Abstract
N-glycosylation, a post-translational modification whereby glycans are covalently linked to select Asn residues of target proteins, occurs in all three domains of life. Across evolution, the N-linked glycans are initially assembled on phosphorylated cytoplasmically-oriented polyisoprenoids, with polyprenol (mainly C55 undecaprenol) fulfilling this role in Bacteria and dolichol assuming this function in Eukarya and Archaea. The eukaryal and archaeal versions of dolichol can, however, be distinguished on the basis of their length, degree of saturation and by other traits. As is true for many facets of their biology, Archaea, best known in their capacity as extremophiles, present unique approaches for synthesizing phosphodolichols. At the same time, general insight into the assembly and processing of glycan-bearing phosphodolichols has come from studies of the archaeal enzymes responsible. In this review, these and other aspects of archaeal phosphodolichol biology are addressed.
Keywords: Archaea, dolichol, isoprene, N-glycosylation, oligosaccharyltransferase, prenyltransferase
1. Introduction
Life on Earth can be divided into three domains, namely Eukarya, Bacteria and Archaea, [1]. The Archaea, first reported in 1977 [2], were initially thought of as extremophiles, given their abilities to thrive in some of the harshest environments on the planet, including those characterized by extremes of temperature, pH or salinity. However, it has since become clear that Archaea are major residents of so-called ‘normal niches’, such as ocean waters, soil and even intestinal flora [3–5]. In terms of their biology, Archaea can be considered a mosaic, presenting traits shared by Eukarya and/or Bacteria, together with elements that distinguish this form of life, such as the unique lipids comprising the archaeal membrane [6].
As in Eukarya and Bacteria, the archaeal cell is also surrounded by a phospholipid-based membrane. Yet, whereas eukaryal and bacterial phospholipids essentially comprise fatty acid side chains linked to a 1,2-sn-glycerol-3-phosphate backbone via ester bonds, their archaeal counterparts instead correspond to isoprenoid hydrocarbon side chains linked to a 2,3-sn-glycerol-1-phosphate backbone via ether bonds [6]. While such lipids are organized into the bilayer structure that delineates most archaeal cells, some Archaea are instead surrounded by membranes based on a lipid monolayer comprising tetraether lipids in which both ends of the isoprenoid side chains are ether-bound to glycerol phosphate backbones [7]. It is thought that the unusual physicochemical properties of their membrane lipids contribute to the abilities of Archaea to withstand the physical challenges presented by the hostile surroundings that they often inhabit, although how such distinctive phospholipid composition would benefit non-extremophilic archaea remains unclear [8–10]. For more information on archaeal phospholipids, the reader is directed to reviews on the topic [6,7,11–13].
In addition to isoprenoid-based phospholipids and glycolipids, the archaeal plasma membrane also includes isoprenoid-based non-bilayer-forming lipids. These include carotenoids, such as C50 bacterioruberins and C40 carotenes [14], retinals, such as those associated with bacteriorhodopsin and other sensory and transport rhodopsins [15], and phosphodolichols, lipids involved in protein N-glycosylation [16,17]. In the following, current knowledge on the phosphodolichols that participate in archaeal N-glycosylation is reviewed.
2. The lipid glycan carriers of N-glycosylation across evolution
Long held to be an exclusively eukaryal post-translational modification, it is now clear that both Bacteria and Archaea can also perform N-glycosylation, i.e., the covalent attachment of mono- to polysaccharides to select Asn residues of target proteins [18]. Archaea, however, display a degree of diversity in all aspects of N-glycosylation that is unparalleled in either Eukarya or Bacteria [19]. Such diversity is evident at the level of the lipid carrier upon which N-linked glycans are assembled.
At present, the process of N-glycosylation is best understood in higher Eukarya [20,21]. Here, the sugar N-acetylglucosamine (GlcNAc)-1-phosphate, donated by UDP-GlcNAc, is first added to dolichol phosphate (DolP) to yield dolichol pyrophosphate (DolPP)-bound GlcNAc-1-phosphate. Six additional nucleotide-activated sugars are next added to yield heptasaccharide-charged DolPP. This moiety is then flipped across the endoplasmic reticulum (ER) membrane to face the ER lumen by an ATP-independent flippase that remains to be described. At this point, seven additional sugars, each delivered from a distinct DolP carrier charged on the cytoplasmic face of the ER membrane and flipped to face the ER lumen, are added to yield a 14-member branched oligosaccharide that is ultimately transferred to select Asn residues of a nascent polypeptide entering the ER lumen by the multimeric oligosaccharyltransferase, with Stt3 serving as the catalytic subunit of the complex.
In Bacteria, where N-glycosylation is apparently restricted to only certain groups, the process involved has been best described in the pathogen Campylobacter jejuni [22,23]. Here, some 60 different proteins are modified by a heptasaccharide assembled by a pathway reminiscent of its eukaryal counterpart. In C. jejuni, N-glycosylation begins with the generation of di-N-acetylbacillosamine-charged undecaprenol pyrophosphate (UndPP) via the fusion of a UDP-charged version of the sugar with undecaprenol phosphate on the cytoplasmic face of the plasma membrane. Following glycan extension through the addition of six more sugars, the UndPP-linked heptasaccharide is flipped across the membrane in a reaction involving the ABC transporter PglK. Once on the outer surface of the plasma membrane, the glycan is delivered to select Asn residues of target proteins by PglB, the bacterial oligosaccharyltransferase.
In Archaea, where the first example of non-eukaryal N-glycosylation was reported [24], the process remains the least well understood. In part, this is due to the fact that the vast majority of identified strains cannot be grown in the laboratory, and of the small number of cultivatable strains, genetic tools are only available for few. Furthermore, the N-linked glycans decorating archaeal glycoproteins demonstrate enormous variability in terms of composition and architecture, reflecting the variety of N-glycosylation processes employed in Archaea [19,25]. Still, what is known reveals that as in the other two domains, N-glycosylation in Archaea begins with the assembly of a glycan on one or more phosphorylated dolichol carriers. For instance, the first four sugars of the N-linked pentasaccharide decorating glycoproteins in the halophile (‘salt-loving’ organism) Haloferax volcanii (optimal growth in 1.5–2.5 M NaCl [26]) are assembled on a common DolP carrier, while the final sugar is delivered from a distinct DolP [27]. As such, DolP serves both as lipid glycan carrier and glycan donor in this organism. By contrast, the similar N-linked pentasaccharide attached to glycoproteins of a second haloarchaea also originating from the Dead Sea, Haloarcula marismortui (optimal growth in 3.5–4 M NaCl [28]), is completely assembled on a single DolP carrier [29]. In yet another halophilic archaea, Halobacterium salinarum (optimal growth in 3.9 M NaCl [30]), the surface (S)-layer glycoprotein, the building block of the S-layer surrounding the cell, is simultaneously modified by two distinct N-linked glycans, one initially assembled on DolP, the second on DolPP [31,32]. In the thermoacidophile Sulfolobus acidocaldarius (optimal growth at 80°C and pH 2 [33]), DolPP is charged with the same hexasaccharide as N-linked to glycoproteins in this organism, as well as its derivatives, although DolP and hexose-charged DolP have also been detected [34,35]. In the related species Sulfolobus solfataricus (optimal growth at 87°C and pH 3.5–5 [36]), DolPP is charged with the first six sugars of the heptasaccharide N-linked to glycoproteins in this organism; the final sugar could thus be derived from hexose-charged DolP [37]. As such, it appears that N-glycosylation in Archaea relies on a variety of lipid-linked glycan processing strategies.
The dolichols and polyprenols that serve as lipid glycan carriers across evolution (Fig 1; Table 1) are examples of polyisoprenoids, a family of hydrophobic polymers containing linearly linked isoprene subunits, members of which are found in all organisms [38,39]. While both contain up to 25 isoprene subunits and present a hydroxyl group at the so-called α end, dolichols include a saturated α position isoprene subunit, whereas polyprenols, including C55 undecaprenol, do not. The dolichols used in eukaryal N-glycosylation can, however, be distinguished from their archaeal counterparts in that all of the dolichols shown to participate in archaeal N-glycosylation to date also present an additional saturated isoprene subunit at the ω position, namely that isoprene farthest from the α end of the molecule [27,29,32,34,35,37,40,41]. The significance of saturation of the ω position isoprene is not clear, since in vitro studies revealed how C55,60 DolP saturated solely at the α position could be glycan-charged using enzymes involved in N-glycosylation in the methanogen Methanococcus voltae (grown in the laboratory in a 80%H2/20% CO2 atmosphere [42]). Moreover, this glycan-charged lipid could serve as substrate for the archaeal oligosaccharyltransfase AglB. At the same time, in the thermophile Pyrococcus horikoshii (optimal growth at 98°C [43]), both undecaprenol phosphate and dolichol phosphate were reported to serve as substrates for DolP mannose synthase, the enzyme that charges DolP with mannose, raising questions as to the importance of α position isoprene saturation in archaeal N-glycosylation [44].
Fig 1. Lipid glycan carriers used across evolution.
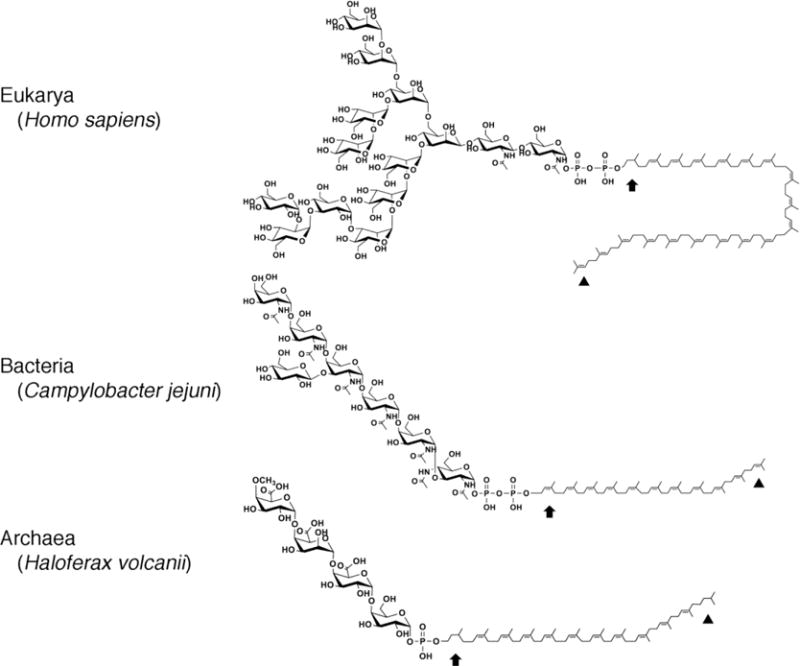
Examples of lipid glycan carriers from Eukarya (human C95 DolPP (trans3cis16 isoprenes) charged with the tetradecasaccharide GlcNAc2-Man9-Glc3 where Glc is glucose, GlcNAc is N-acetylglucosamine and Man is mannose), Bacteria (C. jejuni C55 UndPP (trans3cis8 isoprenes) charged with the (GalNAc-α1,4-GalNAc-α1,4-[Glc-β-1,3]GalNAc-α1,4-GalNAc-α1,4-GalNAc-α1,3-diNAcBac where diNAcBac is 2,4-diacetamido-2,4,6-trideoxy-D-glucopyranose, GalNAc is N-acetylgalactosamine and Glc is glucose) and Archaea (Hfx. volcanii C60 DolP (trans4cis8 isoprenes, predicted) charged with methyl-O-4-GlcA-β-1,4-GalA-α1,4-GlcA-β1,4-glucose where GalA is galacturonic acid and GlcA is glucuronic acid). In each lipid, the arrow depicts the α position isoprene while the arrowhead depicts the ω position isoprene. In the eukaryal and archaeal lipids, the α position isoprene is saturated, whereas the ω position isoprene is saturated only in the archaeal lipid.
