Abstract
7-Hydroxy-2,3-dihydrobenzofuran derivatives, metabolites of a carbamate insecticide carbofuran, and five other phenolic inhibitors of indoleacetic acid (IAA) oxidase interfered with IAA-induced spectral change in the Soret band of horseradish peroxidase (HRP). The onset of IAA degradation required transformed HRP intermediates. The inhibitors, when added before IAA, protected HRP from reacting with IAA, thus preventing formation of highly reactive enzyme intermediates, and consequently, IAA degradation. When added after IAA, the inhibitors quickly reversed the IAA-induced spectral change of HRP and inhibited further IAA degradation.
The phenolic inhibitors differed in stability and reactivity. 7-Hydroxy-2,2-dimethyl-2,3-dihydrobenzofuran, 3,7-dihydroxy-2,2-dimethyl-2,3-dihydrobenzofuran, catechol, protocatechuic acid, caffeic acid, ferulic acid, and scopoletin belonged to one group which produced only a temporary inhibition to IAA-induced spectral change of HRP and IAA degradation since the inhibitors were metabolized in the reaction. The length of the lag was dependent on the IAA, inhibitor, and enzyme concentrations. 3-Keto-7-hydroxy-2,2-dimethyl-2,3-dihydrobenzofuran and 3-keto-carbofuran belonged to the other group which produced a persistent inhibition.
