Abstract
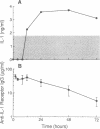
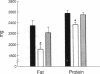
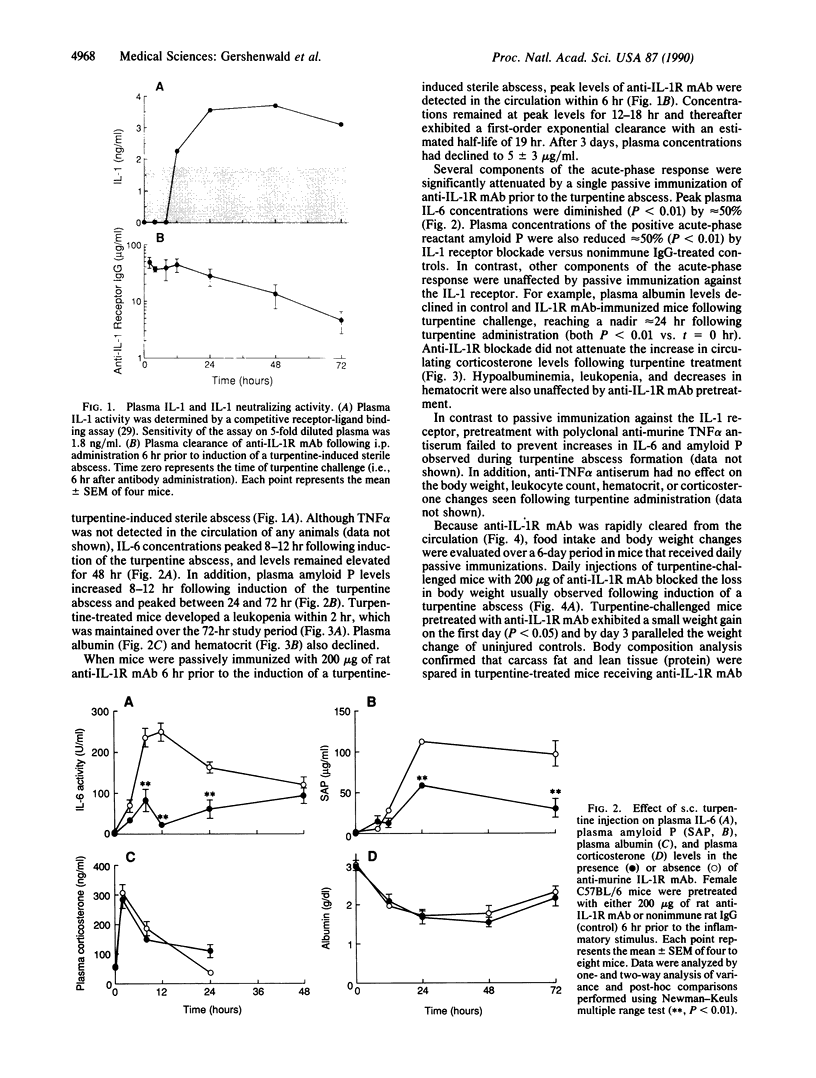
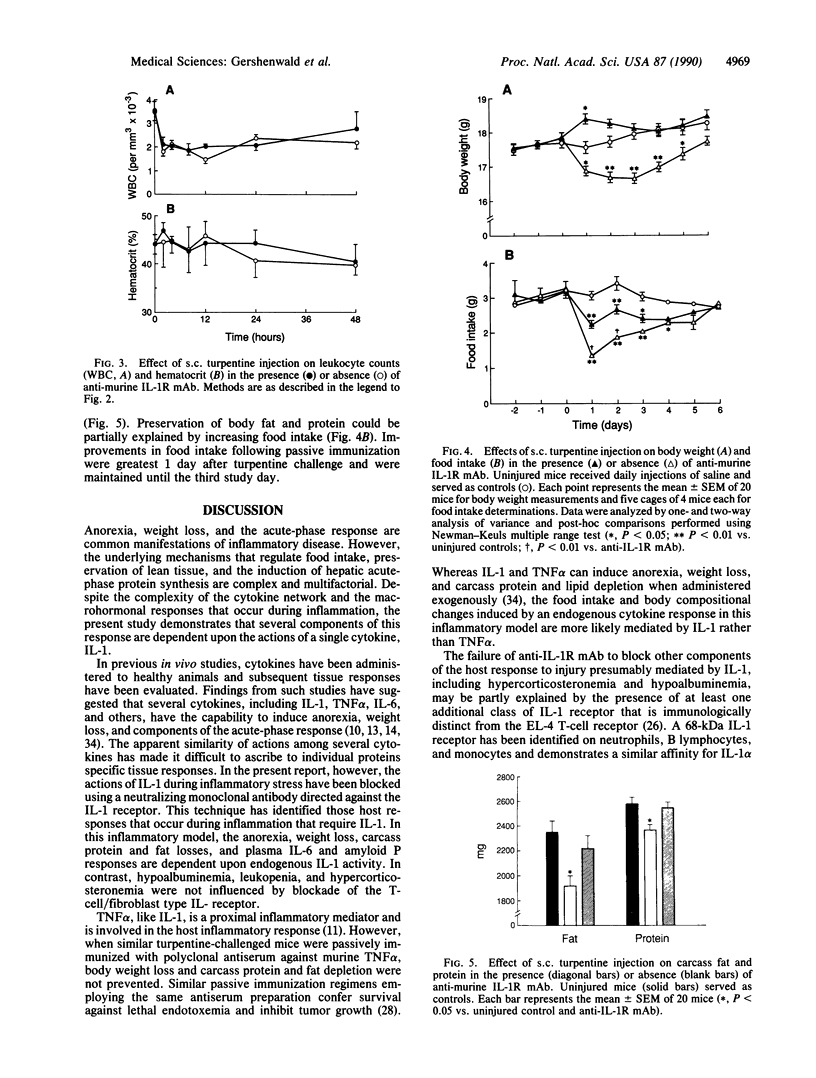
Cytokines, including interleukin 1 (IL-1), tumor necrosis factor alpha, and interleukin 6, are often produced in response to tissue injury and contribute to several host responses such as weight loss, anorexia, and acute-phase protein synthesis. However, the role of IL-1 in specific tissue responses is unclear. To test our hypothesis that specific in vivo blockade of IL-1's action might inhibit the catabolic host changes associated with inflammation, mice were passively immunized with a monoclonal antibody directed against the murine IL-1 receptor prior to initiation of a turpentine-induced sterile abscess. This antibody prevents IL-1-mediated proliferation of murine thymocytes in vitro by inhibiting IL-1 alpha and IL-1 beta by way of competition for a common receptor. Weight loss following turpentine challenge was prevented by daily injections of anti-IL-1 receptor monoclonal IgG. Body composition analysis confirmed that lean tissue and fat were preserved by passive immunization. Furthermore, pretreatment with an anti-IL-1 receptor monoclonal antibody significantly attenuated the plasma amyloid P and interleukin 6 responses but did not affect the decline in plasma albumin or the increase in circulating corticosterone. Passive immunization of similar mice with polyclonal antisera against another cytokine, tumor necrosis factor alpha, failed to prevent either the weight loss or hepatic acute-phase protein changes observed in this inflammatory model. These findings suggest that IL-1 orchestrates weight loss and body compositional changes during inflammation and contributes to the induction of interleukin 6 and acute-phase protein synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Bagby G. C., Jr, Dinarello C. A., Wallace P., Wagner C., Hefeneider S., McCall E. Interleukin 1 stimulates granulocyte macrophage colony-stimulating activity release by vascular endothelial cells. J Clin Invest. 1986 Nov;78(5):1316–1323. doi: 10.1172/JCI112717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H., Richards C., Gauldie J. Interaction among hepatocyte-stimulating factors, interleukin 1, and glucocorticoids for regulation of acute phase plasma proteins in human hepatoma (HepG2) cells. J Immunol. 1987 Dec 15;139(12):4122–4128. [PubMed] [Google Scholar]
- Beisel W. R. Metabolic response to infection. Annu Rev Med. 1975;26:9–20. doi: 10.1146/annurev.me.26.020175.000301. [DOI] [PubMed] [Google Scholar]