Table 1.
A comparison of lipid glycan carriers used in N-glycosylation across evolution
| Eukarya | Bacteria | Archaea | |
|---|---|---|---|
| Lipid glycan carrier | DolP DolPP | UndPP | DolP DolPP |
| Length | C70–C105 | C50–C60 | C30–C70 |
| Positions of saturated isoprenes | Alpha-position isoprene | None | Alpha- and omega-position isoprenes; variable number of internal position isoprenes |
| Location | ER membrane | Plasma membrane | Plasma membrane |
| Topology of sugar addition | Sugars added on both sides of the membrane | Sugars added only on the cytosolic side of the membrane | Sugars added on the cytosolic side of the membrane and in some species, on the external side of the membrane |
| Number of lipid glycan carriers used per N-glycosylation event | Multiple | Single | Single or more in some species |
| Chemical modification of lipid-linked glycan | No | No | In some species |
| Recycling after glycan transfer to target protein | DolPP is dephosphorylated to DolP, flipped to face the ER lumen and recharged to yield DolPP-glycan | UndPP is dephosphophorylated to UndP, flipped to face the cytoplasm and recharged to yield UndPP-glycan | Unknown |
3. The diversity of archaeal phosphodolichols
In addition to the saturated ω position isoprene subunit that differentiates archaeal dolichol from the eukaryal version of this lipid, the dolichols employed in archaeal N-glycosylation also present various species-specific characteristics. These differences are mostly manifested in terms of dolichol length, degree of phosphorylation and the extent of internal isoprene subunit saturation (Fig 2).
Fig 2. Phosphodolichols used in archaeal N-glycosylation.
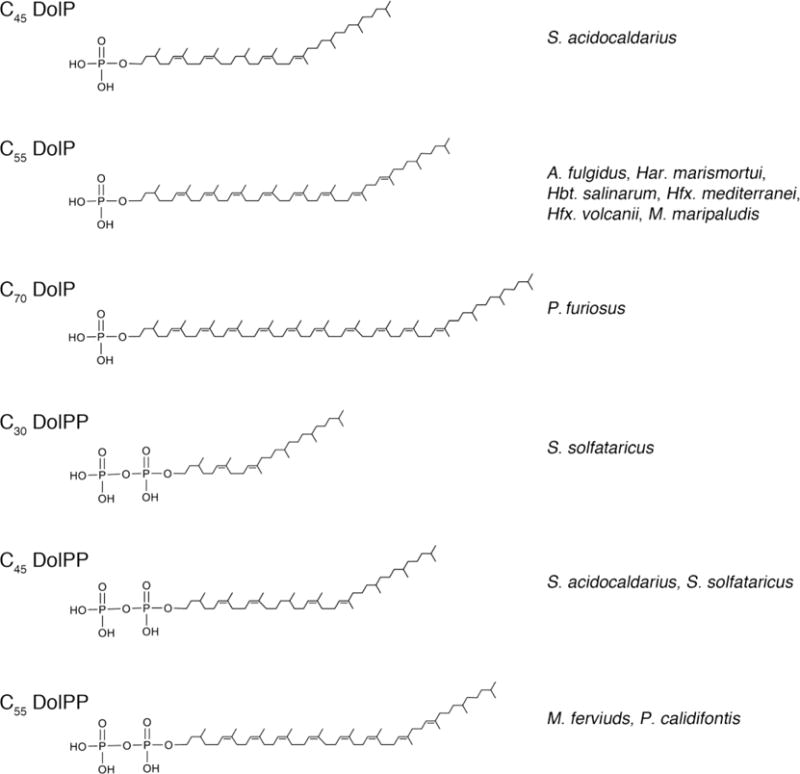
Archaea N-glycosylation relies on phosphodolichols that differ in terms of length, degree of phosphorylation and the extent of internal isoprene subunit saturation. Shown are major phosphodolichol species used in N-glycosylation in different Archaea.
To date, phosphorylated dolichols, many charged with the same glycans as N-linked to glycoproteins in the same species, have been detected in Archaea representing several phenotypes and belonging to different phyla. The first phosphorylated dolichols detected in Archaea were in Hbt. salinarum, soon after this halophile provided the first example of non-eukaryal N-glycosylation, namely the S-layer glycoprotein [24,45,46]. Here, C55,60 DolP is modified by a sulfated tetra/pentasaccharide N-linked to ten N-glycosylation sites of the protein, while C55–60 DolPP is modified by a distinct sulfated pentasaccharide that apparently assembles into a chain when linked to the Asn-2 position of the protein [31,32,46,47]. It is, however, not known whether the chain of repeating sulfated pentasaccharides is assembled on a single DolPP carrier (and if so, on which side of the membrane) and only then transferred to the protein, or alternatively, whether such polymerization results from the transfer of the subunit glycan to the protein from a series of glycan-charged DolPP carriers assembled on the cytoplasmic face of the membrane and then flipped to face the exterior, where N-glycosylation transpires [48].
Recently, saturation of the α and ω position isoprenes of Hbt. salinarum C55–60 DolP and C55,60 DolPP was demonstrated. In these studies, DolP was detected bearing a disaccharide precursor of the tetra/pentasaccharide, while DolPP was shown to be modified by the first four sugars of the repeating pentasaccharide; DollPP bearing repeats of this glycan was not observed [32]. However, the first demonstration of dolichol saturation at both ends of the lipid was reported in the case C55,60 DolP bearing a tetrasaccharide in Hfx. volcanii [40]. Much later, the tetrasaccharide attached to this DolP was shown to modify at least one Asn of the S-layer glycoprotein when the cells were grown in 1.7 M NaCl [49], considered as low salinity for this halophile [26]. Indeed, the most convincing evidence for the involvement of DolP in archaeal N-glycosylation has come from studies on Hfx. volcanii, where the deletion of genes encoding components of the N-glycosylation pathway yielded the same effects on the glycans linked to both DolP and to a target protein, the S-layer glycoprotein [27]. Subsequently, C55,60 DolP saturated at both the α and ω position isoprenes was observed in other haloarchaea, namely Har. marismortui, where the lipid was modified by the same pentasaccharide as found on the S-layer glycoprotein in this species [29], and Haloferax mediterranei, where the lipid was modified by a disaccharide [32]. Presently, nothing else is known about N-glycosylation in Hfx. mediterranei.
Haloarchaea, such as those considered above, belong to the Euryarchaeota, one of the better-studied archaeal phyla [1,50]. The same phylum also includes the methanogens, namely Archaea able to reduce carbon dioxide with hydrogen to generate methane [51]. To date, phosphorylated dolichols have only been characterized in two species. In Methanococcus maripaludis (grown in the laboratory in a 80% H2/20% CO2 atmosphere [52]), a lipid extract was shown to contain C55 DolP saturated at two non-specified positions and modified by a trisaccharide that corresponds to a precursor of the tetrasaccharide N-linked to glycoproteins in this organism [53,54]. Moreover, the enzyme thought to add the initial sugar to the lipid carrier in M. maripaludis was shown to be more active when presented with C55,60 DolP rather than with longer C85–105 DolP, as found in eukaryotes [54]. In contrast, older studies conducted on Methanothermus fervidus (optimal growth at 83°C in a 80% H2/20% CO2 atmosphere [55]), where the S-layer glycoprotein is modified by an N-linked hexasaccharide, detected sugar-modified C55 DolPP [56].
In addition to halophiles and methanogens, the Euryarchaeota also include thermophilic archaea where phosphodolichols involved in N-glycosylation have been characterized, namely Pyrocococcus furiosus and Archaeoglobus fulgidus. In P. furiosus (optimal growth at 100°C [57]), C60,65,70 DolP modified by glycans containing up to the first six sugars found in the N-linked heptasaccharide that decorates glycoproteins in this organism was described [37,41]. As such, P. furiosus DolP is the longest phosphodolichol involved in archaeal N-glycosylation known to date. More striking, however, was the finding that in addition to being saturated at the a and ω position isoprenes, P. furiosus DolP is also saturated at up to four more internal isoprene positions, with these additional sites of saturation being closer to the ω than the α end of the molecule. In A. fulgidus (optimal growth at 83°C [58]), C55,60 DolP displays saturation of not only the α and ω position isoprenes but also of up to four additional internal isoprene positions [37]. Moreover, A. fulgidus DolP is apparently modified by two distinct heptasaccharides [37]. The different lipid-linked glycans could reflect the fact that A. fulgidus encodes multiple versions of the oligosaccharyltransferase AglB [59], responsible for the transfer of the glycan moiety from the lipid carrier to select Asn residues of modified proteins (see below), raising the possibility that different version of the enzyme process distinct glycan substrates.
In addition to the phosphodolichols involved in N-glycosylation studied in representatives of the Euryarchaeota, these lipids have also been considered in three members of a second well-studied archaeal phylum, the Crenarchaeota [1,50]. S. acidocaldarius is the only crenarchaeote for which insight into the N-glycosylation process is available. Here, C40,45,50 DolP, in some cases charged with one of three different hexoses, and C45 DolPP modified by the hexasaccharide added to target proteins in N-glycosylation, as well as by glycans comprising the first four of five sugars of this structure, were detected [34,35]. In S. acidocaldarius DolP and DolPP, up to six isoprenes are saturated, including those at the α and ω positions. In the related thermoacidophile S. solfataricus, C30 and C45 DolPP modified by the first six sugars of the N-linked heptasaccharide assembled in this species [60] and saturated at the α and ω isoprene positions, as well as at up to four additional internal position isoprenes, were identified [37]. Moreover, it has been suggested that two phosphodolichol glycan carriers used in S. solfataricus N-glycosylation differ not only in length (C30 and C45) but also in terms of stereochemistry [37]. At the same time, Pyrobaculum calidifontis (optimal growth at 90–95°C [61]) was reported to contain C50,55 DolPP modified by a decasaccharide also shown to be N-linked to a glycopeptide generated by proteolytic digestion of a membrane fraction prepared from this organism [37]. Similarly to the phosphodolichols in other crenarchaeotes considered to date, P. calidfontis DolPP is saturated at the α position isoprene, at up to five internal position isoprenes and at the ω position isoprene [37].
Despite the limited number of archaeal species in which phosphodolichols either shown or likely to be involved in N-glycosylation have been characterized, general properties on these lipids may have already been revealed. With the exception of M. fervidus, where older technologies were employed, it would appear that N-glycosylation in the Euryarchaeota relies on DolP as glycan lipid carrier, with the N-linked glycan either being assembled on a single DolP carrier or alternatively, being assembled on the modified protein from precursors delivered from at least two DolP carriers. In contrast, Crenarchaeota rely on DolPP as lipid glycan carrier, with DolP possibly serving as the carrier of individual sugars either delivered to DolPP-linked precursors of N-linked glycans or to glycans already transferred from DolPP carriers to target protein Asn residues. As such, it would appear that euryarchaeotes rely on a more bacterial-like N-glycosylation process, whereas crenarchaeotes rely on a process reminiscent of that employed in Eukarya. Indeed, phylogenetic analyses of known Archaea position the Crenarchaeota proximal to the ancestor of modern-day eukaryotes [62,63].
