Synopsis/abstract
Periviable births are defined as births occurring from 20 0/7 through 25 6/7 weeks of gestation. Among and within developed nations, significant variation exists in the approach to obstetrical and neonatal care for periviable birth. Understanding gestational age-specific survival, including factors that may influence survival estimates and how these estimates have changed over time, may guide approaches to the care of periviable births and inform conversations with families and caregivers. This review provides a historical perspective on survival following periviable birth, summarizes recent and new data on gestational age-specific survival rates, and addresses factors that have a significant impact on survival.
Keywords: Mortality, Perinatal Epidemiology, Preterm Infant, Resuscitation, Stillbirth
Introduction
Periviable births comprise a particularly high-risk group of patients cared for by obstetricians, neonatologists and other caregivers. Periviable birth is currently defined as delivery occurring from 20 0/7 through 25 6/7 weeks’ of gestation.1,2 This review provides a historical perspective into survival of periviable births, summarizes recent data on gestational age-specific survival rates and reviews factors that can have a significant impact on survival. As this review is focused on survival, we do not discuss additional outcomes among surviving periviable infants, such as disability, but acknowledge the importance of considering such outcomes alongside estimates of mortality to inform understanding about prognosis.
Historical perspectives
The survival of extremely low birth weight (ELBW, birth weight ≤1000 g) infants, including periviable infants, has improved consistently over the past seven decades. In the 1940s, death was the expected outcome for all ELBW infants born in developed nations around the world.3 Beginning in the 1950's and 1960s, the probability of survival for ELBW infants among several centers in the US and UK increased to 10 to 30% as understanding of neonatal physiology improved and the provision of neonatal intensive care became more common and more advanced.3,4 In the 1970s, multiple reports from the US and international centers demonstrated improved survival rates for ELBW and extremely preterm infants.3 A single-center study from Illinois of 100 ELBW infants born between 1974 and 1976 and admitted to the neonatal intensive care unit (NICU) reported survival rates of 10% for infants weighing 501–750 g and 48% for infants weighing 751–1000 grams.5 A multicenter study of live births from 1976 to 1978 in New York City reported a survival rate of approximately 50% for singleton live-births weighing 501–1250 g born at level-3 centers.6 In a population-based study from England and Wales, survival of liveborn infants weighing <1000 grams increased from 16% in 1964 to 23% in in 1975.7
In the late 1980s and mid-1990s, prospective cohort studies from the NICHD Neonatal Research Network (NRN)8 and EPICure9 were among the first to systematically evaluate periviable birth outcomes on a relatively large scale in the surfactant era of neonatology. The NICHD NRN reported outcomes of 1804 very low birth weight (VLBW, birth weight 501–1500 g) live births at 8 academic centers in the US from 1989–1990.8 The estimated survival for liveborn infants in this cohort was 18% at less than 23 weeks’ of gestation, 15% at 23 weeks’, 54% at 24 weeks’ and 59% at 25 weeks’. The EPICure study collected outcomes for periviable live births across all maternity units in the UK and Ireland in 1995 (n=4004) and reported survival rates of 40% for births between 20 and 25 weeks’ of gestation.9 Similar to the NICHD NRN study, the EPICure study reported survival approaching 20% for infants born before 24 weeks’ of gestation and survival greater than 60% for births at 25 weeks’ of gestation.
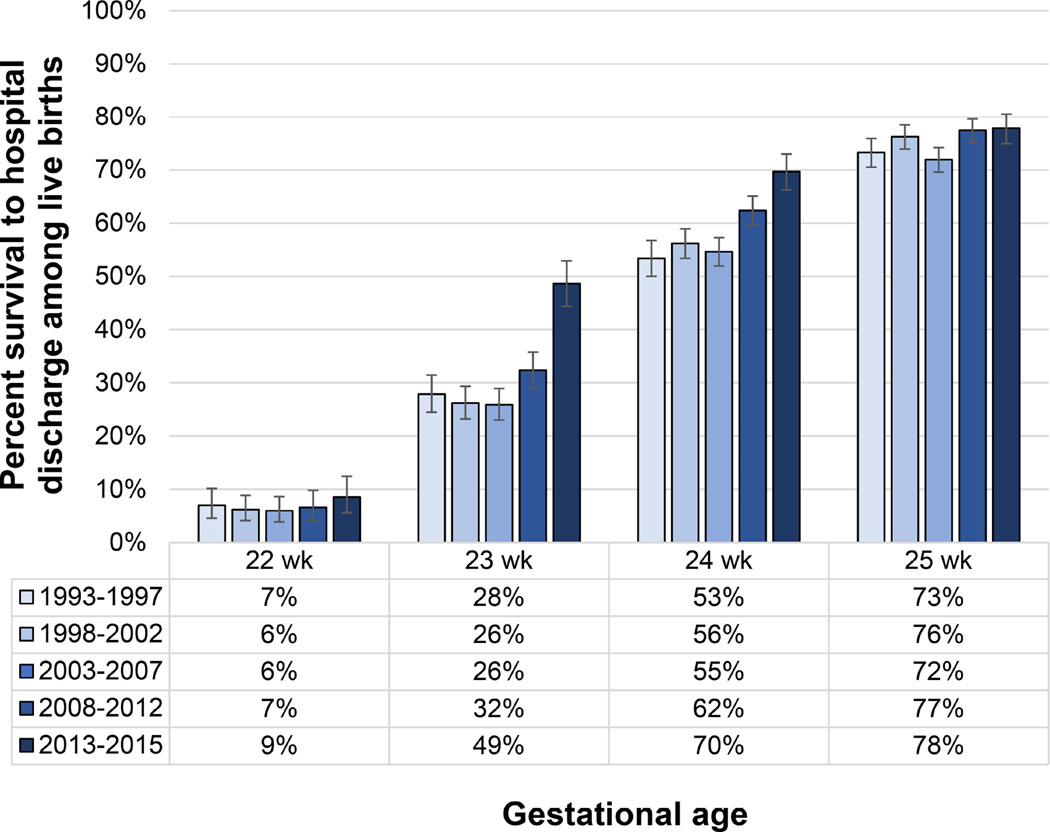
In the early part of the 21st century, data from the NICHD NRN for periviable births from 2003 to 2007 suggested that improvements in survival had plateaued, with no increases in survival rates over the period.10 However, more recent reports from the NICHD NRN (Figure 1) and other centers in the US,11–13 as well as from several other developed nations around the world,14–19 demonstrate incremental improvements in survival following periviable birth, continuing trends established over half a century ago. These studies are discussed in detail below.
Figure 1. Survival from 1993 through 2015 following Live Birth in the NICHD Neonatal Research Network.
Includes all participating centers. Liveborn infants were included regardless of whether active treatment was initiated. Whisker bars indicate 95% confidence intervals calculated with the Clopper-Pearson method.
Data Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. 2015;314(10):1039–1051 for 1993–2012 and courtesy of the NICHD Neonatal Research Network for 2013–2015.
Estimates of gestational-age specific survival
During the past 5 years, large cohort studies from developed nations in North America,11–13,20,21 South America,22 Europe,14,15,23–25 Asia,18,19,26 and Australia27 have reported estimates of gestational age-specific survival following periviable birth. Direct comparisons of estimated survival rates among these studies are limited, however, by potential biases introduced from differences in the data sources, ascertainment of death, selection of denominators, and definitions of live birth.28 Recommendations to improve reporting of birth outcomes have recently been published with the goal of providing more clinically meaningful and comparable estimates of survival.29 These recommendations emphasize the importance of reporting standardized information on the source population and outcomes measured, including the timing of assessment, and measures of statistical uncertainty, such as 95% confidence intervals (CI), when reporting on periviable birth outcomes. With the aforementioned caveats that studies have varied in their methodology and reporting, estimates of gestational age-specific survival for select population-based and multicenter studies that report outcomes among periviable live births or infants admitted to the NICU (Table 1) are shown in Figure 2.
