Abstract
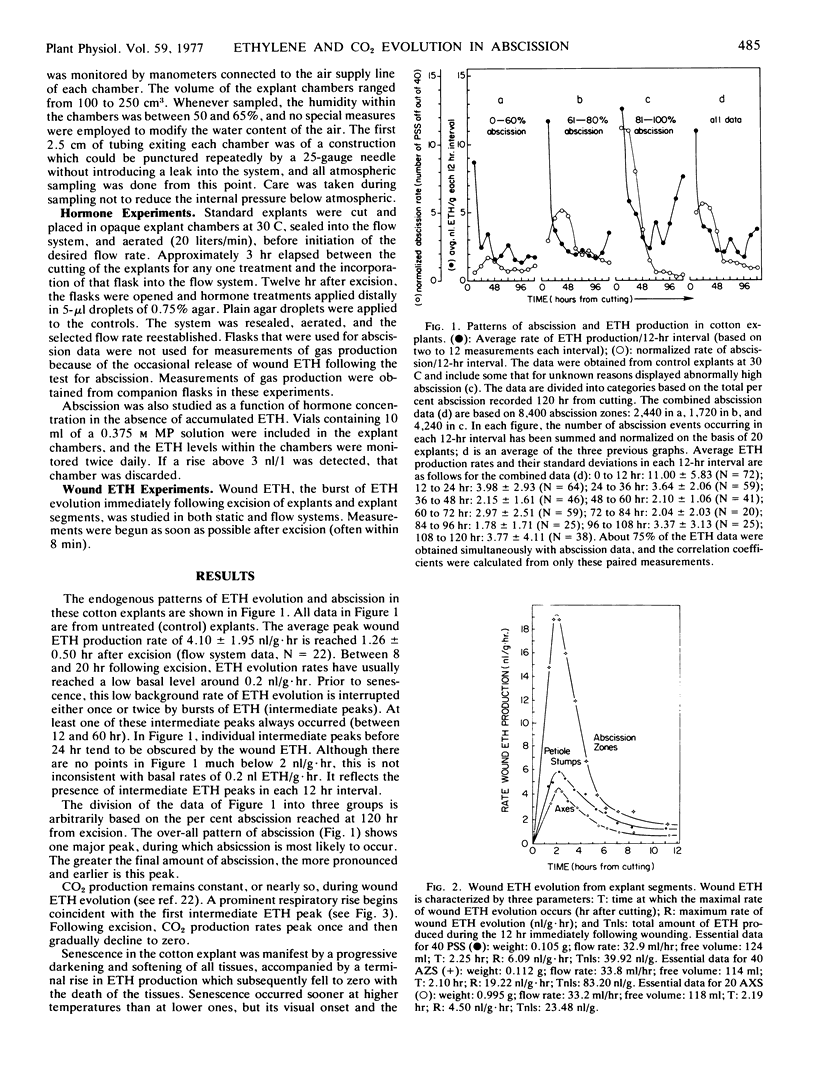
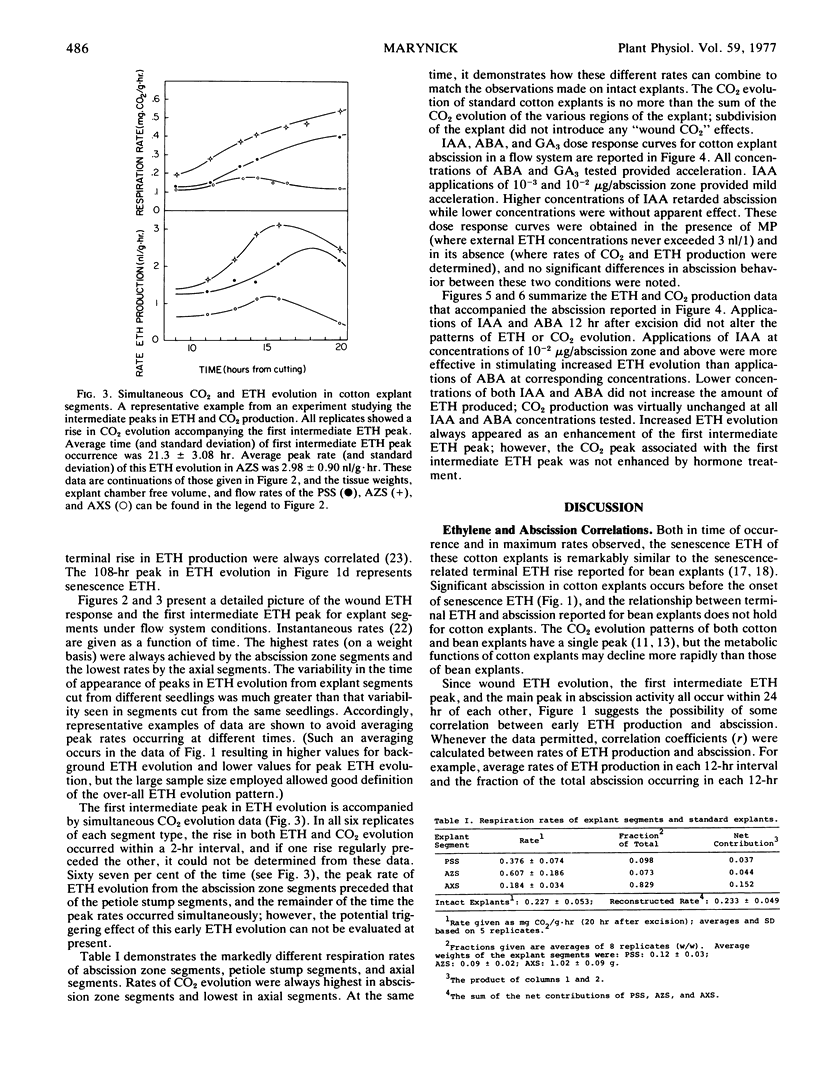
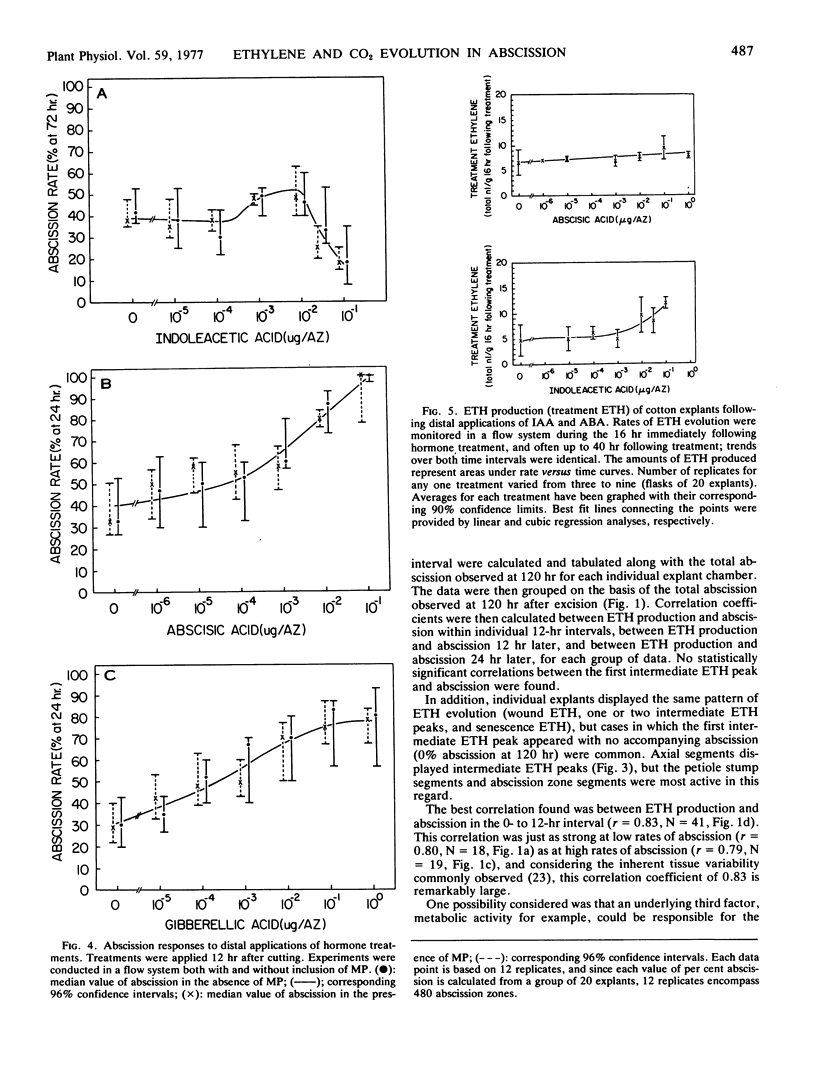
The relationship between abscission and the evolution of ethylene and CO2 was examined in explants and explant segments of cotton seedlings (Gossypium hirsutum L. cv. Acala SJ-1) under both static and flow system conditions, and in the presence and absence of mercuric perchlorate. Explant excision was immediately followed by increased ethylene evolution (wound ethylene); senescence was also accompanied by increased ethylene evolution (senescence ethylene). One or two ethylene peaks were found to interrupt the low background rate of ethylene evolution during the period between excision and senescence. The first intermediate ethylene peak coincided with a rise in CO2 evolution; however, precedence could not be established. No statistical correlations were discovered between either intermediate ethylene peak and abscission. The best statistical correlation was found between wound ethylene and abscission at 12 hr after excision. No positive correlations were found between senescence ethylene and abscission. Implications of these results for the understanding of the role of ethylene in explant abscission are discussed.