In addition, the fact that DolP and DolPP from both thermophilic euryarchaeotes and crenarchaeotes include saturated isoprenes at internal positions points to dolichol isoprene reduction as being important for survival at high temperatures. Such enhanced saturation, relative to what is seen in mesophilic archaea, would reduce the movement of phosphodolichols in the membranes of thermophilic archaea, a property that could be important for the enzyme-mediated processing of these glycan carriers. At the same time, dolichol length does not seem to be sensitive to temperature, since both the shortest (C30) and longest (C70) archaeal phosphodolichols known to date have been detected in thermophiles [37,41].
4. Phosphodolichol biosynthesis in Archaea
Phosphodolichol synthesis occurs in two stages [38,39]. In the first stage, the two building blocks of isoprenoid biogenesis, isopentenyl pyrophosphate (IPP) and its isomer dimethylallyldiphosphate (DMAPP), are generated from acetyl CoA. This is followed by the second stage of dolichol biogenesis in which a series of condensation reactions between DMAPP and several IPP moieties occurs. The resulting polymer is then further processed to yield the phosphorylated dolichols used in archaeal N-glycosylation.
The classic route for generating IPP and DMAPP is known as the mevalonate pathway [64]. Here, three acetyl CoA molecules are condensed and reduced using NADPH to yield mevalonate via the sequential actions of acetoacetyl-CoA thiolase, 3-hydroxy-3-methylglutaryl (HMG)-CoA synthase and HMG-CoA reductase. Mevalonate is subsequently phosphorylated by mevalonate-5-kinase and phosphomevalonate kinase to yield mevalonate-5-phosphate and mevalonate pyrophosphate, respectively. The latter compound is processed by mevalonate pyrophosphate decarboxylase to generate IPP, which is isomerized by IPP isomerase to yield DMAPP (Fig 3).
Fig 3. Pathways of isopentenyl pyrophosphate biosynthesis in Archaea.
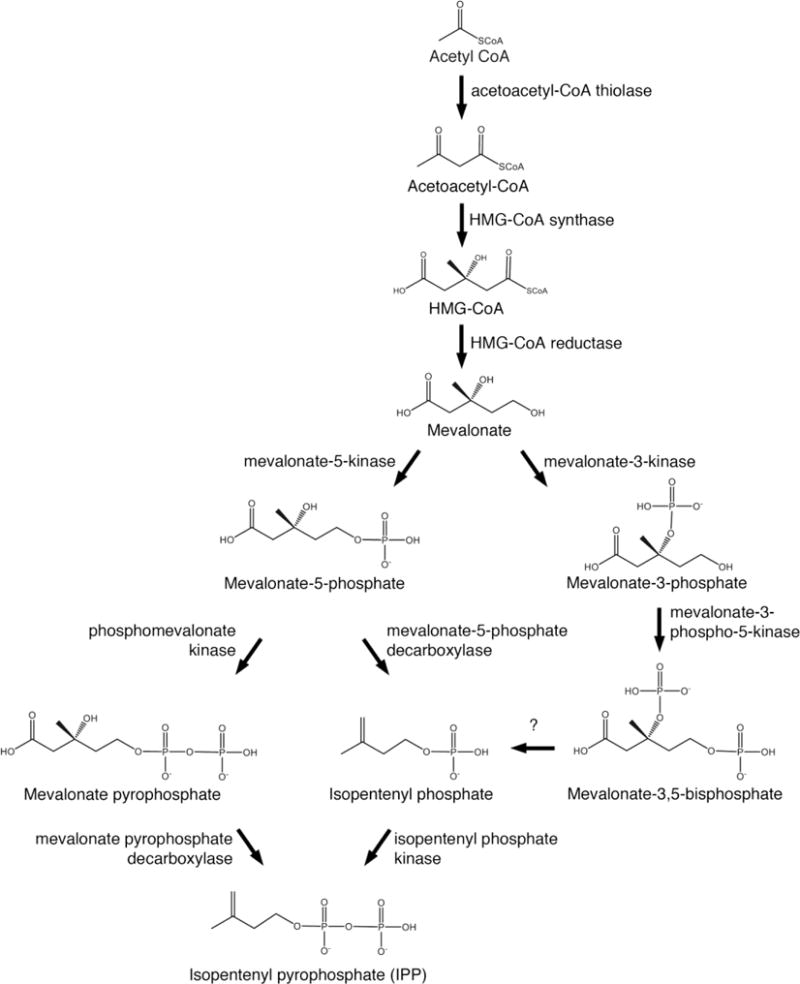
In Archaea, isopentenyl pyrophosphate can be synthesized by different pathways. In the classic mevalonate pathway, mevalonate undergoes two rounds of phosphorylation at the 5 position and then decarboxylation at the 3 position to yield IPP. In a second pathway, mevalonate undergoes phosphorylation at the 5 position, decarboxylation at the 3 position and then a second phosphorylation at the 5 position to generate IPP. In a potentially third pathway, mevalonate is phosphorylated at the 3 position and then at the 5 position. Dephosphorylation at the 3 position, a reaction yet to be observed, would yield isopentenyl phosphate, which would be phosphorylated at the 5 position to produce IPP.
Genomic analyses revealed the presence of the first four enzymes of the classic mevalonate pathway in Archaea, namely acetoacetyl-CoA thiolase, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) synthase, HMG-CoA reductase and mevalonate-5-kinase [65,66]. Subsequent efforts not only confirmed the functionality of archaeal versions of these enzymes but also revealed unique properties in several instances. For example, Hfx. volcanii HMG-CoA reductase is regulated at the transcription level by environmental salinity, pointing to a link between the cell surroundings and the biosynthesis of isoprenoids, including phosphodolichols [67]. Moreover, Hfx. volcanii HMG-CoA reductase is the first such enzyme that is not sensitive to feedback inhibition by its substrate, acetoacetyl-CoA [68]. Likewise, known feedback inhibitors of mevalonate-5-kinases do not inhibit the Methanosarcina mazei enzyme, making it the sole member of a third class of mevalonate kinases defined on the basis of inhibition profile [69].
At the same time, genomic scanning failed to detect archaeal homologues of mevalonate pyrophosphate decarboxylase in most species [65,66,70], suggesting the existence of an alternate route for the conversion of mevalonate-5-phosphate to IPP. Initial support for this hypothesis came with the identification of an isopentenyl phosphate kinase in Methanocaldococcus jannaschii (optimal growth at 85°C in a 80% H2/20% CO2 atmosphere; first isolated from a hydrothermal vent on the Pacific Ocean floor [71]), namely an enzyme that synthesizes IPP via the phosphorylation of isopentenyl phosphate [72]. Genomic analyses revealed that other Archaea also contain the gene encoding this protein, with the activity of recombinant isopentenyl phosphate kinase from Methanothermobacter themautotrophicus (optimal growth at 65–70°C in a 80% H2/20% CO2 atmosphere [73]) and Thermoplasma acidophilum (optimal growth at 59°C and pH 1–2 [74]) being demonstrated [75]. Subsequent efforts uncovered the existence of both phosphomevalonate decarboxylase, responsible for converting mevalonate-5-phosphate into isopentenyl phosphate, and isopentenyl phosphate kinase in Hfx. volcanii, thereby confirmed the existence of an alternate route for IPP biosynthesis in Archaea (Fig 3) [76]. Nonetheless, there are archaeal species that rely on the classic mevalonate pathway despite also encoding components of the alternate pathway, such as S. solfataricus [77]. Here too, Archaea have introduced unusual variations not seen elsewhere. For instance, while the mevalonate pyrophosphate decarboxylase in S. solfataricus exists as a dimer, like its eukaryal and bacterial counterparts, the archaeal enzyme is unique in that a disulfide bond serves to stabilize the dimer in this thermoacidophile [78]. Recently, evidence for the existence of yet another pathway for IPP biosynthesis in Archaea has been presented. In T. acidophilum, mevalonate-3-kinase converts mevalonate into mevalonate-3-phosphate, which is then converted into mevalonate-3,5-bisphosphate by mevalonate-3-phosphate-5-kinase [79,80]. Mevolonate-3-kinase was subsequently described in Picrophilus torridus (optimal growth at 60°C and pH 1.8–2 [81]), with sequence analysis suggesting the enzyme to also exist in other members of the Thermoplasmatales, the order that includes these two species [82]. Presently, however, the mevalonate-3,5-bisphosphate decarboxylase that would be required for the appearance of IPP by this novel route has yet to be identified (Fig 3). Finally, the IPP isomerases used by Archaea to convert IPP into DMAPP are, for the most part, orthologs of a bacterial, rather than the eukaryal version of this enzyme [83]. This, despite the fact that many Bacteria do not use the MVA pathway but rather rely on a distinct pathway for IPP biosynthesis termed the MEP/DOXP pathway, as do plants [84,85]. Members of the order Halobacteriales, however, encode both versions of the isomerase [66].
To generate phosphodolichols from the five-carbon precursors IPP and DMAPP, there is a need for elongation steps in which DMAPP and multiple IPP moieties polymerize to yield an isoprenoid chain, dephosphorylation steps to yield a polyprenol, saturation steps to reduce the isoprene subunit at the α end of the dolichol molecule (and, in Archaea, the isoprene subunit at the ω position, and in some cases, at more internal positions) and finally, phosphorylation steps. While the details of many of these reactions are relatively well described in Eukarya (and in undecaprenol synthesis in Bacteria, where saturation steps are not required), much less is known about the route(s) taken in Archaea.
Polyprenol biosynthesis begins with DMAPP serving as the acceptor for an IPP molecule to generate C10 geranyl pyrophosphate [38,39]. This reaction, as well as the addition of a second IPP to generate C15 farnesyl pyrophosphate, involves trans-condensations catalyzed by isoprenyl pyrophosphate synthases, also termed prenyltransferases, members of an enzyme family responsible for generating polyprenol chains of increasing length [86]. Following the condensation of IPP moieties in trans, additional IPP units are added in cis by a cis-prenyltransferase to generate a trans/cis-polyprenol pyrophosphate. Now found within the membrane, the polyprenol pyrophosphate is next thought to undergo two rounds of dephosphorylation to yield polyprenol [87,88]. In Eukarya, saturation of the α position isoprene subunit is proposed to occur at this stage, yielding dolichol; in Bacteria, no such saturation occurs. Finally, dolichol is phosphorylated by dolichol kinase to generate DolP [87,89]. In Bacteria, an undecaprenol phosphokinase that catalyzes a similar reaction has been described [38,90]. Whereas there is considerable understanding of the steps taken to synthesize IPP and DMAPP in Archaea, insight into how Archaea assemble phosphodolichol from these building blocks remains only partial. Nonetheless, what is currently known has revealed unexpected aspects of each of the latter steps of phosphodolichol assembly, namely elongation, dephosphorylation, saturation and phosphorylation.