Table 1.
Recent Studies Reporting Gestational-Age Specific Survival following Periviable Birth
| Study (publication year) |
EPICure (2012)14 |
EXPRESS (2013)83 |
EPIPAGE- 2 (2015)15 |
Victoria (2016)27 |
NICHD NRNa |
Japan NRN (2013)18 |
SNN (2016)24 |
Pediatrix (2016)20 |
CNN (2013)21 |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Study Type | Population-based cohort studies | Center-based cohort studies | ||||||||
|
Source population |
National | National | National (except 1 region, 2% of births) |
Regional | 18 network centers |
48 tertiary centers |
15 NICUs (95% of births in nation) |
362 NICUs |
National network of NICUs |
|
| Country | UK | Sweden | France | Australia | US | Japan | Switzerland | US | Canada | |
| Year(s)* | 2006 | 2004– 2007 |
2011 | 2010– 2011 |
2013– 2015 |
2003– 2005 |
2009– 2012 |
1997–2013 | 2010–2011 | |
| Sample sizeb (at 22–25 weeks’) |
2034 (1454) |
707 (501) | 5169 (641) | 541 (279) | 2430 | 1057 (1057) |
3068 (450) |
64,896 (17,085) |
6106 (1208) |
|
| GA inclusion | 22–26 weeks’ |
<27 weeks’c |
22–34 weeks’ |
22–27 weeks’ |
22–25 weeks’ |
22–25 weeks’ |
23–31 weeks’ |
22–29 weeks’ |
23–30 weeks’ |
|
| Minimal BW inclusion |
400g | None | None | None | 401g | None | None | None | None | |
| Inception (denominator reported by study) |
Live birth or stillbirth |
Live birth or stillbirth |
Live birth, stillbirth or TOP |
Live birth | Live birth | Live birth | Live birth | NICU admission |
NICU admission |
|
| Denominator for Figure 2 |
Live births | Infants admitted to center |
||||||||
| Outborn, % of denominator |
14.7% - Included |
16.5% - Included |
21.0% - Included |
15.5% - Included |
Not reported |
9.3% - Included |
4.9% - Included |
18.5% - Excluded |
18.1% - Included |
|
| Exclusions | TOP | TOP, birth outside country |
No verbal consent |
TOP, birth defects |
Outborn | Admitted after 28d, birth defects |
Outborn, transfer, birth defects |
Multipled | ||
|
Survival assessment |
Survival to d/c |
Survival to 1 year |
Survival to d/c |
Survival to d/c |
Survival to d/c |
Survival to d/c |
Survival to d/c |
Survival to d/c |
Survival to d/c |
|
Abbreviations: NICHD, National Institute of Child Health and Human Development; NRN, Neonatal Research Network; EPIPAGE-2, Etude Epidémiologique sur les Petits Ages Gestationnels 2; EXPRESS, Extremely Preterm Infants in Sweden Study; SNN, Swiss Neonatal Network; CNN, Canadian Neonatal Network; NICU, neonatal intensive care unit; TOP, termination of pregnancy; d/c, hospital discharge;
Data on mortality among inborn live births from 2013–2015 courtesy of the NICHD NRN.
Sample size of live births for year(s) listed; number of periviable births in Figure 2 noted in parenthesis.
Stillbirths at 22 0/7–26 6/7 included; no lower gestational age bounds for live births.
Infants declared moribund, infants receiving palliative care, infants with lethal birth defects or infants with missing birth date or missing/ambiguous gender.
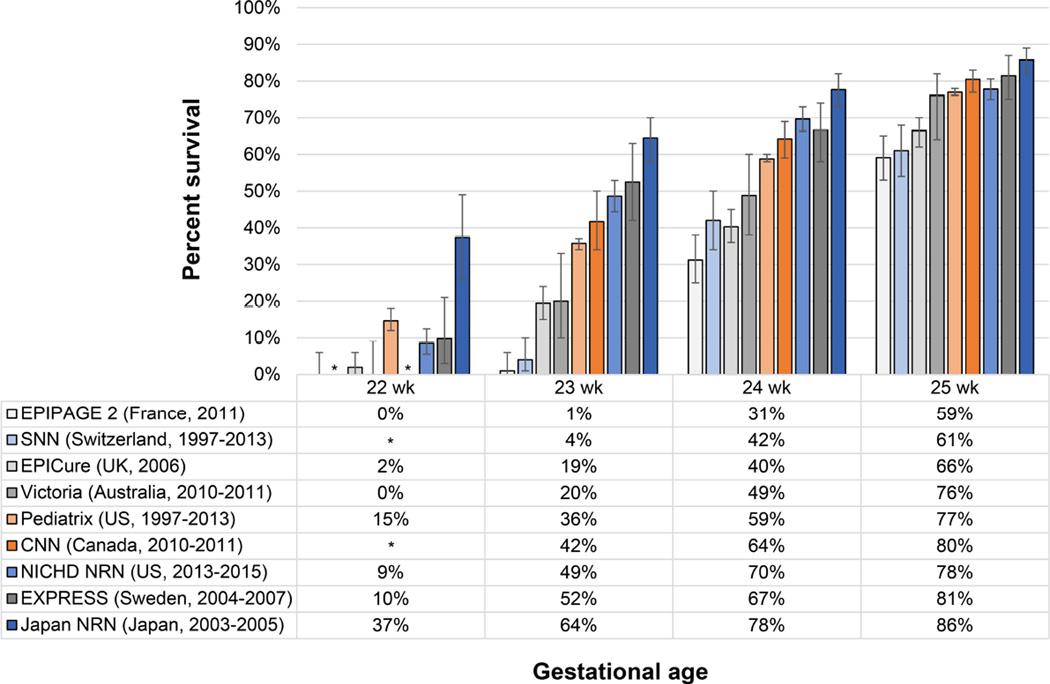
Figure 2. Gestational Age-Specific Survival following Live Birth by Study Type.
Data are shown for population-based cohorts (gray hues), center-based cohorts reporting survival for live births (blue hues) and center-based cohorts reporting survival for infants admitted to the NICU (orange hues). Characteristics of the data sources are reported in Table 1. Whisker bars indicate 95% confidence intervals calculated with the Clopper-Pearson method. *Estimates not reported by the study.
These studies show a wide variation in survival rates following live birth at periviable gestational ages, ranging from 0–37% at 22 weeks’, 1–64% at 23 weeks’, 31–78% at 24 weeks’ and 59–86% at 25 weeks’ of gestation. The variation in survival rates following periviable birth among cohorts in developed nations (Figure 2) is much greater in magnitude than the increases in survival over time within the NICHD NRN (Figure 1). Variation in approaches to perinatal care and other factors that may explain the large amount of variation in periviable survival are discussed below. In general, we have categorized this variation as resulting from between-study differences in: national and institutional recommendations and guidelines for perinatal care, cohort characteristics, maternal-infant characteristics, and antenatal and postnatal treatment, including decisions about the initiation and withdrawal of care.
Recommendations and guidelines for perinatal care
Some of the variation in survival observed in Figure 2 may be attributable to variation in the approach to perinatal care based on guideline statements from professional organizations and scientific societies. In a systematic review of 31 national or international guidelines for perinatal care of periviable births in highly developed countries, there was substantial variation in recommendations. Sixty-eight percent of guideline statements supported comfort care at 22 weeks’ of gestation and 65% supported active treatment and resuscitation at 25 weeks’ of gestation.30 At 23 and 24 weeks’ of gestation, there was more variability among recommendations, including for comfort care, routine active treatment, individualized care, and active treatment based on parental wishes. Many of these guidelines statements include reporting of country-specific survival rates, although as we discuss below, substantial within-country variation in survival rates have also been reported.