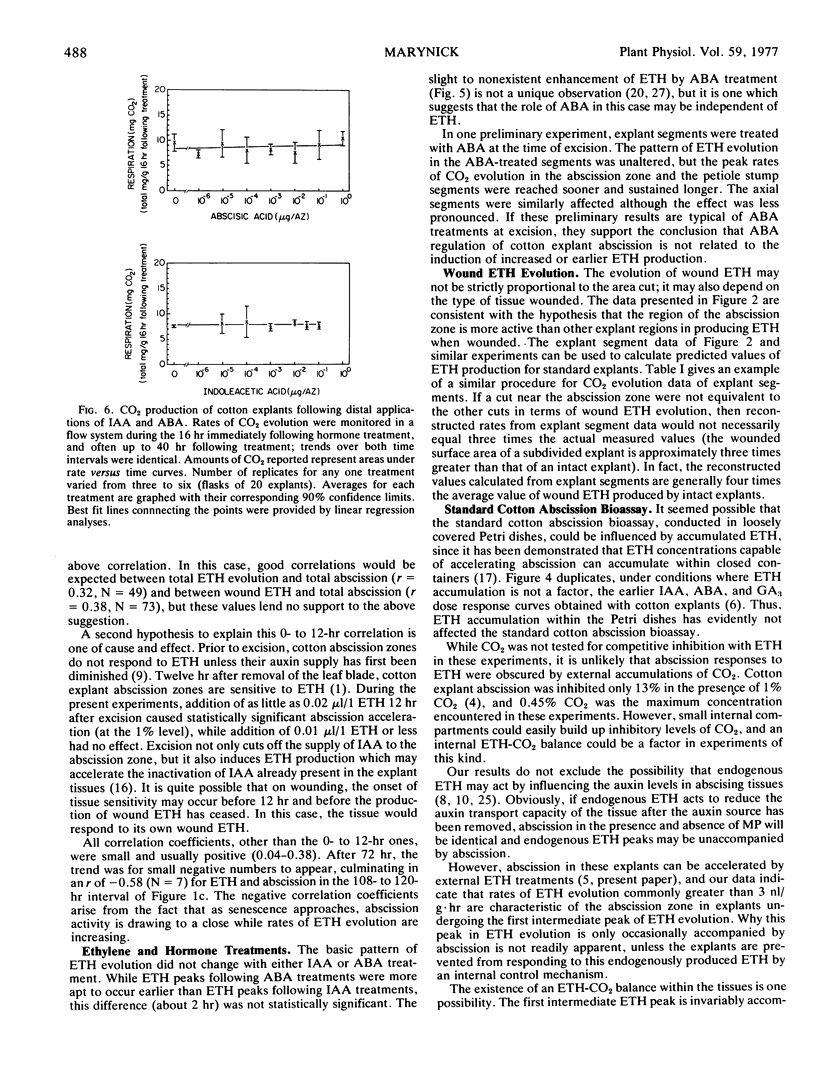
Relationships between a number of different explant treatments and ethylene evolution were also examined. Ethylene production in response to indoleacetic acid applications, abscisic acid applications, and different types of wounding is summarized. It was concluded that the results of the standard abscission bioassay (conducted in Petri dishes) have not been influenced by unnatural ethylene accumulations.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles F. B., Gahagan H. E. Abscission: the role of ethylene, ethylene analogues, carbon dioxide, and oxygen. Plant Physiol. 1968 Aug;43(8):1255–1258. doi: 10.1104/pp.43.8.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M. Abscission: support for a role of ethylene modification of auxin transport. Plant Physiol. 1973 Jul;52(1):1–5. doi: 10.1104/pp.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M. Abscission: the initial effect of ethylene is in the leaf blade. Plant Physiol. 1975 Feb;55(2):322–327. doi: 10.1104/pp.55.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M., Morgan P. W. Abscission: the role of ethylene modification of auxin transport. Plant Physiol. 1971 Aug;48(2):208–212. doi: 10.1104/pp.48.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeschl J. D. Concentration dependencies of some effects of ethylene on etiolated pea, peanut, bean, and cotton seedlings. Plant Physiol. 1975 Apr;55(4):670–677. doi: 10.1104/pp.55.4.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Osborne D. J. Ethylene, the natural regulator of leaf abscission. Nature. 1970 Mar 14;225(5237):1019–1022. doi: 10.1038/2251019a0. [DOI] [PubMed] [Google Scholar]
- Jordan W. R., Morgan P. W., Davenport T. L. Water Stress Enhances Ethylene-mediated Leaf Abscission in Cotton. Plant Physiol. 1972 Dec;50(6):756–758. doi: 10.1104/pp.50.6.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipe J. A., Morgan P. W. Ethylene, a regulator of young fruit abscission. Plant Physiol. 1973 May;51(5):949–953. doi: 10.1104/pp.51.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marynick D. S. A Mathematical Treatment of Rate Data Obtained in Biological Flow Systems under Nonsteady State Conditions. Plant Physiol. 1975 Nov;56(5):680–683. doi: 10.1104/pp.56.5.680. [DOI] [PMC free article] [PubMed] [Google Scholar]