The sequential addition of IPP subunits to a DMAPP acceptor is catalyzed by distinct prenyltransferases that catalyze condensation reactions as a function of substrate length [86]. To generate dolichol, the so-called short-chain prenyltransferases are required [91]. In most Archaea, the only members of this group are C20 geranylgeranyl pyrophosphate synthases [92,93]. These, however, can assemble trans-polyprenol pyrophosphates of different lengths, as exemplified by the bi-functional enzyme from M. themautotrophicus that is able to synthesize both farnesyl pyrophosphate and geranylgeranyl pyrophosphate [94]. Similar bi-functional activity was reported for geranylgeranyl pyrophosphate synthases from S. acidocaldarius and Thermococcus kodakarensis (optimal growth at 95°C [95]) [96,97]. Based on such observations, it has been proposed that archaeal geranylgeranyl pyrophosphate synthases represent the ancestor of the farnesyl pyrophosphate synthases and geranylgeranyl pyrophosphate synthases presently found in Eukarya and Bacteria [98,99]. This hypothesis has, however, been questioned by others [100]. Finally, in S. acidocaldarius, genes encoding IPP isomerase (saci0091), geranylgeranyl pyrophosphate synthase (saci0092) and AglH, the glycosyltransferase responsible for adding the first sugar of the N-linked glycan to DolPP (saci0093), lay adjacent to each other in the genome, with saci0092 and saci0093 being found on the same polycistronic mRNA [101,102]. This gene arrangement offers support for close interplay between phosphodolichol biosynthesis and N-glycosylation in this thermoacidophile.
In general, the substrate (or primer) of cis-prenyltransferases in Eukarya and Bacteria is C15 trans-farnesyl pyrophosphate [38,39,91], although evidence for the broader selectively of these enzymes in plants has been offered [103,104]. In Archaea, both C15 trans-farnesyl pyrophosphate and C20 trans-geranylgeranyl pyrophosphate serve as cis-prenyltransferase substrates [92]. In S. acidocaldarius, apparently only trans-geranylgeranyl pyrophosphate serves this role [92]. The recent report of one of the three cis-prenyltransferases in Methanosarcina acetivorans being able to catalyze both head-to-tail and non-head-to-tail condensation reactions [105] could reflect yet another unique aspect of archaeal phosphodolichol biosynthesis. In the hyperthermophile Aeropyrum pernix (optimal growth at 90–95°C [106]), the cis-prenyltransferase is unusual in that the substrate is the C25 trans-geranylfarnesyl pyrophosphate [107]. This could indicate the use of a unique lipid glycan carrier in this species. Accordingly, it is of note that when 168 archaeal genomes were considered for the presence of aglB, encoding the archaeal oligosaccharyltransferase responsible for the transfer of glycans from phosphodolichol carriers to target protein Asn residues, A. pernix was only one of two species where no such gene was detected, raising the question of whether N-glycosylation occurs in this organism [108]. However, if N-glycosylation indeed occurs in A. pernix, the process may rely on an oligosaccharyltransferase distinct from AglB. Lastly, it has been reported that in mammalian cells, cis-prenyltransferases require interaction with the NogoB receptor for stabilization of the enzyme [109]. In plants, where cis-prenyltransferases exist as enzyme families, it was shown that select family members that contribute to dolichol biogenesis also interact with a NogoB receptor homologue [103,110]. Although phylogenetic analysis has raised the possibility of such a protein in Archaea [105], evidence for the involvement of a NogoB receptor homologue in archaeal cis-prenyltransferase activity has yet to be presented.
Once the polyprenol pyrophosphates destined to become phosphodolichols have been assembled, it is believed that they must next undergo dephosphorylation, saturation and phosphorylation [111], although conclusive evidence that this is indeed the order of events in Archaea remains wanting. With this in mind, a scheme of the possible steps taken during the latter phase of archaeal DolP biosynthesis, using S. acidocaldarius as a model system [34], is presented in Fig 4. Indeed, it should be noted that alternate pathways leading to the appearance of DolP exist in other forms of life [112], and, therefore, possibly in Archaea as well. Nonetheless, in this review it is assumed that in Archaea, polyprenol pyrophosphates are subjected to dephosphorylation, saturation and then phosphorylation to generate DolP.
Fig 4. Potential pathways for the biosynthesis of DolP in Archaea.
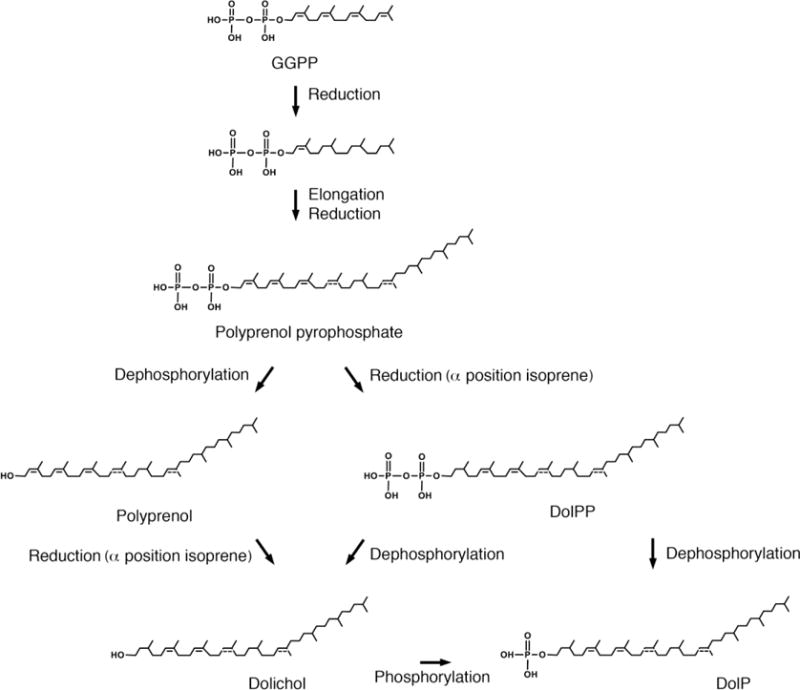
The biogenesis of DolP in S. acidocaldarius offers an example of the potential pathways used for synthesizing phosphodolichols in Archaea. Initially (not shown), three isopentenyl pyrophosphates and dimethylallyldiphosphate combine to form geranylgeranyl pyrophosphate (GGPP). Where internal phosphodolichol isoprenes are reduced, as in S. acidocaldarius, three of the four double bonds in GGPP may be reduced at this stage; the α position isoprene remains unsaturated. Elongation occurs when additional isoprene subunits (four-six in the case of S. acidocaldarius) are added to the α end of GGPP to generate polyprenol pyrophosphate. The generation of dolichol requires the removal of the two phosphate groups, followed by reduction of the α position isoprene subunit (and possibly other subunits elsewhere, as in the case of S. acidocaldarius phosphodolichols; when the position of a double bond is only speculated in a S. acidocaldarius lipid in the figure, a dotted line appears). Dolichol would undergo phosphorylation to yield dolichol phosphate (DolP). Alternatively, polyprenol pyrophosphate could undergo reduction reactions, followed by removal of one phosphate group to yield DolP or both phosphate groups, followed by re-phosphorylation, again yielding DolP, as thought to occur in Eukarya. Abbreviations used: DolP, dolichol phosphate; DolPP, dolichol pyrophosphate; GGPP, geranylgeranyl pyrophosphate.
The conversion of polyprenol pyrophosphates to polyprenol requires dephosphorylation. In Eukarya, the enzyme(s) responsible has(have) yet to be described, although polyprenol pyrophosphate phosphatase and polyprenol phosphatase activities have been observed in whole-cell homogenates [113]. In Bacteria, undecaprenol pyrophosphate is converted to undecaprenol phosphate by the actions of UppP [114]. The resulting mono-phosphorylated polymer serves as the bacterial lipid glycan carrier. In Archaea, it is assumed that dephosphorylation also occurs. Indeed, undecaprenol (and dolichol) has been observed in M. acetivorans [115]. Likewise, the detection of dolichol in S. acidocaldarius [34] could reflect the initial dephosphorylation of polyprenol pyrophosphates, although here too, the enzyme(s) responsible remain(s) to be identified. Finally, while roles for polyprenol and dolichol have been proposed in Bacteria and Eukarya, respectively [17], it is not known whether dolichol serves any biological function in Archaea.
In the presumed next step in phosphodolichol biosynthesis, saturation of the isoprene subunit found at the α position of polyprenol is thought to occur. In Archaea, saturation of the isoprene subunit at the ω position would likely also occur at this point, as would the saturation of other isoprene subunits found at more internal positions in those in species where highly saturated phosphodolichols exist. A polyprenol reductase responsible for saturation of the α position isoprene subunit of polyprenol has been identified in Eukarya [116]. The enzyme, encoded by the SRD5A3 gene in humans, has no archaeal homologue. As such, it would appear that Archaea rely on a distinct enzyme for such activity. Furthermore, given that eukaryal cells lacking SRD5A3 can still generate some 30% of the dolichol found in normal cells [116], it would seem that at least one more eukaryal polyprenol reductase must exist. One can speculate that Archaea, lacking a SRD5A3 gene, could instead contain homologues of this(these) additional polyprenol reductase(s), considering the high number of saturated isoprenes in archaeal phosphodolichols. To date, archaeal proteins showing polyprenol reductase activity have been described. S. acidocaldarius contains a geranylgeranyl reductase that is able to saturate three of the four double bonds in geranylgeranyl pyrophosphate, leaving the Δ2 double bond intact and thereby yielding a substrate for prenyltransferases in a reaction that would result in the removal of the pyrophosphate group from the geranylgeranyl pyrophosphate substrate [117]. Recent crystallographic analysis of S. acidocaldarius geranylgeranyl reductase bound to geranylgeranyl pyrophosphate has provided insight into the catalytic mechanism of this enzyme [118]. In contrast to the geranylgeranyl reductase of T. acidophilum, which uses NADPH as electron donor [119], the S. acidocaldarius enzyme does not use either NADPH or NADH, and as such, the source of electrons used in the saturation reaction in this species is not known [117]. In the case of M. acetivorans geranylgeranyl reductase, ferrodoxin is thought to serve as electron donor [120]. In addition, reductases that saturate the ω position isoprene subunit of polyprenol, all members of the geranylgeranyl reductase family, have also been identified. These enzymes, however, affect the saturation status of other lipids as well, raising the question of whether these reductases are truly components of the phopshodolichol biosynthesis pathway in Archaea [115,121]. Indeed, the observation that S. acidocaldarius contains versions of C45 DolP presenting saturation at the α position isoprene subunit, at the α and ω position isoprene subunits and at these two positions together as well as at one-four more internal positions, suggests that multiple reductases contribute to phosphodolichol biosynthesis here [34]. Finally, considering that two or more of the isoprene subunits comprising archaeal dolichols are saturated, it has been proposed that archaeal dolichols should be called archaeoprenols, so as to distinguish them from their less saturated eukaryal counterparts [122].
In what is assumed to be the final step of DolP biosynthesis, dolichol is phosphorylated. In Eukarya, this reaction is catalyzed by a CTP-dependent dolichol kinase [123,124]. Homology-based searches of archaeal genomes have identified homologues of eukaryal dolichol kinase in only a limited number of species, implying that dolichol phosphorylation in Archaea relies on a distinct enzyme.