Degradation of IAA required both the heme group and apoprotein of HRP. Reconstituted enzyme from bovine hemin and apoprotein or HRP after unfolding by urea or guanidine treatment were inhibited by the inhibitors in a way similar to the native HRP. The inhibition was reversible by higher concentrations of IAA, but the plot of 1/v versus 1/s and 1/v versus i were curvilinear, reflecting the complex nature of a competitive inhibition.
Full text
PDF
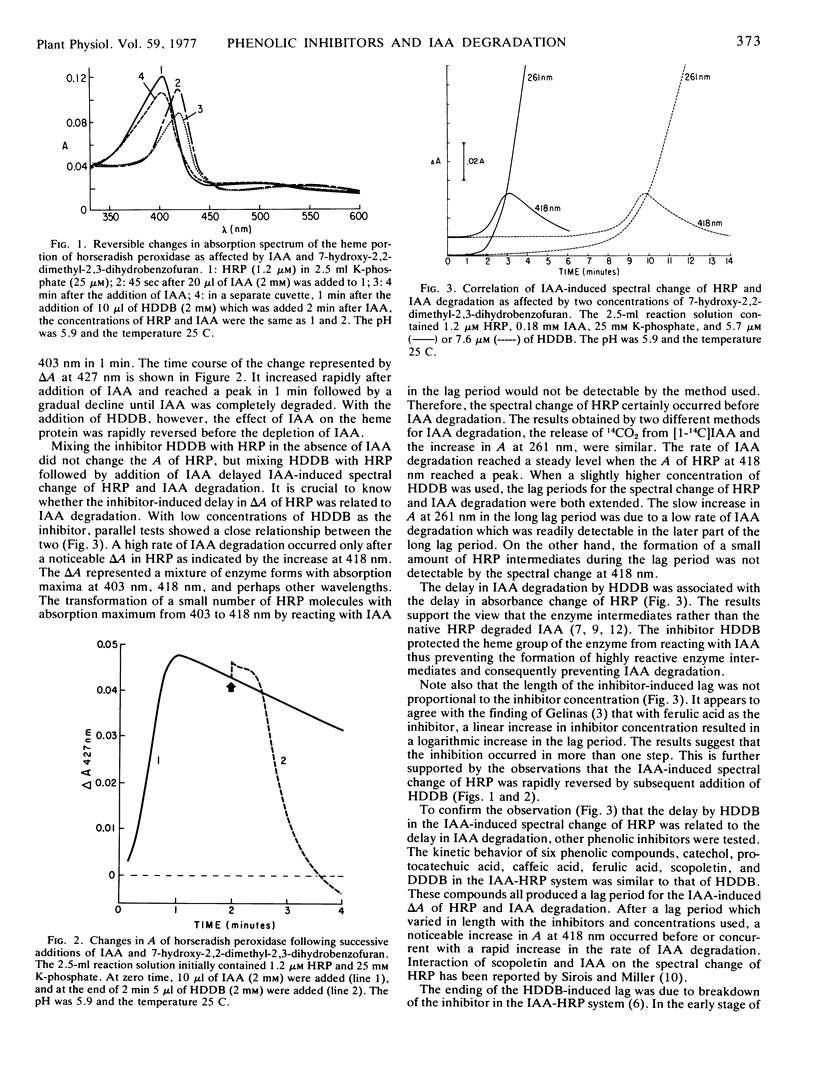
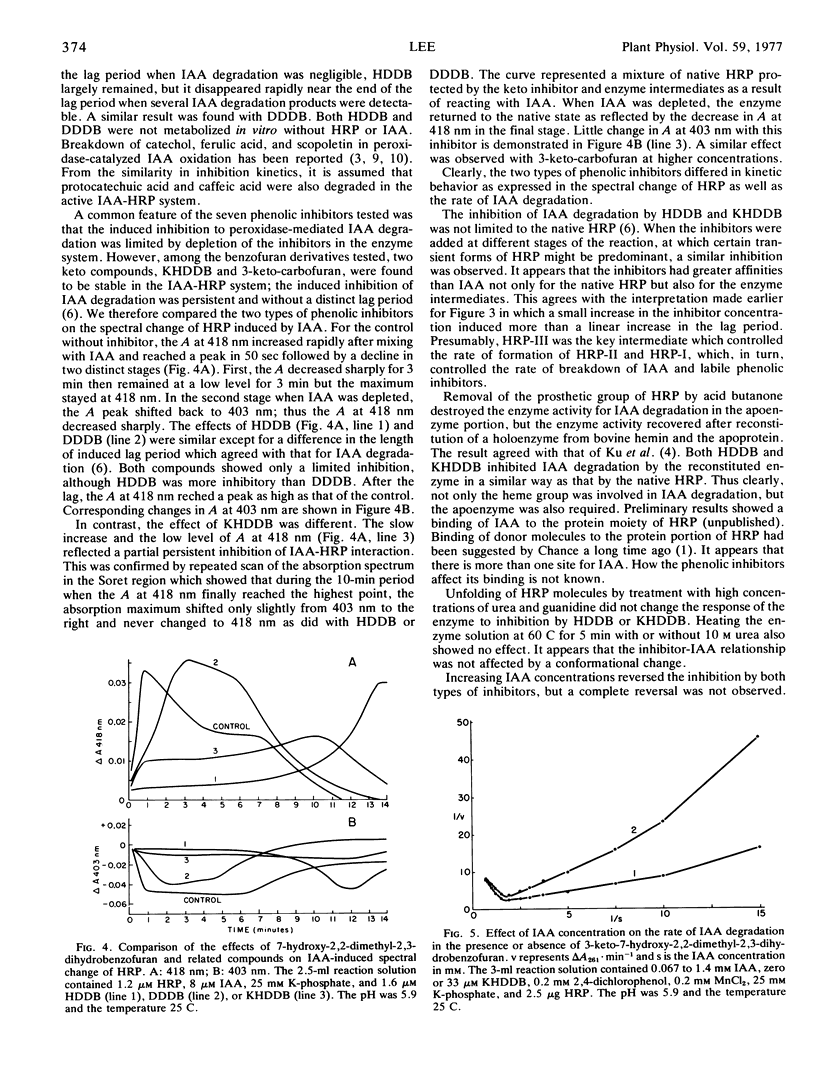
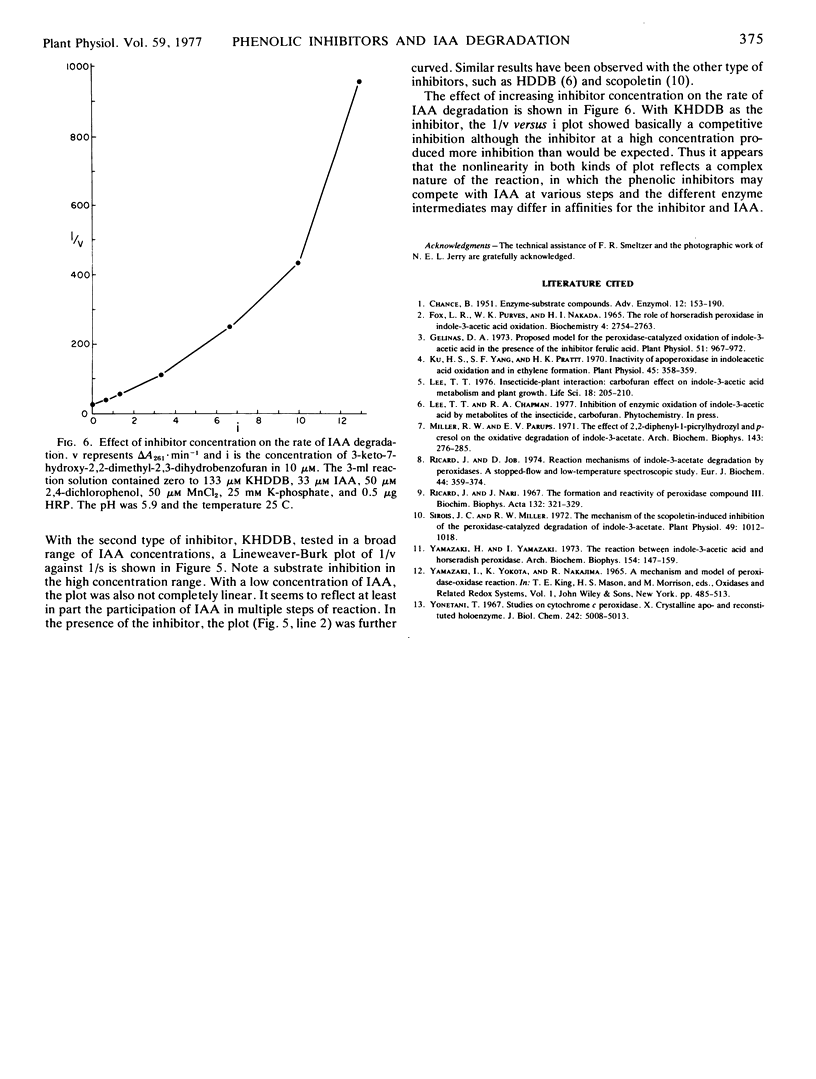
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANCE B. Enzyme-substrate compounds. Adv Enzymol Relat Subj Biochem. 1951;12:153–190. doi: 10.1002/9780470122570.ch3. [DOI] [PubMed] [Google Scholar]
- Fox L. R., Purves W. K., Nakada H. I. The role of horseradish peroxidase in indole-3-acetic acid oxidation. Biochemistry. 1965 Dec;4(12):2754–2763. doi: 10.1021/bi00888a028. [DOI] [PubMed] [Google Scholar]
- Gelinas D. A. Proposed Model for the Peroxidase-Catalyzed Oxidation of Indole-3-acetic Acid in the Presence of the Inhibitor Ferulic Acid. Plant Physiol. 1973 May;51(5):967–972. doi: 10.1104/pp.51.5.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku H. S., Yang S. F., Pratt H. K. Inactivity of apoperoxidase in indoleacetic acid oxidation and in ethylene formation. Plant Physiol. 1970 Mar;45(3):358–359. doi: 10.1104/pp.45.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. T. Insecticide - plant interaction: carbofuran effect on indole-3-acetic acid metabolism and plant growth. Life Sci. 1976 Jan 15;18(2):205–210. doi: 10.1016/0024-3205(76)90026-6. [DOI] [PubMed] [Google Scholar]