- Bird T. A., Gearing A. J., Saklatvala J. Murine interleukin 1 receptor. Direct identification by ligand blotting and purification to homogeneity of an interleukin 1-binding glycoprotein. J Biol Chem. 1988 Aug 25;263(24):12063–12069. [PubMed] [Google Scholar]
- Chizzonite R., Truitt T., Kilian P. L., Stern A. S., Nunes P., Parker K. P., Kaffka K. L., Chua A. O., Lugg D. K., Gubler U. Two high-affinity interleukin 1 receptors represent separate gene products. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8029–8033. doi: 10.1073/pnas.86.20.8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Brown J. F., Young K. M., Cooley A. J., Kurtz R. S. Effects of murine recombinant interleukin 1 alpha on the host response to bacterial infection. J Immunol. 1988 Feb 1;140(3):962–968. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and the pathogenesis of the acute-phase response. N Engl J Med. 1984 Nov 29;311(22):1413–1418. doi: 10.1056/NEJM198411293112205. [DOI] [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Fong Y., Moldawer L. L., Marano M., Wei H., Barber A., Manogue K., Tracey K. J., Kuo G., Fischman D. A., Cerami A. Cachectin/TNF or IL-1 alpha induces cachexia with redistribution of body proteins. Am J Physiol. 1989 Mar;256(3 Pt 2):R659–R665. doi: 10.1152/ajpregu.1989.256.3.R659. [DOI] [PubMed] [Google Scholar]
- Fong Y., Moldawer L. L., Marano M., Wei H., Tatter S. B., Clarick R. H., Santhanam U., Sherris D., May L. T., Sehgal P. B. Endotoxemia elicits increased circulating beta 2-IFN/IL-6 in man. J Immunol. 1989 Apr 1;142(7):2321–2324. [PubMed] [Google Scholar]
- Harvey K. B., Moldawer L. L., Bistrian B. R., Blackburn G. L. Biological measures for the formulation of a hospital prognostic index. Am J Clin Nutr. 1981 Oct;34(10):2013–2022. doi: 10.1093/ajcn/34.10.2013. [DOI] [PubMed] [Google Scholar]
- Hesse D. G., Tracey K. J., Fong Y., Manogue K. R., Palladino M. A., Jr, Cerami A., Shires G. T., Lowry S. F. Cytokine appearance in human endotoxemia and primate bacteremia. Surg Gynecol Obstet. 1988 Feb;166(2):147–153. [PubMed] [Google Scholar]
- Hurme M., Palkama T., Sihvola M. Interleukin-4 inhibits interleukin-1 synthesis by a posttranscriptional mechanism. Biochem Biophys Res Commun. 1988 Dec 30;157(3):861–866. doi: 10.1016/s0006-291x(88)80954-9. [DOI] [PubMed] [Google Scholar]
- Keith L. D., Winslow J. R., Reynolds R. W. A general procedure for estimation of corticosteroid response in individual rats. Steroids. 1978 Apr;31(4):523–531. doi: 10.1016/0039-128x(78)90034-x. [DOI] [PubMed] [Google Scholar]