In the US, a recent consensus statement by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine provides general guidance regarding obstetric and neonatal active treatment for fetuses and infants at periviable gestational ages.2 At 20 to 21 weeks’ of gestation, provision of antenatal corticosteroids, cesarean delivery for fetal indication and neonatal assessment for resuscitation are not recommended. At 22 weeks’ of gestation, the authors recommend that clinicians “consider” neonatal assessment for resuscitation but do not recommend antenatal corticosteroids or cesarean delivery. At 23 weeks’ of gestation, the authors recommend that clinicians consider all general measures of neonatal and obstetric active treatment but do not give a firm recommendation for any of them. At 24 weeks’ of gestation, cesarean delivery should be considered, and all other measures of neonatal and obstetric active treatment are recommended. At 25 weeks’ of gestation, cesarean delivery and other active neonatal and obstetric measures are recommended. Given the recent publication of this guideline statement in the US, it is too soon to assess the effect of these recommendations on clinical practice.
Additional Factors influencing survival rates for periviable infants
Cohort selection
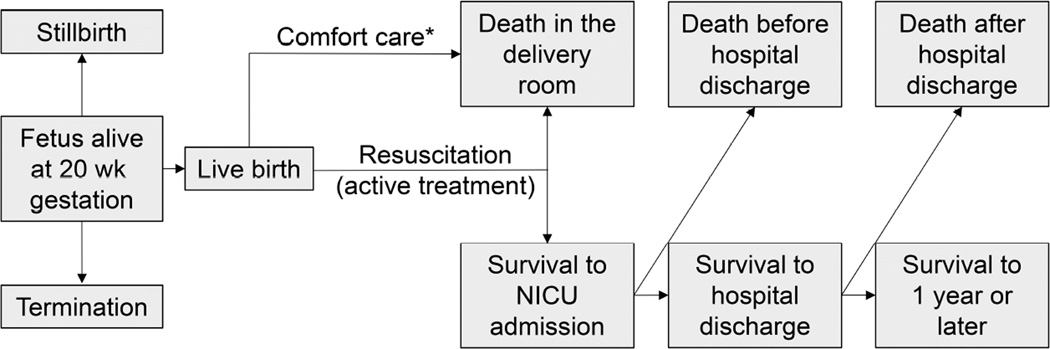
Differences in the conduct of cohort studies are important to understand when interpreting and comparing gestational age-specific survival rates. This is particularly relevant when studies use different numerators (e.g. death in the delivery room, death before 28 d, death before hospital discharge, death before 1 year) and denominators (e.g. fetus alive at maternal admission and >20 weeks’ of gestation, all live births, inborn live births, live births receiving active treatment, infants admitted to the NICU) to estimate mortality rates (Figure 3). The appropriate numerator and denominator depend on the relevant period at risk.29 For a woman pregnant with a fetus alive at 20 weeks’ of gestation, studies reporting outcomes with a denominator of infants admitted to the NICU do not reflect all of the potential birth outcomes of her fetus (Figure 3). Likewise, a high stillbirth rate may be obscured by reporting outcomes only among live births, and a high delivery room death rate could be obscured by reporting only outcomes for infants admitted to the NICU (Figure 4). In contrast, if the population of interest is infants receiving active treatment after live birth, the stillbirth rate has ordinarily been assumed to be irrelevant. However, exclusion of stillbirths can lead to imperfect risk adjustment when comparing populations that differ in prenatal and antepartum care and the proportion of fetuses at high risk for death after birth.
Figure 3. Potential Birth Outcomes for a Fetus Alive at 20 weeks’ of Gestation.
*Although uncommon, survival to NICU admission may occur following initial provision of comfort care.
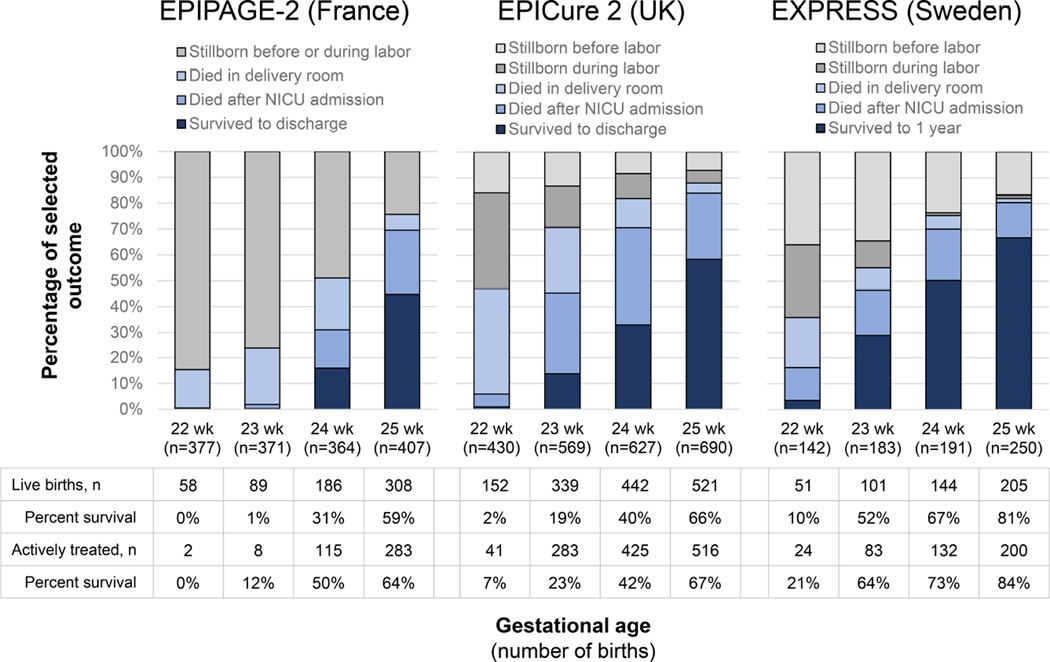
Figure 4. Periviable Birth Outcomes by Gestational Age in Population-based Studies.
Data from population-based studies in France,15,56 the United Kingdom (UK)14,82 and Sweden83 evaluating outcomes of live births and stillbirths in Table 1. Pregnancies with termination not included. Active treatment definitions vary by study but include measures of delivery room intervention or admission for neonatal care.
Study selection criteria—particularly with regards to selection of infants based on admission to a hospital or birth within a geographic population, decisions regarding the inclusion of outborn infants in hospital-based studies, and the exclusion of infants born below a lower bounds of birth weight or with the diagnosis of a birth defect—can impact the estimated mortality rates.
In addition, differences in case-mix among cohorts studied can lead to biased comparisons of risk-adjusted mortality due to Simpson’s paradox, which occurs when populations differing in case-mix are grouped together and can lead to reversal of an association demonstrated when groups are compared separately.31 This paradox has been demonstrated in studies using risk-adjustment to compare standardized mortality ratios among different populations.32,33
Place of birth
As previously discussed, country of birth has a large impact on the probability of survival.34 Take, for example, the nearly universal death of infants born alive at 23 weeks’ of gestation in France compared to the approximately 50% reported survival among live births in Sweden (Figure 2). Some of this variation may be accounted for by national guideline statements and cultural preferences regarding perinatal care of periviable births,35 as previously discussed. However, even within countries, there is substantial variation in survival among live births.