Once the lipid-linked glycan has been transferred by the oligosaccharyltransferase to a target protein, the lipid carrier can be recycled for subsequent rounds of glycan-charging. In Eukarya, for example, the DolPP that remains following N-glycosylation undergoes dephosphorylation to yield DolP, which is subsequently returned to the cytoplasmic leaflet of ER, where it is again recruited as a glycan carrier [125,126]. In Archaea, incubation of glycan-charged DolPP from S. solfataricus or P. calidifontis with a membrane fraction containing the oligosaccharyltransferase AglB and an peptide that contained an Asn residue suitable for glycosylation resulted in the appearance of DolP, suggesting that a dephosphorylation reaction, similar to that which occurs in Eukarya, also transpires in Archaea where DolPP serves as the lipid glycan carrier [37]. Indeed, externally oriented pyrophosphatases from S. acidocaldarius and Sulfolobus tododaii (optimal growth at 80°C and pH 2.5–3 [127]) have been described and proposed to participate in DolPP recycling [128–130].
5. Phosphodolichol-processing enzymes that participate in archaeal N-glycosylation
In terms of both glycan composition and glycan architecture, archaeal N-glycosylation presents a degree of diversity not seen in either Eukarya or Bacteria [19]. Fittingly, what is known of archaeal N-glycosylation pathways reveals the involvement of numerous species-specific components [25]. In contrast, the oligosaccharyltransferase AglB is highly conserved across Archaea. Indeed, like PglB, the bacterial oligosaccharyltransferase, and Stt3, the catalytic subunit of the eukaryal oligosaccharyltransferase, AglB also contains the WWDXG and other motifs important for activity. AglB, moreover, presents a topology similar to those of PglB and Stt3 [131,132]. Solution of the crystal structures of the complete AglB protein from A. fulgidus, as well as of the catalytic domain from P. furiosus and those of the three A. fulgidus AglB paralogs by the Kohda group [131,133–135] has not only provided insight into how AglB catalyzes the glycosylation of target Asn residues but also into how the enzyme interacts with the glycan-bearing phosphodolichol carrier. Analysis of the electron density map of full-length A. fulgidus AglB revealed substantial electron density, modeled as a sulfate ion, 5 Å from the zinc ion found in the catalytic site of the enzyme (that, during the crystallization process, had apparently replaced the magnesium ion normally associated with the enzyme) [135]. This sulfate group interacted with His-81, His-162, Trp-215 and Arg-246, residues that are conserved in other AglB sequences. Site-directed mutagenesis showed the importance of the positive charges of His-81, His-162 and Arg-246. Based on these observations, it was assumed that the sulfate ion mimicked the phosphate group of the DolP that serves as the glycan lipid carrier in A. fulgidus N-glycosylation [37]. Accordingly, a docking model showed a good fit of the DolP dolichol chain within the hydrophobic groove on the protein surface leading to these residues. Once a crystal structure of AglB from an archaeal species that relies on DolPP as glycan lipid carrier becomes available, it will be of interest to see if the same architecture applies.
Flippases, catalyzing the translocation of glycan-charged and glycan-free phosphodolichols across the membrane [136], are also components of N-glycosylation pathways in intimate contact with DolP and/or DolPP. In Archaea, the only known protein proposed to act as a flippase or to play a flippase-assisting role is Hfx. volcanii AglR [137]. Upon deletion of the encoding gene, only the first four sugars of the N-linked pentasaccharide normally decorating glycoproteins in this species were protein-bound. It was previously shown that this tetrasaccharide is assembled on a common DolP carrier, while the fifth sugar (mannose) is derived from its own DolP carrier [27]. Moreover, in the aglR-deleted strain, mannose-charged DolP accumulated. As such, it would appear that AglR is involved in translocating mannose-charged DolP across the membrane. Accumulation of tetraaccharide-charged DolP was, however, also noted in the absence of AglR, pointing to a more general flippase or flippase-related role for the protein. Further support for AglR acting as a flippase or playing a flippase-supporting role comes from sequence homology searches that revealed the similarity of AglR to Wzx proteins. In Bacteria, members of this group of proteins are thought to act as flippases responsible for translocating lipid-linked O-antigen precursor oligosaccharides across the membrane during lipopolysaccharide biosynthesis [138].
6. Conclusions and future prospects
Until recently, the study of Archaea had been hampered by the inability to cultivate the vast majority of identified strains in the laboratory, as well as the few cultivatable strains for which genetic tools were available. However, as recent advances have expanded the list of species amenable to molecular studies, it is likely that novel solutions employed by these unusual organisms to a variety of biological challenges will be revealed. In terms of phosphodolichols, it is conceivable that not only will the enzymes catalyzing all of the steps of known pathways be defined but that additional archaeal-specific steps will be identified. In addition, other more specific mysteries may be solved. For instance, both DolP and DolPP serve as lipid glycan carriers in Hbt. salinarum [31]. It is presently unclear whether both are processed by the same oligosaccharyltransferase. Since genome analysis reveals only a single aglB gene in this organism [58, 108], it is possible that a novel oligosaccharyltransferase will be identified. Indeed, studies on Hfx. volcanii N-glycosylation suggest that such an enzyme exists [49].
In summary, life in extreme conditions, such as those encountered by many Archaea, often calls for unique biological solutions. The routes used for archaeal N-glycosylation and the biosynthesis of the phosphodolichols used as lipid glycan carriers in this post-translational modification have provided and will likely to continue to provide novel examples of Nature’s creativity in the face of such challenges.
Highlights.
In Archaea, N-linked glycans are assembled on phosphorylated dolichols
Archaeal phosphodolichols present traits unique to this domain of life
Archaeal phosphodolichols vary in terms of length, phosphorylation and saturation
Steps in archaeal phosphodolichol biogenesis are often unique to this domain
Acknowledgments
J.E. was supported by grants from the Israel Science Foundation (ISF) (grant 109/16), the ISF within the ISF-UGC joint research program framework (grant 2253/15), the ISF-NSFC joint research program (grant 2193/16) and the German-Israeli Foundation for Scientific Research and Development (grant I-1290- 416.13/2015). Z.G. was supported by the LIPID MAPS Large Scale Collaborative Grant (GM-069338) and grant EY023666 from NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA. 1990;87:4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woese CR, Fox GE. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci USA. 1977;74:5088–5090. doi: 10.1073/pnas.74.11.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeLong EF. Everything in moderation: archaea as ‘non-extremophiles’. Curr Opin Genet Dev. 1998;8:649–654. doi: 10.1016/s0959-437x(98)80032-4. [DOI] [PubMed] [Google Scholar]
- 4.Chaban B, Ng SY, Jarrell KF. Archaeal habitats–from the extreme to the ordinary. Can J Microbiol. 2006;52:73–116. doi: 10.1139/w05-147. [DOI] [PubMed] [Google Scholar]
- 5.Gaci N, Borrel G, Tottey W, O’Toole PW, Brugére JF. Archaea and the human gut: new beginning of an old story. World J Gastroenterol. 2014;20:16062–16078. doi: 10.3748/wjg.v20.i43.16062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koga Y, Morii H. Biosynthesis of ether-type polar lipids in archaea and evolutionary considerations. Microbiol Mol Biol Rev. 2007;71:97–120. doi: 10.1128/MMBR.00033-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chong PL. Archaebacterial bipolar tetraether lipids: Physico-chemical and membrane properties. Chem Phys Lipids. 2010;163:253–265. doi: 10.1016/j.chemphyslip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 8.van de Vossenberg JL, Driessen AJ, Konings WN. The essence of being extremophilic: the role of the unique archaeal membrane lipids. Extremophiles. 1998;2:163–170. doi: 10.1007/s007920050056. [DOI] [PubMed] [Google Scholar]
- 9.Konings WN, Albers SV, Koning S, Driessen AJ. The cell membrane plays a crucial role in survival of bacteria and archaea in extreme environments. Antonie Van Leeuwenhoek. 2002;81:61–72. doi: 10.1023/a:1020573408652. [DOI] [PubMed] [Google Scholar]
- 10.Tenchov B, Vescio EM, Sprott GD, Zeidel ML, Mathai JC. Salt tolerance of archaeal extremely halophilic lipid membranes. J Biol Chem. 2006;281:10016–10023. doi: 10.1074/jbc.M600369200. [DOI] [PubMed] [Google Scholar]
- 11.Koga Y, Morii H. Recent advances in structural research on ether lipids from archaea including comparative and physiological aspects. Biosci Biotechnol Biochem. 2005;69:2019–2034. doi: 10.1271/bbb.69.2019. [DOI] [PubMed] [Google Scholar]
- 12.Jain S, Caforio A, Driessen AJ. Biosynthesis of archaeal membrane ether lipids. Front Microbiol. 2014;5:641. doi: 10.3389/fmicb.2014.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villanueva L, Damsté JS, Schouten S. A re-evaluation of the archaeal membrane lipid biosynthetic pathway. Nat Rev Microbiol. 2014;12:438–448. doi: 10.1038/nrmicro3260. [DOI] [PubMed] [Google Scholar]
- 14.Calegari-Santos R, Diogo RA, Fontana JD, Bordin Bonfim TM. Carotenoid production by halophilic archaea under different culture conditions. Curr Microbiol. 2016;72:641–651. doi: 10.1007/s00284-015-0974-8. [DOI] [PubMed] [Google Scholar]
- 15.Hoff WD, Jung KH, Spudich JL. Molecular mechanism of photosignaling by archaeal sensory rhodopsins. Annu Rev Biophys Biomol Struct. 1997;26:223–258. doi: 10.1146/annurev.biophys.26.1.223. [DOI] [PubMed] [Google Scholar]