- Kohase M., May L. T., Tamm I., Vilcek J., Sehgal P. B. A cytokine network in human diploid fibroblasts: interactions of beta-interferons, tumor necrosis factor, platelet-derived growth factor, and interleukin-1. Mol Cell Biol. 1987 Jan;7(1):273–280. doi: 10.1128/mcb.7.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurell C. B. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21–37. doi: 10.3109/00365517209102748. [DOI] [PubMed] [Google Scholar]
- Le J., Vilcek J. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Invest. 1987 Mar;56(3):234–248. [PubMed] [Google Scholar]
- McIntyre K. W., Unowsky J., DeLorenzo W., Benjamin W. Enhancement of antibacterial resistance of neutropenic, bone marrow-suppressed mice by interleukin-1 alpha. Infect Immun. 1989 Jan;57(1):48–54. doi: 10.1128/iai.57.1.48-54.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel S. B. Interleukin 1 and T cell activation. Immunol Rev. 1982;63:51–72. doi: 10.1111/j.1600-065x.1982.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Moldawer L. L., Andersson C., Gelin J., Lundholm K. G. Regulation of food intake and hepatic protein synthesis by recombinant-derived cytokines. Am J Physiol. 1988 Mar;254(3 Pt 1):G450–G456. doi: 10.1152/ajpgi.1988.254.3.G450. [DOI] [PubMed] [Google Scholar]
- Moldawer L. L., Gelin J., Scherstén T., Lundholm K. G. Circulating interleukin 1 and tumor necrosis factor during inflammation. Am J Physiol. 1987 Dec;253(6 Pt 2):R922–R928. doi: 10.1152/ajpregu.1987.253.6.R922. [DOI] [PubMed] [Google Scholar]
- Moldawer L. L., Lowry S. F., Cerami A. Cachectin: its impact on metabolism and nutritional status. Annu Rev Nutr. 1988;8:585–609. doi: 10.1146/annurev.nu.08.070188.003101. [DOI] [PubMed] [Google Scholar]
- Moldawer L. L., Sobrado J., Blackburn G. L., Bistrian B. R. A rationale for administering leukocyte endogenous mediator to protein malnourished, hospitalized patients. J Theor Biol. 1984 Jan 21;106(2):119–133. doi: 10.1016/0022-5193(84)90013-4. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganelli K. A., Stern A. S., Kilian P. L. Detergent solubilization of the interleukin 1 receptor. J Immunol. 1987 Apr 1;138(7):2249–2253. [PubMed] [Google Scholar]
- Sherry B. A., Gelin J., Fong Y., Marano M., Wei H., Cerami A., Lowry S. F., Lundholm K. G., Moldawer L. L. Anticachectin/tumor necrosis factor-alpha antibodies attenuate development of cachexia in tumor models. FASEB J. 1989 Jun;3(8):1956–1962. doi: 10.1096/fasebj.3.8.2721856. [DOI] [PubMed] [Google Scholar]