The level, volume and quality of neonatal care provided at a given center or hospital has potential impacts on extremely preterm survival. Variation in neonatal care and outcomes by center,10,36,37 region,38,38 and country39 has been well documented, and the level and volume of neonatal care provided has been associated with a center’s rate of death or serious morbidity.40,41 However, the level of neonatal care is not necessarily associated with some aspects of quality care, as demonstrated in a recent study of 134 California NICUs.42 Within limits, greater availability of neonatal intensive care, measured by the number of neonatologists working in a given region, is associated with a decreased neonatal mortality rate.43 However, the potential benefits of increased availability of neonatal intensive care may be offset by the associated de-regionalization of care that can occur as the number of NICUs increase, with a greater proportion of high-risk births occurring in lower volume centers.44 In addition, hospital recognition for nursing excellence, a surrogate measure of quality of nursing care, is associated with a lower risk of early mortality in the first 7 days of life for very low birth weight infants, but not mortality before discharge.45
Variation in the provision of active treatment
Intensive care is necessary for neonatal survival at periviable gestational ages. In the U.S., the American Academy of Pediatrics statement on antenatal counselling recommends that decision-making in the delivery room be individualized and family-centered for births at 22 to 24 weeks’ of gestation, taking into account known fetal and maternal conditions and parental beliefs.46 However, individual attitudes and biases of providers may impact shared-decision making with families about whether to initiate or withhold active treatment. In a UK study that evaluated attitudes of 25 neonatologists towards resuscitation and care of extremely preterm infants, providers were grouped as having one of three types of attitudes towards decision making: 1) treatments should not be limited based on gestational age and technology should be used to help improve treatment of suffering and disability; 2) treatment should be provided based primarily on gestational age; 3) treatment should be withheld or withdrawn based on quality of life principles to prevent disability.47 Understanding these attitudes and biases, and minimizing them when discussing outcome data regarding survival with parents are important aspects of counseling about the outcomes of periviable birth.48
Biases affect decisions about care, and they may also affect the presentation of information for family counseling. One example of the latter is framing bias, which involves only discussing the probability of survival or the probability of mortality instead of presenting both potential outcomes.49 Another examples is mis-estimation of the probability of adverse outcomes. This was highlighted in a study in Victoria, Australia, where obstetric and neonatal provider estimates of survival and disability were compared to actual outcome rates within the same region and found to overestimate poor outcomes.50 This was consistent with findings from a prior US study.51 The use of model-based outcome estimates based on systematically collected data from multiple centers and incorporating several maternal and infant factors—including whether infants received active treatment—may be useful in decreasing these types of biases, as we discuss below.52
Effect of obstetrical and neonatal active treatment
Active treatment of fetuses and liveborn infants is one of the most important determinants of early survival for periviable births. In a multicenter study of 24 academically-affiliated hospitals in the US, the proportion of live births receiving active treatment at 22 and 23 weeks’ of gestation ranged from 0 to 100% and 25 to 100%, respectively, among individual hospitals.37 The variation in hospital rate of active treatment accounted for 78% of the observed differences in hospital survival rates for infants born at 22 or 23 weeks’ of gestation. In contrast, only 22% and 1% of the differences in survival rates at 24 and 25 weeks’ of gestation, respectively, could be explained by variation in the hospital rate of active treatment. At some of the hospitals, most infants born at 22 weeks’ of gestation received active treatment whereas at others no infants born at 22 weeks’ did, a difference that may be reflective of institutional policies, clinician attitudes, or parental preferences.
In a national study in Sweden, regional rates of obstetric and neonatal active treatment were used to estimate the effects on survival among fetuses alive at the mother’s admission.53 The proportion of key obstetric interventions (birth at a level 3 unit, antenatal corticosteroids, cesarean delivery, and tocolysis) and neonatal interventions (surfactant administration, neonatologist present for delivery, intubation after birth, and NICU referral) were used to estimate the intensity of perinatal care and associate this intensity with the risk of stillbirth, death within 12 hours of birth, and death before 1 yr of age. The range of activity scores, reflecting the intensity of active treatment, were 72 to 100. For each 5 point increase in activity score, the risk of stillbirth (adjusted OR 0.90; 95% CI 0.83–0.97), death within 12 hours (0.75; 0.68–0.86) and death before 1 year (0.83; 0.75–0.91) all decreased significantly after accounting for several other characteristics known to affect fetal and infant outcomes. Regions of Sweden providing a high-intensity of active treatment at 22 to 24 weeks’ of gestation (activity score 96–100) had a mortality rate of 35%, compared to a mortality rate of 59% among regions providing a low intensity of active treatment (activity score 74–80).
Importantly, active treatment occurs both before and after birth. In another study of live births before 27 weeks’ of gestation in Sweden, the effect of individual components of active obstetric treatment on death within 24 hours after live birth were estimated.54 The risk of death decreased with each additional week of gestation (adjusted OR 0.3; 95% CI 0.2–0.4) after adjusting for differences in pregnancy and delivery characteristics; this decrease in risk of early mortality was similar to that seen with two components of obstetric active treatment: administration of antenatal corticosteroids (adjusted OR 0.3; 95% CI 0.1–0.6) and cesarean delivery (adjusted OR 0.4; 95% CI 0.2–0.9). In addition, observational studies have consistently demonstrated a lower risk of death among neonates born <24 weeks’ of gestation exposed to antenatal corticosteroids.55 In a population based study of infants at 22 to 26 weeks’ of gestation in France, 96% of those infants who had neonatal intensive care withheld or withdrawn died in the delivery room compared to 1% of those who received intensive treatment, including oxygen therapy and endotracheal intubation.56 At 22 weeks’ and 23 weeks’ of gestation, 96% and 91% of live births, respectively, had intensive care withheld or withdrawn and the limitation in active treatment mirrored the 96% and 92% of live births at these gestational ages that died in the delivery room. In the previously discussed study evaluating the effects of active treatment in a cohort of academically-affiliated hospitals in the US, overall survival rates at 22 weeks’ of gestation increased four-fold when restricting the denominator of analysis from all live births to those receiving active treatment, from 5% (95% CI 3–8) to 23% (95% CI 14–34), highlighting the importance of this factor to the survival estimate.37 All infants born alive at 22 to 25 weeks’ of gestation who did not receive active treatment died before hospital discharge, with nearly all (97–100% depending on gestational age at birth) dying within 12 hours of birth and all dying within 24 hours of birth. In contrast, among those that received active treatment, 41% of infants born alive at 22 weeks’ of gestation, and 20% of infants born alive at 23 weeks’ of gestation died within 12 hours of birth, with a much smaller proportion (2–8%) dying within the first 12 hours at 24 and 26 weeks’ of gestation.37 Therefore, recent recommendations emphasize the importance of stratifying outcomes by those infants that do and do not receive active treatment.29
A clearer understanding of decisions surrounding active treatment for periviable infants may facilitate better parental counseling and decision-making. Large, systematic cohort studies that measure key characteristics and outcomes of periviable infants receiving intensive care versus those receiving comfort care may be used to estimate the benefits of and burdens of intensive care. One such analysis is described below.52 A strength of this analysis is the avoidance of a “self-fulfilling prophecy” for infants not given intensive care by estimating the proportion of additional infants who would have survived had such care been provided (the “maximum potential survival rate”). This method assumes that infants who died without receiving intensive care would have the same potential survival rate as infants given intensive care had they received it, which may be an overestimate. Therefore, the true survival rate had all periviable infants been given intensive care will likely be between the observed mortality rate and the maximum estimated survival rate.
Antenatal factors
Beyond the gestational age at birth and provision of active treatment, which are often correlated, a number of other factors influence the likelihood of survival. Important antenatal factors that influence the prognosis of survival include estimated fetal weight, sex, plurality, and receipt of antenatal corticosteroids, with higher estimated fetal weight, female sex, singleton gestation and receipt of antenatal corticosteroids associated with higher probability of survival.52 As mentioned above, these factors can be used in antenatal counseling to predict the probability of survival if intensive care is provided (available at https://www.nichd.nih.gov/about/org/der/branches/ppb/programs/epbo/Pages/index.aspx). Importantly, although the NICHD NRN extremely preterm birth outcome estimator has been externally validated across large populations including California, in the U.S., and Victoria, in Australia,57–60 individual centers may have better or worse outcomes.61 Additionally, after birth, other factors, such as a the level of respiratory support and other clinical variables discussed below, increase in prognostic value for prediction of survival, while the prognostic value of baseline characteristics, such as gestational age, decrease.62
Delivery room factors
In the delivery room, the Apgar score, traditionally associated with neonatal survival,63 may be influenced by the provision or withholding of active treatment. The receipt of chest compressions or epinephrine in the delivery room is associated with a higher risk of mortality,64 although this effect may vary by gestational age.65 The visual assessment of extremely preterm neonates <26 weeks’ of gestation in the first seconds to minutes after birth by neonatal providers is a poor predictor of survival66 and should not be used as a reliable prognostic characteristic to determine whether to provide or withhold resuscitative efforts.