- 16.Guan Z, Eichler J. Liquid chromatography/tandem mass spectrometry of dolichols and polyprenols, lipid sugar carriers across evolution. Biochim Biophys Acta. 2011;1811:800–806. doi: 10.1016/j.bbalip.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartley MD, Imperiali B. At the membrane frontier: a prospectus on the remarkable evolutionary conservation of polyprenols and polyprenyl-phosphates. Arch Biochem Biophys. 2012;517:83–97. doi: 10.1016/j.abb.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiro RG. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12:43R–56R. doi: 10.1093/glycob/12.4.43r. [DOI] [PubMed] [Google Scholar]
- 19.Eichler J. Extreme sweetness: protein glycosylation in Archaea. Nat Rev Microbiol. 2013;11:151–156. doi: 10.1038/nrmicro2957. [DOI] [PubMed] [Google Scholar]
- 20.Weerapana E, Imperiali B. Asparagine-linked protein glycosylation: from eukaryotic to prokaryotic systems. Glycobiology. 2006;16:91R–101R. doi: 10.1093/glycob/cwj099. [DOI] [PubMed] [Google Scholar]
- 21.Aebi M. N-linked protein glycosylation in the ER. Biochim Biophys Acta. 2013;1833:2430–2437. doi: 10.1016/j.bbamcr.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Nothaft H, Szymanski CM. Protein glycosylation in bacteria: sweeter than ever. Nat Rev Microbiol. 2010;8:765–778. doi: 10.1038/nrmicro2383. [DOI] [PubMed] [Google Scholar]
- 23.Nothaft H, Szymanski CM. Bacterial protein N-glycosylation: new perspectives and applications. J Biol Chem. 2013;288:6912–6920. doi: 10.1074/jbc.R112.417857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mescher MF, Strominger JL. Purification and characterization of a prokaryotic glucoprotein from the cell envelope of Halobacterium salinarium. J Biol Chem. 1976;251:2005–2014. [PubMed] [Google Scholar]
- 25.Jarrell KF, Ding Y, Meyer BH, Albers SV, Kaminski L, Eichler J. N-linked glycosylation in Archaea: A structural, functional and genetic analysis. Microbiol Mol Biol Rev. 2014;78:304–341. doi: 10.1128/MMBR.00052-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mullakhanbhai MF, Larsen H. Halobacterium volcanii spec. nov., a Dead Sea halobacterium with a moderate salt requirement. Arch Microbiol. 1975;104:207–214. doi: 10.1007/BF00447326. [DOI] [PubMed] [Google Scholar]
- 27.Guan Z, Naparstek S, Kaminski L, Konrad Z, Eichler J. Distinct glycan-charged phosphodolichol carriers are required for the assembly of the pentasaccharide N-linked to the Haloferax volcanii S-layer glycoprotein. Mol Microbiol. 2010;78:1294–1303. doi: 10.1111/j.1365-2958.2010.07405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ginzburg M, Sachs L, Ginzburg BZ. Ion metabolism in a Halobacterium. I. Influence of age of culture on intracellular concentrations. J Gen Physiol. 1970;55:187–207. doi: 10.1085/jgp.55.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calo D, Guan Z, Naparstek S, Eichler J. Different routes to the same ending: Comparing the N-glycosylation processes of Haloferax volcanii and Haloarcula marismortui, two halophilic archaea from the Dead Sea. Mol Microbiol. 2011;81:1166–1177. doi: 10.1111/j.1365-2958.2011.07781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng C, Zhu JC, Liu Y, Yang Y, Zhu JY, Huang YP, Shen P. Investigation of the influence of NaCl concentration on Halobacterium salinarum growth. J Therm Anal Calorim. 2006;84:625–630. [Google Scholar]
- 31.Lechner J, Wieland F. Structure and biosynthesis of prokaryotic glycoproteins. Annu Rev Biochem. 1989;58:173–194. doi: 10.1146/annurev.bi.58.070189.001133. [DOI] [PubMed] [Google Scholar]
- 32.Cohen-Rosenzweig C, Guan Z, Shaanan B, Eichler J. Substrate promiscuity: AglB, the archaeal oligosaccharyltransferase, can process a variety of lipid-linked glycans. Appl Environ Microbiol. 2014;80:486–496. doi: 10.1128/AEM.03191-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brock TD, Brock KM, Belly RT, Weiss RL. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Microbiol. 1972;84:54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- 34.Guan Z, Meyer BH, Albers SV, Eichler J. The thermoacidophilic archaeon Sulfolobus acidocaldarius contains an unsually short, highly reduced dolichyl phosphate. Biochim Biophys Acta. 2011;181:607–616. doi: 10.1016/j.bbalip.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guan Z, Delago A, Nussbaum P, Meyer B, Albers SV, Eichler J. N-glycosylation in the thermoacidophilic archaeon Sulfolobus acidocaldarius involves a short dolichol pyrophosphate carrier. FEBS Lett. 2016;590:3168–3178. doi: 10.1002/1873-3468.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zillig W, Stetter KO, Wunderl S, Schulz W, Priess H, Scholz I. The Sulfolobus-“Caldariella” group: taxonomy on the basis of the structure of DNA-dependent RNA polymerases. Arch Microbiol. 1980;125:259–269. [Google Scholar]
- 37.Taguchi Y, Fujinami D, Kohda D. Comparative analysis of archaeal lipid-linked oligosaccharides that serve as oligosaccharide donors for Asn glycosylation. J Biol Chem. 2016;291:11042–11054. doi: 10.1074/jbc.M115.713156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swiezewska E, Danikiewicz W. Polyisoprenoids: Biosynthesis, structure and function. Prog Lipid Res. 2005;44:235–258. doi: 10.1016/j.plipres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Jones MB, Rosenberg JN, Betenbaugh MJ, Krag SS. Structure and synthesis of polyisoprenoids used in N-glycosylation across the three domains of life. Biochim Biophys Acta. 2009;1790:485–494. doi: 10.1016/j.bbagen.2009.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuntz C, Sonnenbichler J, Sonnenbichler I, Sumper M, Zeitler R. Isolation and characterization of dolichol-linked oligosaccharides from Haloferax volcanii. Glycobiology. 1997;7:897–904. doi: 10.1093/glycob/7.7.897. [DOI] [PubMed] [Google Scholar]
- 41.Chang MM, Imperiali B, Eichler J, Guan Z. In the hyperthermophilic archaea Pyrococcus furiosus, N-linked glycans are assembled on highly reduced dolichol phosphate carriers. PLoS One. 2015;10:e0130482. doi: 10.1371/journal.pone.0130482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalmokoff ML, Jarrell KF, Koval SF. Isolation of flagella from the archaebacterium Methanococcus voltae by phase separation with Triton X–114. J Bacteriol. 1988;170:1752–1758. doi: 10.1128/jb.170.4.1752-1758.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.González JM, Masuchi Y, Robb FT, Ammerman JW, Maeder DL, Yanagibayashi M, Tamaoka J, Kato C. Pyrococcus horikoshii sp. nov., a hyperthermophilic archaeon isolated from a hydrothermal vent at the Okinawa Trough. Extremophiles. 1998;2:123–130. doi: 10.1007/s007920050051. [DOI] [PubMed] [Google Scholar]
- 44.Urushibata Y, Ebisu S, Matsui I. A thermostable dolichol phosphoryl mannose synthase responsible for glycoconjugate synthesis of the hyperthermophilic archaeon Pyrococcus horikoshii. Extremophiles. 2008;12:665–676. doi: 10.1007/s00792-008-0173-7. [DOI] [PubMed] [Google Scholar]
- 45.Mescher MF, Strominger JL. Structural (shape-maintaining) role of the cell surface glycoprotein of Halobacterium salinarium. Proc Natl Acad Sci USA. 1976;73:2687–2691. doi: 10.1073/pnas.73.8.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lechner J, Wieland F, Sumper M. Biosynthesis of sulfated saccharides N-glycosidically linked to the protein via glucose. Purification and identification of sulfated dolichyl monophosphoryl tetrasaccharides from halobacteria. J Biol Chem. 1985;260:860–866. [PubMed] [Google Scholar]
- 47.Paul G, Wieland F. Sequence of the halobacterial glycosaminoglycan. J Biol Chem. 1987;262:9587–9593. [PubMed] [Google Scholar]
- 48.Sumper M. Halobacterial glycoprotein biosynthesis. Biochim Biophys Acta. 1987;906:69–79. doi: 10.1016/0304-4157(87)90005-0. [DOI] [PubMed] [Google Scholar]
- 49.Kaminski L, Guan Z, Yurist-Doutsch S, Eichler J. Two distinct N-glycosylation pathways process the Haloferax volcanii S-layer glycoprotein upon changes in environmental salinity. mBio. 2013;4:e00716–e00713. doi: 10.1128/mBio.00716-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brochier-Armanet C, Forterre P, Gribaldo S. Phylogeny and evolution of the Archaea: one hundred genomes later. Curr Opin Microbiol. 2011;14:274–281. doi: 10.1016/j.mib.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 51.Thauer RK. Biochemistry of methanogenesis: a tribute to Marjory Stephenson. 1998 Marjory Stephenson Prize Lecture. Microbiology. 1998;144:2377–2406. doi: 10.1099/00221287-144-9-2377. [DOI] [PubMed] [Google Scholar]
- 52.Jones WJ, Paynter MJB, Gupta R. Characterization of Methanococcus maripaludis sp. nov., a new methanogen isolated from salt marsh sediment. Arch Microbiol. 1983;135:91–97. [Google Scholar]
- 53.Kelly J, Logan SM, Jarrell KF, VanDyke DJ, Vinogradov E. A novel N-linked flagellar glycan from Methanococcus maripaludis. Carbohydr Res. 2009;344:648–653. doi: 10.1016/j.carres.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 54.Larkin A, Chang MM, Whitworth GE, Imperiali B. Biochemical evidence for an alternate pathway in N-linked glycoprotein biosynthesis. Nat Chem Biol. 2013;9:367–373. doi: 10.1038/nchembio.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stetter KO, Thomm M, Winter J, Wildgruber G, Huber H, Zillig W, Jané-Covic D, König H, Palm P, Wunderl S. Methanothermus fervidus, sp. nov., a novel extremely thermophilic methanogen isolated from an Icelandic hot spring, Zentralbl. Bakteriol Hyg I Abt Orig. 1981;C2:166–178. [Google Scholar]
- 56.Hartmann E, König H. Uridine and dolichyl diphosphate activated oligosaccharides are intermediates in the biosynthesis of the S-layer glycoprotein of Methanothermus fervidus. Arch Microbiol. 1989;151:274–281. [Google Scholar]
- 57.Fiala G, Stetter KO. Pyrococcus furiosus sp. nov. represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100°C. Arch Microbiol. 1986;145:56–61. [Google Scholar]
- 58.Stetter KO. Archaeoglobus fulgidus gen. nov., sp. nov.: a new taxon of extremely thermophilic archaebacteria. Syst Appl Microbiol. 1988;10:172–173. [Google Scholar]
- 59.Magidovich H, Eichler J. Glycosyltransferases and oligosaccharyltransferases in Archaea: putative components of the N-glycosylation pathway in the third domain of life. FEMS Microbiol Lett. 2009;300:122–130. doi: 10.1111/j.1574-6968.2009.01775.x. [DOI] [PubMed] [Google Scholar]
- 60.Palmieri G, Balestrieri M, Peter-Katalinić J, Pohlentz G, Rossi M, Fiume I, Pocsfalvi G. Surface-exposed glycoproteins of hyperthermophilic Sulfolobus solfataricus P2 show a common N-glycosylation profile. J Proteome Res. 2013;12:2779–2790. doi: 10.1021/pr400123z. [DOI] [PubMed] [Google Scholar]