- Shiozawa S., Yoshikawa N., Iijima K., Negishi K. A sensitive radioimmunoassay for circulating alpha-interferon in the plasma of healthy children and patients with measles virus infection. Clin Exp Immunol. 1988 Sep;73(3):366–369. [PMC free article] [PubMed] [Google Scholar]
- Sims J. E., March C. J., Cosman D., Widmer M. B., MacDonald H. R., McMahan C. J., Grubin C. E., Wignall J. M., Jackson J. L., Call S. M. cDNA expression cloning of the IL-1 receptor, a member of the immunoglobulin superfamily. Science. 1988 Jul 29;241(4865):585–589. doi: 10.1126/science.2969618. [DOI] [PubMed] [Google Scholar]
- Sipe J. D. The molecular biology of interleukin 1 and the acute phase response. Adv Intern Med. 1989;34:1–20. [PubMed] [Google Scholar]
- Tellado-Rodriguez J., Christou N. V. Clinical assessment of host defense. Surg Clin North Am. 1988 Feb;68(1):41–55. doi: 10.1016/s0039-6109(16)44431-2. [DOI] [PubMed] [Google Scholar]
- Tellado J. M., Garcia-Sabrido J. L., Hanley J. A., Shizgal H. M., Christou N. V. Predicting mortality based on body composition analysis. Ann Surg. 1989 Jan;209(1):81–87. doi: 10.1097/00000658-198901000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Wei H., Manogue K. R., Fong Y., Hesse D. G., Nguyen H. T., Kuo G. C., Beutler B., Cotran R. S., Cerami A. Cachectin/tumor necrosis factor induces cachexia, anemia, and inflammation. J Exp Med. 1988 Mar 1;167(3):1211–1227. doi: 10.1084/jem.167.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdal D. L., Call S. M., Jackson J. L., Dower S. K. Affinity purification and chemical analysis of the interleukin-1 receptor. J Biol Chem. 1988 Feb 25;263(6):2870–2877. [PubMed] [Google Scholar]
- Vogel S. N., Douches S. D., Kaufman E. N., Neta R. Induction of colony stimulating factor in vivo by recombinant interleukin 1 alpha and recombinant tumor necrosis factor alpha 1. J Immunol. 1987 Apr 1;138(7):2143–2148. [PubMed] [Google Scholar]
- Waage A., Brandtzaeg P., Halstensen A., Kierulf P., Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989 Jan 1;169(1):333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucali J. R., Dinarello C. A., Oblon D. J., Gross M. A., Anderson L., Weiner R. S. Interleukin 1 stimulates fibroblasts to produce granulocyte-macrophage colony-stimulating activity and prostaglandin E2. J Clin Invest. 1986 Jun;77(6):1857–1863. doi: 10.1172/JCI112512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer J. W., Barza M., Wolff S. M., Dinarello C. A. A low dose of recombinant interleukin 1 protects granulocytopenic mice from lethal gram-negative infection. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1620–1623. doi: 10.1073/pnas.85.5.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]