Factors beyond the delivery room
On the day of birth, variables such as receipt of surfactant and mechanical ventilation, outborn versus. inborn status, and illness severity, which may be respresented by the Score for Neonatal Acute Physiology version 2,67 in addition to baseline factors including gestational age, small for gestational age, gender, and exposure to antenatal corticosteroids are important factors that can predict survival with and without morbidity of extremely preterm infants.21 Beyond the first week of life through 28 days of age, the type of respiratory support (high-frequency ventilation, conventional ventilation, continuous positive airway pressure, nasal cannula or none) and the highest fraction of inspired oxygen that an infant receives become the most significant predictors of survival, while the prognostic value of birth weight and gestational age decreases.62,68 At 28 days of age, the highest fraction of inspired oxygen, the number of episodes of late-onset culture-negative infection, days of parenteral feeding and days of CPAP are all independent negative predictors of survival to hospital discharge, with the highest fraction of inspired oxygen carrying the most weight.62
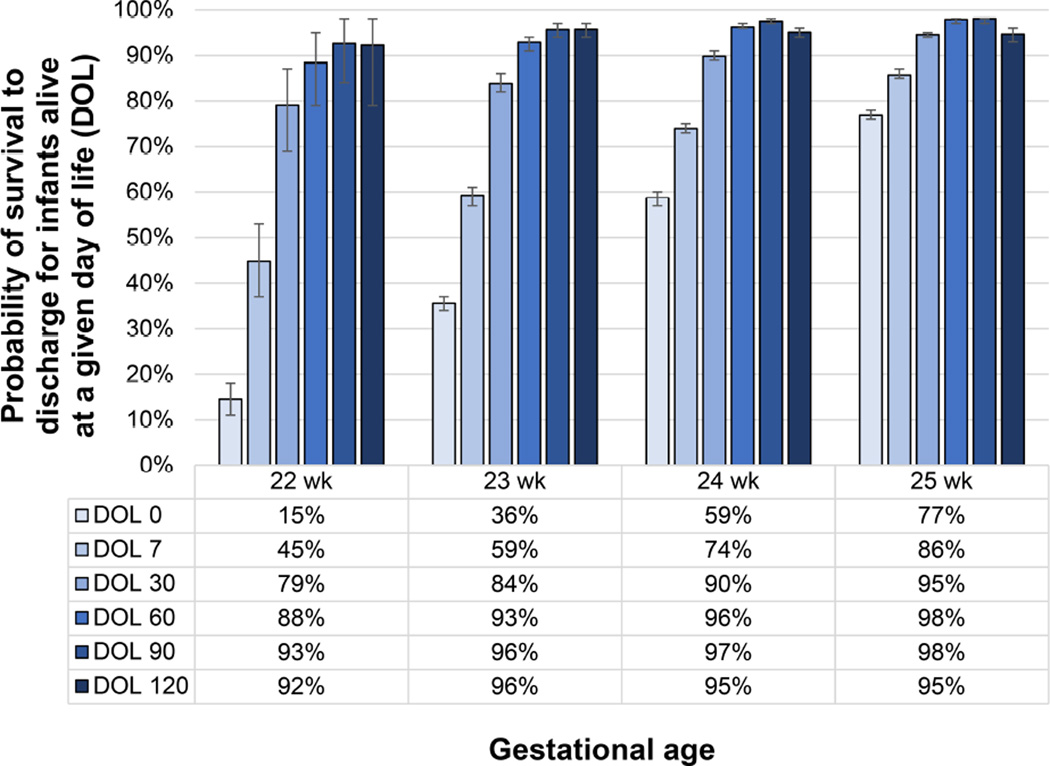
The timing of death varies by gestation age and, as an infant survives beyond the first few days of life, the probability of survival increases substantially69 and continues to increase thereafter until 90 postnatal days (Figure 5).20 For infants born at 22 weeks’ of gestation between 2000 and 2011, 90% of all in-hospital deaths occurred within 12 hours of life and 1.4% occurred after 28 days of age, based on data from a multicenter study from the NICHD NRN.12 The high frequency of early mortality likely reflects the frequent approach of comfort care for these infants. The proportion of deaths within 12 hours of birth among those infants with in-hospital death continues to decrease with each additional week of gestation, from 56% at 23 weeks’ to 26% at 24 weeks’ and 18% at 25 weeks’ of gestation. Similarly, the proportion of deaths occurring after 28 days of age increases, from 8% at 23 weeks’ to 21% at 24 weeks’ and 27% at 25 weeks’. Important aspects of care during this period include fluid administration, nutrition, cardiorespiratory support and prevention of intraventricular hemorrhage (IVH),70 although randomized trials that include periviable infants are needed to identify additional interventions to improve outcomes in this population.
Figure 5. Changes in Probability of Survival to Discharge Among Infants Alive at a Given Day of Life.
Figure denotes changes in probability of survival to hospital discharge for infants who survive to 7 days of life (DOL) and beyond from a cohort of 64,896 infants in 362 US NICUs. Whisker bars indicate 95% confidence intervals calculated with the Clopper-Pearson method
Data from Hornik CP, Sherwood AL, Cotten CM, Laughon MM, Clark RH, Smith PB. Daily mortality of infants born at less than 30weeks' gestation. Early Hum Dev. 2016;96:27–30.
Racial and social associations with survival
Among women in high-income countries, an adverse socioeconomic background is associated with twice the risk of stillbirth compared to women who are not from a disadvantaged background. Access to neonatal intensive care is one important mediator of the racial and social disparity in birth outcomes and is influenced by race, insurance status and whether a woman received early prenatal care.71 In addition, black race has been consistently linked with a higher overall risk of adverse birth outcomes,72,73 although not gestational age-specific mortality at <32 weeks’ of gestation,74 and is a risk-factor for death after discharge from the NICU among ELBW infants.75 However, one study, from the NICHD NRN, found that rehospitalization of ELBW infants was not associated with race/ethnicity after controlling for low family income, type of insurance, center, and other potential confounding variables.76
Withdrawal of life-sustaining treatment
Approaches to withdrawal of life sustaining treatment, which result in the majority of deaths after infants are admitted to the NICU,77,78 can explain some of the variation in reported rates of survival. Variation in the percentage of deaths following end-of-life decisions among European population-based cohorts varied significantly, with 81% of deaths following end-of-life decisions at ≤24 weeks’ of gestation in France compared to 55% at ≤25 weeks’ of gestation in the UK.79 In a prospective observational study of 19 NICUs in Canada, 84% of all deaths occurred following a discussion of withdrawal of life-sustaining support and 41% were due to a prognosis of poor quality of life in the event of survival, while 35% were due to inevitability of death in the short-term and 24% to prevent prolonged suffering with death likely.80 Neurological complications of prematurity, particularly the presence of severe IVH (grade 3 or 4) or periventricular leukomalacia were common indications for withdrawal of life-sustaining treatment, although the proportion of infants with neurological injury who underwent discussions varied from 10% to 86% across centers and 65% of the withdrawal of care was due to concerns regarding quality of life if the infant survived. The prognostic value accorded to indicators such as evidence for neurological injury on an early cranial ultrasound may differ in clinical practice, as many infants who survive with either severe IVH or cystic periventricular leukomalacia on early cranial ultrasound are found to have no or mild impairment (51%) compared to those with a composite measure of neurodevelopmental impairment (28%).81
Conclusion
In conclusion, survival among periviable births has improved since the 1950s, including over the past decade. There is wide variation in survival of periviable live births across developed countries and across different NICUs in the same country, although estimates of gestational age-specific survival are influenced by a number of factors that limit unbiased comparisons. Provision of active treatment, particularly at 22 and 23 weeks’ of gestation, varies widely among hospitals and developed nations, and this has a significant impact on reported survival rates. However, many other factors affect outcomes for infants born at periviable gestations. Improved reporting of outcomes following periviable birth may yield better understanding of the effects of obstetric and neonatal care in this area.
