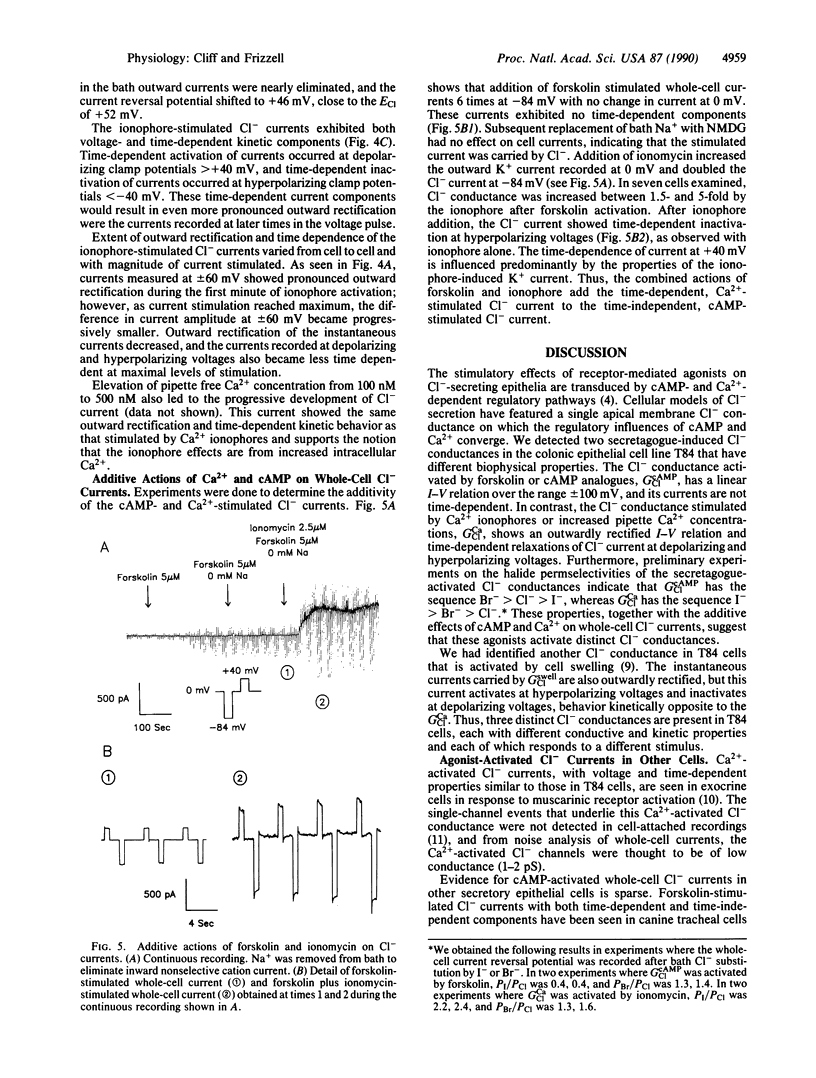
We studied the cAMP- and Ca2(+)-activated secretory Cl- conductances in the Cl(-)-secreting colonic epithelial cell line T84 using the whole-cell patch-clamp technique. Cl- and K+ currents were measured under voltage clamp. Forskolin or cAMP increased Cl- current 2-15 times with no change in K+ current. The current-voltage relation for cAMP-activated Cl- current was linear from -100 to +100 mV and showed no time-dependent changes in current during voltage pulses. Ca2+ ionophores or increased pipette Ca2+ increased both Cl- and K+ currents 2-30 times. The Ca2(+)-activated Cl- current was outwardly rectified, activated during depolarizing voltage pulses, and inactivated during hyperpolarizing voltage pulses. Addition of ionophore after forskolin further increased Cl- conductance 1.5-5 times, and the current took on the time-dependent characteristics of that stimulated by Ca2+. Thus, cAMP and Ca2+ activate Cl- conductances with different properties, implying that these second messengers activate different Cl- channels or that they induce different conductive and kinetic states in the same Cl- channel.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahinski A., Nairn A. C., Greengard P., Gadsby D. C. Chloride conductance regulated by cyclic AMP-dependent protein kinase in cardiac myocytes. Nature. 1989 Aug 31;340(6236):718–721. doi: 10.1038/340718a0. [DOI] [PubMed] [Google Scholar]
- Dharmsathaphorn K., McRoberts J. A., Mandel K. G., Tisdale L. D., Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol. 1984 Feb;246(2 Pt 1):G204–G208. doi: 10.1152/ajpgi.1984.246.2.G204. [DOI] [PubMed] [Google Scholar]
- Duszyk M., French A. S., Man S. F. The 20-pS chloride channel of the human airway epithelium. Biophys J. 1990 Feb;57(2):223–230. doi: 10.1016/S0006-3495(90)82525-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. G., Marty A. Calcium-dependent chloride currents in isolated cells from rat lacrimal glands. J Physiol. 1986 Sep;378:437–460. doi: 10.1113/jphysiol.1986.sp016229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzell R. A., Halm D. R., Rechkemmer G., Shoemaker R. L. Chloride channel regulation in secretory epithelia. Fed Proc. 1986 Nov;45(12):2727–2731. [PubMed] [Google Scholar]
