Abstract
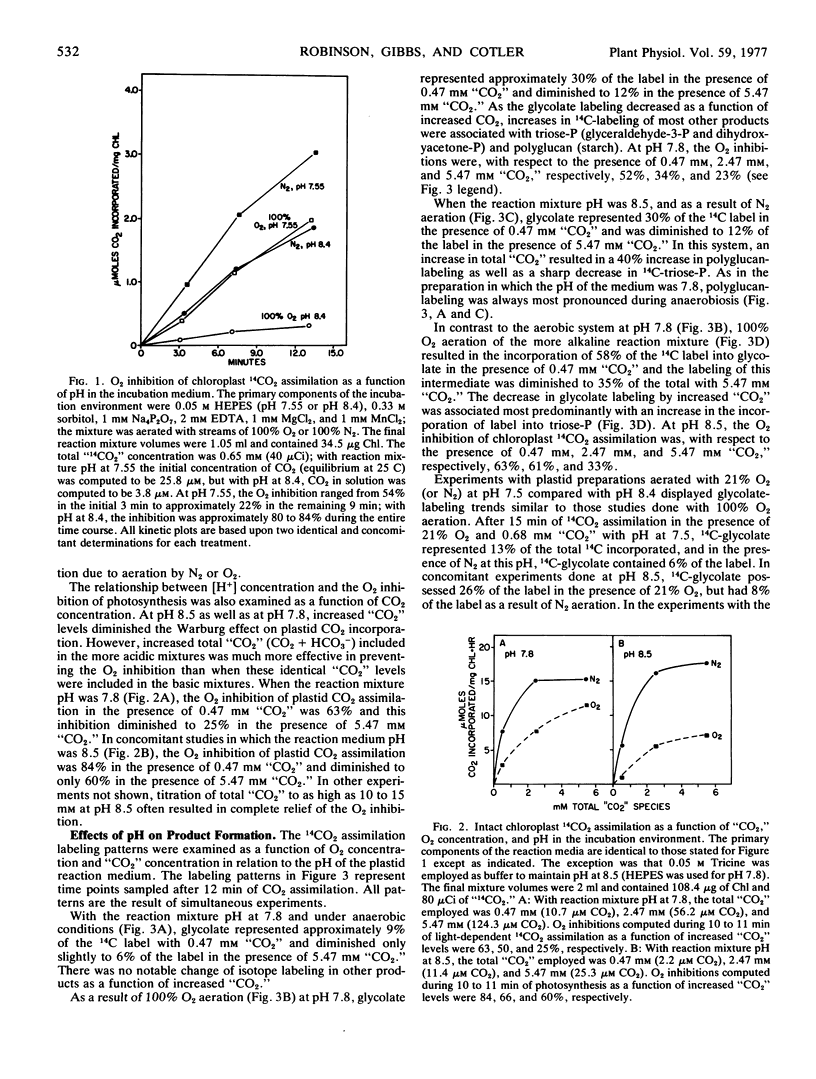
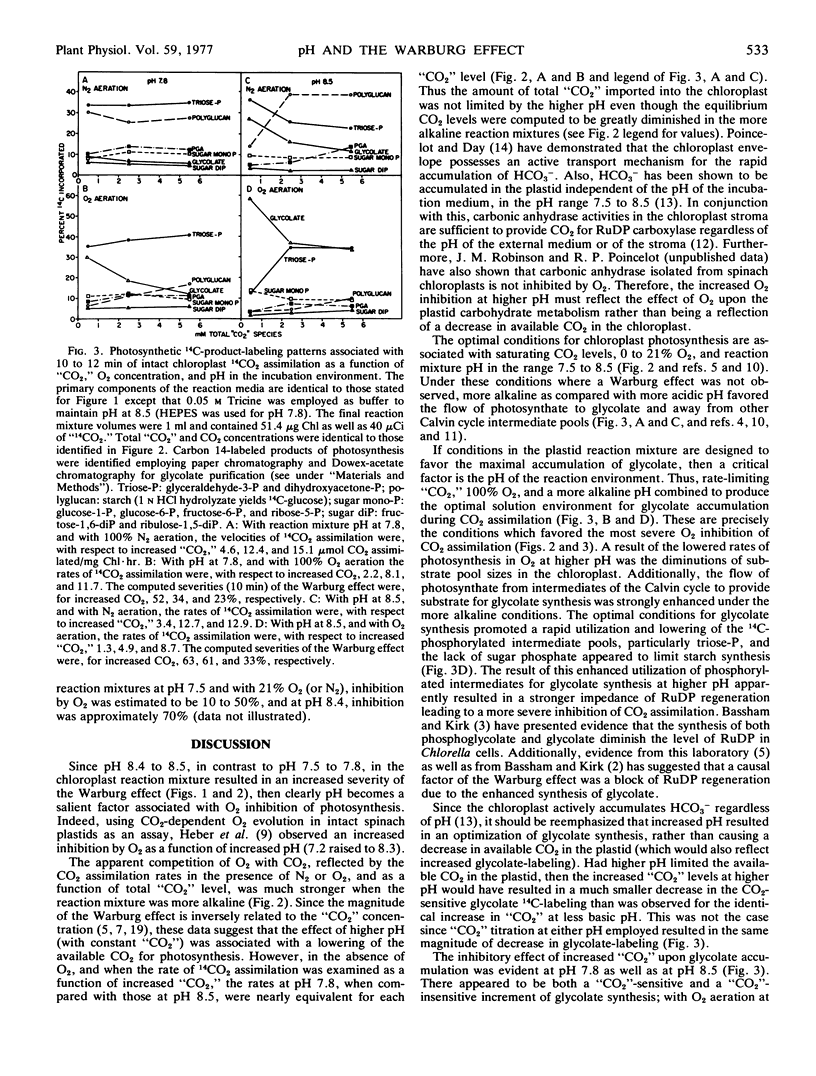
The influence of pH upon the O2 inhibition of 14CO2 photoassimilation (Warburg effect) was examined in intact spinach (Spinacia oleracea) chloroplasts. With conditions which favored the Warburg effect, i.e. rate-limiting CO2 and 100% O2, O2 inhibition was greater at pH 8.4 to 8.5 than at pH 7.5 to 7.8. At pH 8.5, as compared with 7.8, there was an enhanced 14C-labeling of glycolate, and a decrease of isotope in some phosphorylated Calvin cycle intermediates, particularly triose-phosphate. The 14C-labeling of starch was also more inhibited by O2 at higher pH. The enhanced synthesis of glycolate during 14CO2 assimilation at higher pH resulted in a diminution in the level of phosphorylated intermediates of the Calvin cycle, and this was apparently a causal factor of the increased severity of the Warburg effect.