Key Points.
Estimates of gestational age-specific survival vary significantly across hospitals, regions and countries and are influenced by a number of factors that can make unbiased comparisons challenging.
Survival among live periviable births at 22 to 25 weeks of gestation has incrementally improved since the 1950s, with continued gains over the last decade.
Provision of active treatment, particularly at 22 and 23 weeks of gestation, varies widely among centers and countries, and this variation has a substantial impact on reported survival rates.
Improved reporting of survival rates for periviable births may yield a better understanding of birth outcomes for periviable births occurring at 20 to 25 weeks of gestation.
Acknowledgments
The review was supported, in part, by the National Institutes of Health under award K23 HL128942 (R.M.P.). The authors would like to acknowledge the Neonatal Research Network, including Rosemary Higgins, M.D., Abhik Das, Ph.D. and the GDB subcommittee, for graciously providing recent data on periviable survival.
Abbreviations
- CI
confidence interval
- ELBW
extremely low birth weight
- NICHD
National Institute of Child Health and Human Development
- NICU
neonatal intensive care unit
- NRN
Neonatal Research Network
- OR
odds ratio
- VLBW
very low birth weight
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors have no conflicts of interest to report.
References
- 1.Raju TN, Mercer BM, Burchfield DJ, Joseph GF., Jr Periviable birth: executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Academy of Pediatrics, and American College of Obstetricians and Gynecologists. Obstet Gynecol. 2014;123(5):1083–1096. doi: 10.1097/AOG.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 2.American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine et al. Periviable birth: Interim update. Am J Obstet Gynecol. 2016;215(2):B2–B12. e11. doi: 10.1016/j.ajog.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 3.Stewart AL, Reynolds EO, Lipscomb AP. Outcome for infants of very low birthweight: survey of world literature. Lancet. 1981;1(8228):1038–1040. doi: 10.1016/s0140-6736(81)92198-x. [DOI] [PubMed] [Google Scholar]
- 4.Rawlings G, Stewart A, Reynolds EO, Strang LB. Changing prognosis for infants of very low birth weight. Lancet. 1971;1(7698):516–519. doi: 10.1016/s0140-6736(71)91124-x. [DOI] [PubMed] [Google Scholar]
- 5.Bhat R, Raju TN, Vidyasagar D. Immediate and long-term outcome of infants less than 1000 grams. Crit Care Med. 1978;6(3):147–150. doi: 10.1097/00003246-197805000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Paneth N, Kiely JL, Wallenstein S, Marcus M, Pakter J, Susser M. Newborn intensive care and neonatal mortality in low-birth-weight infants: a population study. N Engl J Med. 1982;307(3):149–155. doi: 10.1056/NEJM198207153070303. [DOI] [PubMed] [Google Scholar]
- 7.Gordon RR. Neonatal and "perinatal" mortality rates by birth weight. Br Med J. 1977;2(6096):1202–1204. doi: 10.1136/bmj.2.6096.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hack M, Wright LL, Shankaran S, et al. Very-low-birth-weight outcomes of the National Institute of Child Health and Human Development Neonatal Network, November 1989 to October 1990. Am J Obstet Gynecol. 1995;172(2 Pt 1):457–464. doi: 10.1016/0002-9378(95)90557-x. [DOI] [PubMed] [Google Scholar]
- 9.Costeloe K, Hennessy E, Gibson AT, Marlow N, Wilkinson AR. The EPICure study: outcomes to discharge from hospital for infants born at the threshold of viability. Pediatrics. 2000;106(4):659–671. doi: 10.1542/peds.106.4.659. [DOI] [PubMed] [Google Scholar]
- 10.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. 2015;314(10):1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel RM, Kandefer S, Walsh MC, et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. 2015;372(4):331–340. doi: 10.1056/NEJMoa1403489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horbar JD, Carpenter JH, Badger GJ, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics. 2012;129(6):1019–1026. doi: 10.1542/peds.2011-3028. [DOI] [PubMed] [Google Scholar]
- 14.Costeloe KL, Hennessy EM, Haider S, Stacey F, Marlow N, Draper ES. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies) BMJ. 2012;345:e7976. doi: 10.1136/bmj.e7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ancel PY, Goffinet F, Group E-W, et al. Survival and morbidity of preterm children born at 22 through 34 weeks' gestation in France in 2011: results of the EPIPAGE-2 cohort study. JAMA Pediatr. 2015;169(3):230–238. doi: 10.1001/jamapediatrics.2014.3351. [DOI] [PubMed] [Google Scholar]
- 16.Shah PS, Sankaran K, Aziz K, et al. Outcomes of preterm infants <29 weeks gestation over 10-year period in Canada: a cause for concern? J Perinatol. 2012;32(2):132–138. doi: 10.1038/jp.2011.68. [DOI] [PubMed] [Google Scholar]
- 17.Kusuda S, Fujimura M, Uchiyama A, Totsu S, Matsunami K Japan Neonatal Research Network. Trends in morbidity and mortality among very-low-birth-weight infants from 2003 to 2008 in Japan. Pediatr Res. 2012;72(5):531–538. doi: 10.1038/pr.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishii N, Kono Y, Yonemoto N, Kusuda S, Fujimura M Japan Neonatal Research Network. Outcomes of infants born at 22 and 23 weeks' gestation. Pediatrics. 2013;132(1):62–71. doi: 10.1542/peds.2012-2857. [DOI] [PubMed] [Google Scholar]
- 19.Su BH, Hsieh WS, Hsu CH, et al. Neonatal outcomes of extremely preterm infants from taiwan: comparison with Canada, Japan, and the USA. Pediatr Neonatol. 2015;56(1):46–52. doi: 10.1016/j.pedneo.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Hornik CP, Sherwood AL, Cotten CM, Laughon MM, Clark RH, Smith PB. Daily mortality of infants born at less than 30weeks' gestation. Early Hum Dev. 2016;96:27–30. doi: 10.1016/j.earlhumdev.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge WJ, Mirea L, Yang J, et al. Prediction of neonatal outcomes in extremely preterm neonates. Pediatrics. 2013;132(4):e876–e885. doi: 10.1542/peds.2013-0702. [DOI] [PubMed] [Google Scholar]
- 22.Guinsburg R, Branco de Almeida MF, Dos Santos Rodrigues Sadeck L, et al. Proactive management of extreme prematurity: disagreement between obstetricians and neonatologists. J Perinatol. 2012;32(12):913–919. doi: 10.1038/jp.2012.28. [DOI] [PubMed] [Google Scholar]
- 23.Serenius F, Kallen K, Blennow M, et al. Neurodevelopmental outcome in extremely preterm infants at 2.5 years after active perinatal care in Sweden. JAMA. 2013;309(17):1810–1820. doi: 10.1001/jama.2013.3786. [DOI] [PubMed] [Google Scholar]
- 24.Chen F, Bajwa NM, Rimensberger PC, Posfay-Barbe KM, Pfister RE Swiss Neonatal Network. Thirteen-year mortality and morbidity in preterm infants in Switzerland. Arch Dis Child Fetal Neonatal Ed. 2016;101(5):F377–F383. doi: 10.1136/archdischild-2015-308579. [DOI] [PubMed] [Google Scholar]