- 61.Amo T, Paje ML, Inagaki A, Ezaki S, Atomi H, Imanaka T. Pyrobaculum calidifontis sp. nov., a novel hyperthermophilic archaeon that grows in atmospheric air. Archaea. 2002;1:113–121. doi: 10.1155/2002/616075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guy L, Ettema TJ. The archaeal ‘TACK’ superphylum and the origin of eukaryotes. Trends Microbiol. 2011;19:580–587. doi: 10.1016/j.tim.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 63.Spang A, Saw JH, Jørgensen SL, Zaremba-Niedzwiedzka K, Martijn J, Lind AE, van Eijk R, Schleper C, Guy L, Ettema TJ. Complex archaea that bridge the gap between prokaryotes and eukaryotes. Nature. 2015;521:173–179. doi: 10.1038/nature14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miziorko HM. Enzymes of the mevalonate pathway of isoprenoid biosynthesis. Arch Biochem Biophys. 2011;505:131–143. doi: 10.1016/j.abb.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smit A, Mushegian A. Biosynthesis of isoprenoids via mevalonate in Archaea: the lost pathway. Genome Res. 2000;10:1468–1484. doi: 10.1101/gr.145600. [DOI] [PubMed] [Google Scholar]
- 66.Boucher Y, Kamekura M, Doolittle WF. Origins and evolution of isoprenoid lipid biosynthesis in archaea. Mol Microbiol. 2004;52:515–527. doi: 10.1111/j.1365-2958.2004.03992.x. [DOI] [PubMed] [Google Scholar]
- 67.Bidle KA, Hanson TE, Howell K, Nannen J. HMG-CoA reductase is regulated by salinity at the level of transcription in Haloferax volcanii. Extremophiles. 2007;11:49–55. doi: 10.1007/s00792-006-0008-3. [DOI] [PubMed] [Google Scholar]
- 68.VanNice JC, Skaff DA, Wyckoff GJ, Miziorko HM. Expression in Haloferax volcanii of 3-hydroxy-3-methylglutaryl coenzyme A synthase facilitates isolation and characterization of the active form of a key enzyme required for polyisoprenoid cell membrane biosynthesis in halophilic archaea. J Bacteriol. 2013;195:3854–3862. doi: 10.1128/JB.00485-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Primak YA, Du M, Miller MC, Wells DH, Nielsen AT, Weyler W, Beck ZQ. Characterization of a feedback-resistant mevalonate kinase from the archaeon Methanosarcina mazei. Appl Environ Microbiol. 2011;77:7772–7778. doi: 10.1128/AEM.05761-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lombard J, Moreira D. Origins and early evolution of the mevalonate pathway of isoprenoid biosynthesis in the three domains of life. Mol Biol Evol. 2011;28:87–99. doi: 10.1093/molbev/msq177. [DOI] [PubMed] [Google Scholar]
- 71.Jones WJ, Leigh JA, Mayer F, Woese CR, Wolfe RS. Methanococcus jannaschii sp. nov., an extremely thermophilic methanogen from a submarine hydrothermal vent. Arch Microbiol. 1983;136:254–261. [Google Scholar]
- 72.Grochowski LL, Xu H, White RH. Methanocaldococcus jannaschii uses a modified mevalonate pathway for biosynthesis of isopentenyl diphosphate. J Bacteriol. 2006;188:3192–3198. doi: 10.1128/JB.188.9.3192-3198.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zeikus JG, Wolfe RS. Methanobacterium thermoautotrophicus sp. n., an anaerobic, autotrophic, extreme thermophile. J, Bacteriol. 1972;109:707–715. doi: 10.1128/jb.109.2.707-713.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Darland G, Brock TD, Samsonoff W, Conti SF. A thermophilic, acidophilic mycoplasma isolated from a coal refuse pile. Science. 1970;170:1416–1418. doi: 10.1126/science.170.3965.1416. [DOI] [PubMed] [Google Scholar]
- 75.Chen M, Poulter CD. Characterization of thermophilic archaeal isopentenyl phosphate kinases. Biochemistry. 2010;49:207–217. doi: 10.1021/bi9017957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vannice JC, Skaff DA, Keightley A, Addo J, Wyckoff GJ, Miziorko HM. Identification in Haloferax volcanii of phosphomevalonate decarboxylase and isopentenyl phosphate kinase as catalysts of the terminal ezymatic reactions in an archaeal alternate mevalonate pathway. J Bacteriol. 2014;196:1055–1063. doi: 10.1128/JB.01230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nishimura H, Azami Y, Miyagawa M, Hashimoto C, Yoshimura T, Hemmi H. Biochemical evidence supporting the presence of the classical mevalonate pathway in the thermoacidophilic archaeon Sulfolobus solfataricus. J Biochem. 2013;153:415–420. doi: 10.1093/jb/mvt006. [DOI] [PubMed] [Google Scholar]
- 78.Hattori A, Unno H, Goda S, Motoyama K, Yoshimura T, Hemmi H. In vivo formation of the protein disulfide bond that enhances the thermostability of diphosphomevalonate decarboxylase, an intracellular enzyme from the hyperthermophilic archaeon Sulfolobus solfataricus. J Bacteriol. 2015;197:3463–3471. doi: 10.1128/JB.00352-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Azami Y, Hattori A, Nishimura H, Kawaide H, Yoshimura T, Hemmi H. (R)-mevalonate 3-phosphate is an intermediate of the mevalonate pathway in Thermoplasma acidophilum. J Biol Chem. 2014;289:15957–15967. doi: 10.1074/jbc.M114.562686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vinokur JM, Korman TP, Cao Z, Bowie JU. Evidence of a novel mevalonate pathway in archaea. Biochemistry. 2014;53:4161–4168. doi: 10.1021/bi500566q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schleper C, Pühler G, Kühlmorgen B, Zillig W. Life at extremely low pH. Nature. 1995;375:741–742. doi: 10.1038/375741b0. [DOI] [PubMed] [Google Scholar]
- 82.Rossoni L, Hall SJ, Eastham G, Licence P, Stephens G. The putative mevalonate diphosphate decarboxylase from Picrophilus torridus is in reality a mevalonate-3-kinase with high potential for bioproduction of isobutene. Appl Environ Microbiol. 2015;81:2625–2634. doi: 10.1128/AEM.04033-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamashita S, Hemmi H, Ikeda Y, Nakayama T, Nishino T. Type 2 isopentenyl diphosphate isomerase from a thermoacidophilic archaeon Sulfolobus shibatae. Eur J Biochem. 2004;271:1087–1093. doi: 10.1111/j.1432-1033.2004.04010.x. [DOI] [PubMed] [Google Scholar]
- 84.Eisenreich W, Rohdich F, Bacher A. Deoxyxylulose phosphate pathway to terpenoids. Trends Plant Sci. 2001;6:78–84. doi: 10.1016/s1360-1385(00)01812-4. [DOI] [PubMed] [Google Scholar]
- 85.Rohdich F, Bacher A, Eisenreich W. Perspectives in anti-infective drug design. The late steps in the biosynthesis of the universal terpenoid precursors, isopentenyl diphosphate and dimethylallyl diphosphate. Bioorg Chem. 2004;32:292–308. doi: 10.1016/j.bioorg.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 86.Kellogg BA, Poulter CD. Chain elongation in the isoprenoid biosynthetic pathway. Curr Opin Chem Biol. 1997;1:570–578. doi: 10.1016/s1367-5931(97)80054-3. [DOI] [PubMed] [Google Scholar]
- 87.Schenk B, Fernandez F, Waechter CJ. The ins(ide) and out(side) of dolichyl phosphate biosynthesis and recycling in the endoplasmic reticulum. Glycobiology. 2001;11:61R–70R. doi: 10.1093/glycob/11.5.61r. [DOI] [PubMed] [Google Scholar]
- 88.Bickford JS, Nick HS. Conservation of the PTEN catalytic motif in the bacterial undecaprenyl pyrophosphate phosphatase, BacA/UppP. Microbiology. 2013;159:2444–2455. doi: 10.1099/mic.0.070474-0. [DOI] [PubMed] [Google Scholar]
- 89.Volpe JJ, Sakakihara Y, Rust RS. Dolichol kinase and the regulation of dolichyl phosphate levels in developing brain. Brain Res. 1987;428:193–200. doi: 10.1016/0165-3806(87)90117-9. [DOI] [PubMed] [Google Scholar]
- 90.Sandermann H, Jr, Strominger JL. C 55 -isoprenoid alcohol phosphokinase: an extremely hydrophobic protein from the bacterial membrane. Proc Natl Acad Sci USA. 1971;68:2441–2443. doi: 10.1073/pnas.68.10.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ogura K, Koyama T. Enzymatic aspects of isoprenoid chain elongation. Chem Rev. 1998;98:1263–1276. doi: 10.1021/cr9600464. [DOI] [PubMed] [Google Scholar]
- 92.Hemmi H, Yamashita S, Shimoyama T, Nakayama T, Nishino T. Cloning, expression, and characterization of cis-polyprenyl diphosphate synthase from the thermoacidophilic archaeon Sulfolobus acidocaldarius. J Bacteriol. 2001;183:401–404. doi: 10.1128/JB.183.1.401-404.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Matsumi R, Atomi H, Driessen AJ, van der Oost J. Isoprenoid biosynthesis in Archaea–biochemical and evolutionary implications. Res Microbiol. 2011;162:39–52. doi: 10.1016/j.resmic.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 94.Chen A, Poulter CD. Purification and characterization of farnesyl diphosphate/geranylgeranyl diphosphate synthase. A thermostable bifunctional enzyme from Methanobacterium thermoautotrophicum. J Biol Chem. 1993;268:11002–11007. [PubMed] [Google Scholar]
- 95.Morikawa M, Izawa Y, Rashid N, Hoaki T, Imanaka T. Purification and characterization of a thermostable thiol protease from a newly isolated hyperthermophilic Pyrococcus sp. Appl Environ Microbiol. 1994;60:4559–4566. doi: 10.1128/aem.60.12.4559-4566.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ohnuma S, Suzuki M, Nishino T. Archaebacterial ether-linked lipid biosynthetic gene. Expression cloning, sequencing, and characterization of geranylgeranyl-diphosphate synthase. J Biol Chem. 1994;269:14792–14797. [PubMed] [Google Scholar]
- 97.Fujiwara S, Yamanaka A, Hirooka K, Kobayashi A, Imanaka T, Fukusaki E. Temperature-dependent modulation of farnesyl diphosphate/geranylgeranyl diphosphate synthase from hyperthermophilic archaea. Biochem Biophys Res Commun. 2004;325:1066–1074. doi: 10.1016/j.bbrc.2004.10.129. [DOI] [PubMed] [Google Scholar]
- 98.Chen A, Kroon PA, Poulter CD. Isoprenyl diphosphate synthases: protein sequence comparisons, a phylogenetic tree, and predictions of secondary structure. Protein Sci. 1994;3:600–607. doi: 10.1002/pro.5560030408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ohnuma S, Hirooka K, Ohto C, Nishino T. Conversion from archaeal geranylgeranyl diphosphate synthase to farnesyl diphosphate synthase. Two amino acids before the first aspartate-rich motif solely determine eukaryotic farnesyl diphosphate synthase activity. J Biol Chem. 1997;272:5192–5198. doi: 10.1074/jbc.272.8.5192. [DOI] [PubMed] [Google Scholar]
- 100.Tachibana A, Yano Y, Otani S, Nomura N, Sako Y, Taniguchi M. Novel prenyltransferase gene encoding farnesylgeranyl diphosphate synthase from a hyperthermophilic archaeon, Aeropyrum pernix. Molecular evolution with alteration in product specificity. Eur J Biochem. 2000;267:321–328. doi: 10.1046/j.1432-1327.2000.00967.x. [DOI] [PubMed] [Google Scholar]