- Miller R. W., Parups E. V. The effect of 2,2-diphenyl-1-picrylhydrazyl and p-cresol on the oxidative degradation of indole-3-acetate. Arch Biochem Biophys. 1971 Mar;143(1):276–285. doi: 10.1016/0003-9861(71)90210-4. [DOI] [PubMed] [Google Scholar]
- Ricard J., Job D. Reaction mechanisms of indole-3-acetate degradation by peroxidases. A stopped-flow and low-temperature spectroscopic study. Eur J Biochem. 1974 May 15;44(2):359–374. doi: 10.1111/j.1432-1033.1974.tb03493.x. [DOI] [PubMed] [Google Scholar]
- Ricard J., Nari J. The formation and reactivity of peroxidase compound 3. Biochim Biophys Acta. 1967 Mar 15;132(2):321–329. doi: 10.1016/0005-2744(67)90151-9. [DOI] [PubMed] [Google Scholar]
- Sirois J. C., Miller R. W. The Mechanism of the Scopoletin-induced Inhibition of the Peroxidase Catalyzed Degradation of Indole-3-acetate. Plant Physiol. 1972 Jun;49(6):1012–1018. doi: 10.1104/pp.49.6.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki H., Yamazaki I. The reaction between indole 3-acetic acid and horseradish peroxidase. Arch Biochem Biophys. 1973 Jan;154(1):147–159. doi: 10.1016/0003-9861(73)90043-x. [DOI] [PubMed] [Google Scholar]
- Yonetani T. Studies on cytochrome c peroxidase. X. Crystalline apo-and reconstituted holoenzymes. J Biol Chem. 1967 Nov 10;242(21):5008–5013. [PubMed] [Google Scholar]