- 25.Zegers MJ, Hukkelhoven CW, Uiterwaal CS, Kollee LA, Groenendaal F. Changing Dutch approach and trends in short-term outcome of periviable preterms. Arch Dis Child Fetal Neonatal Ed. 2016;101(5):F391–F396. doi: 10.1136/archdischild-2015-308803. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal P, Sriram B, Rajadurai VS. Neonatal outcome of extremely preterm Asian infants 28 weeks over a decade in the new millennium. J Perinatol. 2015;35(4):297–303. doi: 10.1038/jp.2014.205. [DOI] [PubMed] [Google Scholar]
- 27.Boland RA, Davis PG, Dawson JA, Doyle LW. Outcomes of infants born at 22–27 weeks' gestation in Victoria according to outborn/inborn birth status. Arch Dis Child Fetal Neonatal Ed. 2016 doi: 10.1136/archdischild-2015-310313. [DOI] [PubMed] [Google Scholar]
- 28.Guillen U, DeMauro S, Ma L, et al. Survival rates in extremely low birthweight infants depend on the denominator: avoiding potential for bias by specifying denominators. Am J Obstet Gynecol. 2011;205(4):329, e321–e327. doi: 10.1016/j.ajog.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 29.Rysavy MA, Marlow N, Doyle LW, et al. Reporting outcomes of extremely preterm births. Pediatrics. 2016;138(3) doi: 10.1542/peds.2016-0689. [DOI] [PubMed] [Google Scholar]
- 30.Guillen U, Weiss EM, Munson D, et al. Guidelines for the management of extremely premature deliveries: a systematic review. Pediatrics. 2015;136(2):343–350. doi: 10.1542/peds.2015-0542. [DOI] [PubMed] [Google Scholar]
- 31.Hernan MA, Clayton D, Keiding N. The Simpson's paradox unraveled. Int J Epidemiol. 2011;40(3):780–785. doi: 10.1093/ije/dyr041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marang-van de Mheen PJ, Shojania KG. Simpson's paradox: how performance measurement can fail even with perfect risk adjustment. BMJ Qual Saf. 2014;23(9):701–705. doi: 10.1136/bmjqs-2014-003358. [DOI] [PubMed] [Google Scholar]
- 33.Manktelow BN, Evans TA, Draper ES. Differences in case-mix can influence the comparison of standardised mortality ratios even with optimal risk adjustment: an analysis of data from paediatric intensive care. BMJ Qual Saf. 2014;23(9):782–788. doi: 10.1136/bmjqs-2013-002608. [DOI] [PubMed] [Google Scholar]
- 34.Patel RM, Rysavy MA. Global variation in neonatal intensive care: does it matter? J Pediatr. 2016;177:6–7. doi: 10.1016/j.jpeds.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Janvier A, Lantos J. Delivery room practices for extremely preterm infants: the harms of the gestational age label. Arch Dis Child Fetal Neonatal Ed. 2016;101(5):F375–F376. doi: 10.1136/archdischild-2016-310466. [DOI] [PubMed] [Google Scholar]
- 36.Horbar JD, Badger GJ, Lewit EM, Rogowski J, Shiono PH. Hospital and patient characteristics associated with variation in 28-day mortality rates for very low birth weight infants. Vermont Oxford Network. Pediatrics. 1997;99(2):149–156. doi: 10.1542/peds.99.2.149. [DOI] [PubMed] [Google Scholar]
- 37.Rysavy MA, Li L, Bell EF, et al. Between-hospital variation in treatment and outcomes in extremely preterm infants. N Engl J Med. 2015;372(19):1801–1811. doi: 10.1056/NEJMoa1410689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith L, Draper ES, Manktelow BN, Pritchard C, Field DJ. Comparing regional infant death rates: the influence of preterm births <24 weeks of gestation. Arch Dis Child Fetal Neonatal Ed. 2013;98(2):F103–F107. doi: 10.1136/fetalneonatal-2011-301359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah PS, Lui K, Sjors G, et al. Neonatal outcomes of very low birth weight and very preterm neonates: an international comparison. J Pediatr. 2016;177:144–152. e146. doi: 10.1016/j.jpeds.2016.04.083. [DOI] [PubMed] [Google Scholar]
- 40.Lapcharoensap W, Gage SC, Kan P, et al. Hospital variation and risk factors for bronchopulmonary dysplasia in a population-based cohort. JAMA Pediatr. 2015;169(2):e143676. doi: 10.1001/jamapediatrics.2014.3676. [DOI] [PubMed] [Google Scholar]
- 41.Jensen EA, Lorch SA. Effects of a birth hospital's neonatal intensive care unit level and annual volume of very low-birth-weight infant deliveries on morbidity and mortality. JAMA Pediatr. 2015;169(8):e151906. doi: 10.1001/jamapediatrics.2015.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Profit J, Gould JB, Bennett M, et al. The association of level of care with NICU quality. Pediatrics. 2016;137(3):e20144210. doi: 10.1542/peds.2014-4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodman DC, Fisher ES, Little GA, Stukel TA, Chang CH, Schoendorf KS. The relation between the availability of neonatal intensive care and neonatal mortality. N Engl J Med. 2002;346(20):1538–1544. doi: 10.1056/NEJMoa011921. [DOI] [PubMed] [Google Scholar]
- 44.Holmstrom ST, Phibbs CS. Regionalization and mortality in neonatal intensive care. Pediatr Clin North Am. 2009;56(3):617–630. doi: 10.1016/j.pcl.2009.04.006. Table of Contents. [DOI] [PubMed] [Google Scholar]
- 45.Lake ET, Staiger D, Horbar J, et al. Association between hospital recognition for nursing excellence and outcomes of very low-birth-weight infants. JAMA. 2012;307(16):1709–1716. doi: 10.1001/jama.2012.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cummings J Committee On F, Newborn. Antenatal counseling regarding resuscitation and intensive care before 25 weeks of gestation. Pediatrics. 2015;136(3):588–595. doi: 10.1542/peds.2015-2336. [DOI] [PubMed] [Google Scholar]
- 47.Gallagher K, Aladangady N, Marlow N. The attitudes of neonatologists towards extremely preterm infants: a Q methodological study. Arch Dis Child Fetal Neonatal Ed. 2016;101(1):F31–F36. doi: 10.1136/archdischild-2014-308071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim UO, Basir MA. Informing and educating parents about the risks and outcomes of prematurity. Clin Perinatol. 2014;41(4):979–991. doi: 10.1016/j.clp.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 49.Haward MF, Murphy RO, Lorenz JM. Message framing and perinatal decisions. Pediatrics. 2008;122(1):109–118. doi: 10.1542/peds.2007-0620. [DOI] [PubMed] [Google Scholar]
- 50.Boland RA, Davis PG, Dawson JA, Doyle LW. What are we telling the parents of extremely preterm babies? Aust N Z J Obstet Gynaecol. 2016;56(3):274–281. doi: 10.1111/ajo.12448. [DOI] [PubMed] [Google Scholar]
- 51.Morse SB, Haywood JL, Goldenberg RL, Bronstein J, Nelson KG, Carlo WA. Estimation of neonatal outcome and perinatal therapy use. Pediatrics. 2000;105(5):1046–1050. doi: 10.1542/peds.105.5.1046. [DOI] [PubMed] [Google Scholar]
- 52.Tyson JE, Parikh NA, Langer J, et al. Intensive care for extreme prematurity--moving beyond gestational age. N Engl J Med. 2008;358(16):1672–1681. doi: 10.1056/NEJMoa073059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Serenius F, Blennow M, Marsal K, Sjors G, Kallen K, Group ES. Intensity of perinatal care for extremely preterm infants: outcomes at 2.5 years. Pediatrics. 2015;135(5):e1163–e1172. doi: 10.1542/peds.2014-2988. [DOI] [PubMed] [Google Scholar]
- 54.Kallen K, Serenius F, Westgren M, Marsal K, Group E. Impact of obstetric factors on outcome of extremely preterm births in Sweden: prospective population-based observational study (EXPRESS) Acta Obstet Gynecol Scand. 2015;94(11):1203–1214. doi: 10.1111/aogs.12726. [DOI] [PubMed] [Google Scholar]