- 101.Wurtzel O, Sapra R, Chen F, Zhu Y, Simmons BA, Sorek R. A single-base resolution map of an archaeal transcriptome. Genome Res. 2010;20:133–141. doi: 10.1101/gr.100396.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meyer BH, Shams-Eldin H, Albers SV. AglH, a thermophilic UDP-N-acetylglucosamine-1-phosphate:dolichyl phosphate GlcNAc-1-phosphotransferase initiating protein N-glycosylation pathway in Sulfolobus acidocaldarius, is capable of complementing the eukaryal Alg7. Extremophiles. 2016 doi: 10.1007/s00792-016-0890-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Akhtar TA, Matsuba Y, Schauvinhold I, Yu G, Lees HA, Klein SE, Pichersky E. The tomato cis-prenyltransferase gene family. Plant J. 2013;73:640–652. doi: 10.1111/tpj.12063. [DOI] [PubMed] [Google Scholar]
- 104.Yamashita S, Yamaguchi H, Waki T, Aoki Y, Mizuno M, Yanbe F, Ishii T, Funaki A, Tozawa Y, Miyagi-Inoue Y, Fushihara K, Nakayama T, Takahashi S. Identification and reconstitution of the rubber biosynthetic machinery on rubber particles from Hevea brasiliensis. Elife. 2016;5:e19022. doi: 10.7554/eLife.19022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ogawa T, Emi K, Koga K, Yoshimura T, Hemmi H. A cis-prenyltransferase from Methanosarcina acetivorans catalyzes both head-to-tail and nonhead-to-tail prenyl condensation. FEBS J. 2016;283:2369–2383. doi: 10.1111/febs.13749. [DOI] [PubMed] [Google Scholar]
- 106.Sako Y, Nomura N, Uchida A, Ishida Y, Morii H, Koga Y, Hoaki T, Maruyama T. Aeropyrum pernix gen. nov., sp. nov., a novel aerobic hyperthermophilic archaeon growing at temperatures up to 100°C. Int J Syst Evol Microbiol. 1996;46:1070–1077. doi: 10.1099/00207713-46-4-1070. [DOI] [PubMed] [Google Scholar]
- 107.Mori T, Ogawa T, Yoshimura T, Hemmi H. Substrate specificity of undecaprenyl diphosphate synthase from the hyperthermophilic archaeon Aeropyrum pernix. Biochem Biophys Res Commun. 2013;436:230–234. doi: 10.1016/j.bbrc.2013.05.081. [DOI] [PubMed] [Google Scholar]
- 108.Kaminski L, Lurie-Weinberger MN, Allers T, Gophna U, Eichler J. Phylogenetic- and genome-derived insight into the evolution of N-glycosylation in Archaea. Mol Phylogenet Evol. 2013;68:327–339. doi: 10.1016/j.ympev.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 109.Harrison KD, Park EJ, Gao N, Kuo A, Rush JS, Waechter CJ, Lehrman MA, Sessa WC. Nogo-B receptor is necessary for cellular dolichol biosynthesis and protein N-glycosylation. EMBO J. 2011;30:2490–2500. doi: 10.1038/emboj.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brasher MI, Surmacz L, Leong B, Pitcher J, Swiezewska E, Pichersky E, Akhtar TA. A two-component enzyme complex is required for dolichol biosynthesis in tomato. Plant J. 2015;82:903–914. doi: 10.1111/tpj.12859. [DOI] [PubMed] [Google Scholar]
- 111.Cantagrel V, Lefeber DJ. From glycosylation disorders to dolichol biosynthesis defects: a new class of metabolic diseases. J Inherit Metab Dis. 2011;34:859–867. doi: 10.1007/s10545-011-9301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Grabinska KA, Cui J, Chatterjee A, Guan Z, Raetz CRH, Robbins PW, Samuelson J. Molecular characterization of the cis-prenyltransferase of Giardia lamblia. Glycobiology. 2010;20:824–832. doi: 10.1093/glycob/cwq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wolf MJ, Rush JS, Waechter CJ. Golgi-enriched membrane fractions from rat brain and liver contain long-chain polyisoprenyl pyrophosphate phosphatase activity. Glycobiology. 1991;1:405–410. doi: 10.1093/glycob/1.4.405. [DOI] [PubMed] [Google Scholar]
- 114.El Ghachi M, Bouhss A, Blanot D, Mengin-Lecreulx D. The bacA gene of Escherichia coli encodes an undecaprenyl pyrophosphate phosphatase activity. J Biol Chem. 2004;279:30106–30113. doi: 10.1074/jbc.M401701200. [DOI] [PubMed] [Google Scholar]
- 115.Ogawa T, Isobe K, Mori T, Asakawa S, Yoshimura T, Hemmi H. A novel geranylgeranyl reductase from the methanogenic archaeon Methanosarcina acetivorans displays unique regiospecificity. FEBS J. 2014;281:3165–3176. doi: 10.1111/febs.12851. [DOI] [PubMed] [Google Scholar]
- 116.Cantagrel V, Lefeber DJ, Ng BG, Guan Z, Silhavy JL, Bielas SL, Lehle L, Hombauer H, Adamowicz M, Swiezewska E, De Brouwer A, Bluemel P, Cegielska J, Houliston SR, Swistun D, Ali BR, Babovic-Vuksanovic D, van Bokhoven H, Wevers RA, Raetz CRH, Freeze HH, Morava E, Al-Gazali L, Gleeson JG. SRD5A3 is required for converting polyprenol to dolichol and is mutated in a congenital glycosylation disorder. Cell. 2010;142:1–15. doi: 10.1016/j.cell.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sato S, Murakami M, Yoshimura T, Hemmi H. Specific partial reduction of geranylgeranyl diphosphate by an enzyme from the thermoacidophilic archaeon Sulfolobus acidocaldarius yields a reactive prenyl donor, not a dead-end product. J Bacteriol. 2008;190:3923–3929. doi: 10.1128/JB.00082-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kung Y, McAndrew RP, Xie X, Liu CC, Pereira JH, Adams PD, Keasling JD. Constructing tailored isoprenoid products by structure-guided modification of geranylgeranyl reductase. Structure. 2014;22:1028–1036. doi: 10.1016/j.str.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 119.Nishimura Y, Eguchi T. Biosynthesis of archaeal membrane lipids: digeranylgeranylglycerophospholipid reductase of the thermoacidophilic archaeon Thermoplasma acidophilum. J Biochem. 2006;139:1073–1081. doi: 10.1093/jb/mvj118. [DOI] [PubMed] [Google Scholar]
- 120.Isobe K, Ogawa T, Hirose K, Yokoi T, Yoshimura T, Hemmi H. Geranylgeranyl reductase and ferredoxin from Methanosarcina acetivorans are required for the synthesis of fully reduced archaeal membrane lipid in Escherichia coli cells. J Bacteriol. 2014;196:417–423. doi: 10.1128/JB.00927-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Naparstek S, Guan Z, Eichler J. A predicted geranylgeranyl reductase reduces the ω-position isoprene of dolichol phosphate in the halophilic archaeon, Haloferax volcanii. Biochim Biophys Acta. 2012;1821:923–933. doi: 10.1016/j.bbalip.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lombard J. Early evolution of polyisoprenol biosynthesis and the origin of cell walls. Peer J. 2016;4:e2626. doi: 10.7717/peerj.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Heller L, Orlean P, Adair WL., Jr Saccharomyces cerevisiae sec59 cells are deficient in dolichol kinase activity. Proc Natl Acad Sci USA. 1992;89:7013–7016. doi: 10.1073/pnas.89.15.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shridas P, Waechter CJ. Human dolichol kinase, a polytopic endoplasmic reticulum membrane protein with a cytoplasmically oriented CTP-binding site. J Biol Chem. 2006;281:31696–31704. doi: 10.1074/jbc.M604087200. [DOI] [PubMed] [Google Scholar]
- 125.Rush JS, Cho SK, Jiang S, Hofmann SL, Waechter CJ. Identification and characterization of a cDNA encoding a dolichyl pyrophosphate phosphatase located in the endoplasmic reticulum of mammalian cells. J Biol Chem. 2002;277:45226–45234. doi: 10.1074/jbc.M207076200. [DOI] [PubMed] [Google Scholar]
- 126.Rush JS, Gao N, Lehrman MA, Waechter CJ. Recycling of dolichyl monophosphate to the cytoplasmic leaflet of the endoplasmic reticulum after the cleavage of dolichyl pyrophosphate on the lumenal monolayer. J Biol Chem Feb. 2008;283:4087–4093. doi: 10.1074/jbc.M707067200. [DOI] [PubMed] [Google Scholar]
- 127.Suzuki T, Iwasaki T, Uzawa T, Hara K, Nemoto N, Kon T, Ueki T, Yamagishi A, Oshima T. Sulfolobus tokodaii sp. nov. (f. Sulfolobus sp. strain 7), a new member of the genus Sulfolobus isolated from Beppu Hot Springs, Japan. Extremophiles. 2002;6:39–44. doi: 10.1007/s007920100221. [DOI] [PubMed] [Google Scholar]
- 128.Meyer W, Schäfer G. Characterization and purification of a membrane-bound archaebacterial pyrophosphatase from Sulfolobus acidocaldarius. Eur J Biochem. 1992;207:741–746. doi: 10.1111/j.1432-1033.1992.tb17104.x. [DOI] [PubMed] [Google Scholar]
- 129.Amano T, Wakagi T, Oshima T. An ecto-enzyme from Sulfolobus acidocaldarius strain 7 which catalyzes hydrolysis of inorganic pyrophosphate, ATP, and ADP: purification and characterization. J Biochem (Tokyo) 1993;114:329–333. doi: 10.1093/oxfordjournals.jbchem.a124176. [DOI] [PubMed] [Google Scholar]
- 130.Manabe F, Itoh YH, Shoun H, Wakagi T. Membrane-bound acid pyrophosphatase from Sulfolobus tokodaii, a thermoacidophilic archaeon: heterologous expression of the gene and characterization of the product. Extremophiles. 2009;13:859–865. doi: 10.1007/s00792-009-0273-z. [DOI] [PubMed] [Google Scholar]
- 131.Igura M, Maita N, Kamishikiryo J, Yamada M, Obita T, Maenaka K, Kohda D. Structure-guided identification of a new catalytic motif of oligosaccharyltransferase. EMBO J. 2008;27:234–243. doi: 10.1038/sj.emboj.7601940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jaffee MB, Imperiali B. Exploiting topological constraints to reveal buried sequence motifs in the membrane-bound N-linked oligosaccharyl transferases. Biochemistry. 2011;50:7557–7567. doi: 10.1021/bi201018d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Matsumoto S, Igura M, Nyirenda J, Matsumoto M, Yuzawa S, Noda N, Inagaki F, Kohda D. Crystal structure of the C-terminal globular domain of oligosaccharyltransferase from Archaeoglobus fulgidus at 1.75 A resolution. Biochemistry. 2012;51:4157–4166. doi: 10.1021/bi300076u. [DOI] [PubMed] [Google Scholar]
- 134.Matsumoto S, Shimada A, Kohda D. Crystal structure of the C-terminal globular domain of the third paralog of the Archaeoglobus fulgidus oligosaccharyltransferases. BMC Struct Biol. 2013;13:11. doi: 10.1186/1472-6807-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Matsumoto S, Shimada A, Nyirenda J, Igura M, Kawano Y, Kohda D. Crystal structures of an archaeal oligosaccharyltransferase provide insights into the catalytic cycle of N-linked protein glycosylation. Proc Natl Acad Sci USA. 2013;110:17868–17873. doi: 10.1073/pnas.1309777110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sanyal S, Menon AK. Flipping lipids: why an’ what’s the reason for? ACS Chem Biol. 2009;4:895–909. doi: 10.1021/cb900163d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kaminski L, Guan Z, Abu-Qarn M, Konrad Z, Eichler J. AglR is required for addition of the final mannose residue of the N-linked glycan decorating the Haloferax volcanii S-layer glycoprotein. Biochim Biophys Acta. 2012;1820:1664–1670. doi: 10.1016/j.bbagen.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Raetz CRH, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]