The 14C-labeling profiles have been interpreted in terms of a “CO2”-sensitive as well as a “CO2”-insensitive mechanism for glycolate synthesis. Both mechanisms functioned optimally at the higher pH and both responded to O2.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BASSHAM J. A., KIRK M. The effect of oxygen on the reduction of CO2 to glycolic acid and other products during photosynthesis by Chlorella. Biochem Biophys Res Commun. 1962 Nov 27;9:376–380. doi: 10.1016/0006-291x(62)90019-0. [DOI] [PubMed] [Google Scholar]
- Bahr J. T., Jensen R. G. Ribulose Diphosphate Carboxylase from Freshly Ruptured Spinach Chloroplasts Having an in Vivo Km[CO(2)]. Plant Physiol. 1974 Jan;53(1):39–44. doi: 10.1104/pp.53.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassham J. A., Kirk M. Sequence of Formation of Phosphoglycolate and Glycolate in Photosynthesizing Chlorella pyrenoidosa. Plant Physiol. 1973 Nov;52(5):407–411. doi: 10.1104/pp.52.5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd W. A., Bidwell R. G. The Effect of pH on the Products of Photosynthesis in CO(2) by Chloroplast Preparations from Acetabularia mediterranea. Plant Physiol. 1971 Jun;47(6):779–783. doi: 10.1104/pp.47.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellyard P. W., Gibbs M. Inhibition of photosynthesis by oxygen in isolated spinach chloroplasts. Plant Physiol. 1969 Aug;44(8):1115–1121. doi: 10.1104/pp.44.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS B. H., EDSALL J. T. RATE OF HYDRATION OF CARBON DIOXIDE AND DEHYDRATION OF CARBONIC ACID AT 25 DEGREES. J Biol Chem. 1963 Oct;238:3502–3507. [PubMed] [Google Scholar]
- Gibbs M. Photorespiration, Warburg effect and glycolate. Ann N Y Acad Sci. 1969 Dec 19;168(2):356–368. doi: 10.1111/j.1749-6632.1969.tb43123.x. [DOI] [PubMed] [Google Scholar]
- Heber U., Andrews T. J., Boardman N. K. Effects of pH and Oxygen on Photosynthetic Reactions of Intact Chloroplasts. Plant Physiol. 1976 Feb;57(2):277–283. doi: 10.1104/pp.57.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth G. M., Tolbert N. E., Jimenez E. Rate of Glycolate Formation During Photosynthesis at High pH. Plant Physiol. 1966 Jan;41(1):143–147. doi: 10.1104/pp.41.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poincelot R. P., Day P. R. Isolation and bicarbonate transport of chloroplast envelope membranes from species of differing net photosynthetic efficiency. Plant Physiol. 1976 Feb;57(2):334–338. doi: 10.1104/pp.57.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poincelot R. P. Intracellular distribution of carbonic anhydrase in spinach leaves. Biochim Biophys Acta. 1972 Feb 28;258(2):637–642. doi: 10.1016/0005-2744(72)90255-0. [DOI] [PubMed] [Google Scholar]
- Poincelot R. P. Uptake of bicarbonate ion in darkness by isolated chloroplast envelope membranes and intact chloroplasts of spinach. Plant Physiol. 1974 Oct;54(4):520–526. doi: 10.1104/pp.54.4.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M., Gibbs M. Photosynthetic intermediates, the warburg effect, and glycolate synthesis in isolated spinach chloroplasts. Plant Physiol. 1974 Jun;53(6):790–797. doi: 10.1104/pp.53.6.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURNER J. S., BRITTAIN E. G. Oxygen as a factor in photosynthesis. Biol Rev Camb Philos Soc. 1962 Feb;37:130–170. doi: 10.1111/j.1469-185x.1962.tb01607.x. [DOI] [PubMed] [Google Scholar]
- ZELITCH I. THE RELATION OF GLYCOLIC ACID SYNTHESIS TO THE PRIMARY PHOTOSYNTHETIC CARBOXYLATION REACTION IN LEAVES. J Biol Chem. 1965 May;240:1869–1876. [PubMed] [Google Scholar]