- 55.Park CK, Isayama T, McDonald SD. Antenatal corticosteroid therapy before 24 weeks of gestation: a systematic review and meta-analysis. Obstet Gynecol. 2016;127(4):715–725. doi: 10.1097/AOG.0000000000001355. [DOI] [PubMed] [Google Scholar]
- 56.Perlbarg J, Ancel PY, Khoshnood B, et al. Delivery room management of extremely preterm infants: the EPIPAGE-2 study. Arch Dis Child Fetal Neonatal Ed. 2016;101(5):F384–F390. doi: 10.1136/archdischild-2015-308728. [DOI] [PubMed] [Google Scholar]
- 57.Lee HC, Green C, Hintz SR, et al. Prediction of death for extremely premature infants in a population-based cohort. Pediatrics. 2010;126(3):e644–e650. doi: 10.1542/peds.2010-0097. [DOI] [PubMed] [Google Scholar]
- 58.Marrs CC, Pedroza C, Mendez-Figueroa H, Chauhan SP, Tyson JE. Infant outcomes after periviable birth: external validation of the Neonatal Research Network estimator with the BEAM trial. Am J Perinatol. 2016;33(6):569–576. doi: 10.1055/s-0035-1569989. [DOI] [PubMed] [Google Scholar]
- 59.Boland RA, Davis PG, Dawson JA, Doyle LW Victorian Infant Collaborative Study Group. Predicting death or major neurodevelopmental disability in extremely preterm infants born in Australia. Arch Dis Child Fetal Neonatal Ed. 2013;98(3):F201–F204. doi: 10.1136/archdischild-2012-301628. [DOI] [PubMed] [Google Scholar]
- 60.Ethridge JK, Jr, Louis JM, Mercer BM. Accuracy of fetal weight estimation by ultrasound in periviable deliveries. J Matern Fetal Neonatal Med. 2014;27(6):557–560. doi: 10.3109/14767058.2013.834324. [DOI] [PubMed] [Google Scholar]
- 61.Alleman BW, Bell EF, Li L, et al. Individual and center-level factors affecting mortality among extremely low birth weight infants. Pediatrics. 2013;132(1):e175–e184. doi: 10.1542/peds.2012-3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ambalavanan N, Carlo WA, Tyson JE, et al. Outcome trajectories in extremely preterm infants. Pediatrics. 2012;130(1):e115–e125. doi: 10.1542/peds.2011-3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Casey BM, McIntire DD, Leveno KJ. The continuing value of the Apgar score for the assessment of newborn infants. N Engl J Med. 2001;344(7):467–471. doi: 10.1056/NEJM200102153440701. [DOI] [PubMed] [Google Scholar]
- 64.Wyckoff MH, Salhab WA, Heyne RJ, et al. Outcome of extremely low birth weight infants who received delivery room cardiopulmonary resuscitation. J Pediatr. 2012;160(2):239–244. e232. doi: 10.1016/j.jpeds.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Handley SC, Sun Y, Wyckoff MH, Lee HC. Outcomes of extremely preterm infants after delivery room cardiopulmonary resuscitation in a population-based cohort. J Perinatol. 2015;35(5):379–383. doi: 10.1038/jp.2014.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manley BJ, Dawson JA, Kamlin CO, Donath SM, Morley CJ, Davis PG. Clinical assessment of extremely premature infants in the delivery room is a poor predictor of survival. Pediatrics. 2010;125(3):e559–e564. doi: 10.1542/peds.2009-1307. [DOI] [PubMed] [Google Scholar]
- 67.Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138(1):92–100. doi: 10.1067/mpd.2001.109608. [DOI] [PubMed] [Google Scholar]
- 68.Laughon MM, Langer JC, Bose CL, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. 2011;183(12):1715–1722. doi: 10.1164/rccm.201101-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abdel-Latif ME, Kecskes Z, Bajuk B Nsw, the NSW and the ACT Neonatal Intensive Care Audit Group. Actuarial day-by-day survival rates of preterm infants admitted to neonatal intensive care in New South Wales and the Australian Capital Territory. Arch Dis Child Fetal Neonatal Ed. 2013;98(3):F212–F217. doi: 10.1136/adc.2011.210856. [DOI] [PubMed] [Google Scholar]
- 70.Barrington KJ. Management during the first 72 h of age of the periviable infant: an evidence-based review. Semin Perinatol. 2014;38(1):17–24. doi: 10.1053/j.semperi.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 71.Bronstein JM, Capilouto E, Carlo WA, Haywood JL, Goldenberg RL. Access to neonatal intensive care for low-birthweight infants: the role of maternal characteristics. Am J Public Health. 1995;85(3):357–361. doi: 10.2105/ajph.85.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.DeFranco EA, Hall ES, Muglia LJ. Racial disparity in previable birth. Am J Obstet Gynecol. 2016;214(3):394, e391–e397. doi: 10.1016/j.ajog.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 73.Schempf AH, Branum AM, Lukacs SL, Schoendorf KC. The contribution of preterm birth to the Black-White infant mortality gap, 1990 and 2000. Am J Public Health. 2007;97(7):1255–1260. doi: 10.2105/AJPH.2006.093708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.MacDorman MF, Mathews TJ. Understanding racial and ethnic disparities in U.S. infant mortality rates. NCHS Data Brief. 2011;74:1–8. [PubMed] [Google Scholar]
- 75.De Jesus LC, Pappas A, Shankaran S, et al. Risk factors for post-neonatal intensive care unit discharge mortality among extremely low birth weight infants. J Pediatr. 2012;161(1):70–74. e71–e72. doi: 10.1016/j.jpeds.2011.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morris BH, Gard CC, Kennedy K, Network NNR. Rehospitalization of extremely low birth weight (ELBW) infants: are there racial/ethnic disparities? J Perinatol. 2005;25(10):656–663. doi: 10.1038/sj.jp.7211361. [DOI] [PubMed] [Google Scholar]
- 77.Verhagen AA, Janvier A, Leuthner SR, et al. Categorizing neonatal deaths: a cross-cultural study in the United States, Canada, and The Netherlands. J Pediatr. 2010;156(1):33–37. doi: 10.1016/j.jpeds.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 78.Weiner J, Sharma J, Lantos J, Kilbride H. How infants die in the neonatal intensive care unit: trends from 1999 through 2008. Arch Pediatr Adolesc Med. 2011;165(7):630–634. doi: 10.1001/archpediatrics.2011.102. [DOI] [PubMed] [Google Scholar]
- 79.Pignotti MS, Berni R. Extremely preterm births: end-of-life decisions in European countries. Arch Dis Child Fetal Neonatal Ed. 2010;95(4):F273–F276. doi: 10.1136/adc.2009.168294. [DOI] [PubMed] [Google Scholar]
- 80.Hellmann J, Knighton R, Lee SK, Shah PS Canadian Neonatal Network End of Life Study Group. Neonatal deaths: prospective exploration of the causes and process of end-of-life decisions. Arch Dis Child Fetal Neonatal Ed. 2016;101(2):F102–F107. doi: 10.1136/archdischild-2015-308425. [DOI] [PubMed] [Google Scholar]
- 81.Hintz SR, Barnes PD, Bulas D, et al. Neuroimaging and neurodevelopmental outcome in extremely preterm infants. Pediatrics. 2015;135(1):e32–e42. doi: 10.1542/peds.2014-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moore T, Hennessy EM, Myles J, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ. 2012;345:e7961. doi: 10.1136/bmj.e7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.EXPRESS Group. Fellman V, Hellstrom-Westas L, et al. One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA. 2009;301(21):2225–2233. doi: 10.1001/jama.2009.771. [DOI] [PubMed] [Google Scholar]