- Giraldez F., Murray K. J., Sepúlveda F. V., Sheppard D. N. Characterization of a phosphorylation-activated Cl-selective channel in isolated Necturus enterocytes. J Physiol. 1989 Sep;416:517–537. doi: 10.1113/jphysiol.1989.sp017775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. A., Harris A., Coleman L., Greenwell J. R., Argent B. E. Two types of chloride channel on duct cells cultured from human fetal pancreas. Am J Physiol. 1989 Aug;257(2 Pt 1):C240–C251. doi: 10.1152/ajpcell.1989.257.2.C240. [DOI] [PubMed] [Google Scholar]
- Halm D. R., Rechkemmer G. R., Schoumacher R. A., Frizzell R. A. Apical membrane chloride channels in a colonic cell line activated by secretory agonists. Am J Physiol. 1988 Apr;254(4 Pt 1):C505–C511. doi: 10.1152/ajpcell.1988.254.4.C505. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harvey R. D., Hume J. R. Autonomic regulation of a chloride current in heart. Science. 1989 May 26;244(4907):983–985. doi: 10.1126/science.2543073. [DOI] [PubMed] [Google Scholar]
- Krouse M. E., Hagiwara G., Chen J., Lewiston N. J., Wine J. J. Ion channels in normal human and cystic fibrosis sweat gland cells. Am J Physiol. 1989 Jul;257(1 Pt 1):C129–C140. doi: 10.1152/ajpcell.1989.257.1.C129. [DOI] [PubMed] [Google Scholar]
- Liedtke C. M. Regulation of chloride transport in epithelia. Annu Rev Physiol. 1989;51:143–160. doi: 10.1146/annurev.ph.51.030189.001043. [DOI] [PubMed] [Google Scholar]
- Marty A., Tan Y. P., Trautmann A. Three types of calcium-dependent channel in rat lacrimal glands. J Physiol. 1984 Dec;357:293–325. doi: 10.1113/jphysiol.1984.sp015501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R., Matthews G., Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988 Aug 11;334(6182):499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Schoppa N., Shorofsky S. R., Jow F., Nelson D. J. Voltage-gated chloride currents in cultured canine tracheal epithelial cells. J Membr Biol. 1989 Apr;108(1):73–90. doi: 10.1007/BF01870427. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res. 1981;7(4):201–224. [PubMed] [Google Scholar]
- Welsh M. J. Electrolyte transport by airway epithelia. Physiol Rev. 1987 Oct;67(4):1143–1184. doi: 10.1152/physrev.1987.67.4.1143. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Li M., McCann J. D. Activation of normal and cystic fibrosis Cl- channels by voltage, temperature, and trypsin. J Clin Invest. 1989 Dec;84(6):2002–2007. doi: 10.1172/JCI114391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrell R. T., Butt A. G., Cliff W. H., Frizzell R. A. A volume-sensitive chloride conductance in human colonic cell line T84. Am J Physiol. 1989 Jun;256(6 Pt 1):C1111–C1119. doi: 10.1152/ajpcell.1989.256.6.C1111. [DOI] [PubMed] [Google Scholar]







