ABSTRACT
The molecular and cellular basis of adult neurogenesis has attracted considerable attention for fundamental and clinical applications because neural stem cells and newborn neurons may, one day, be harnessed to replace neurons and allow cognitive improvement in the diseased brain. In rodents, neural progenitors are located in the dentate gyrus and the sub/periventricular zone. In the dentate gyrus the generation of newborn neurons is associated with plasticity, including regulation of memory. The role of subventricular zone neural precursors that migrate to the olfactory bulb is less characterized. Identifying factors that impact neural stem cell proliferation, migration and differentiation is therefore sine qua non before we can harness their potential. Here, we expand upon our recent results showing that CAR, the coxsackievirus and adenovirus receptor, is among the developing list of key players when it comes to the complex process of integrating newborn neurons into existing circuits in the mature brain.
KEYWORDS: adhesion molecule, adult neuroprecusors, Coxsackievirus and Adenovirus Receptor, neuroinflammation
In mammals, neurogenesis continues in the adult brain in the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG), and in the subventricular zone (SVZ).1 Numerous neurological and psychiatric disorders, including Huntington's disease, anxiety, depression, post-stress traumatic disorders and Alzheimer's disease (AD), display deregulated adult neurogenesis that could be responsible in part for perturbed brain functions. Cell-cell and cell-extracellular matrix interactions mediated by cell adhesion molecules (CAMs) play crucial roles in adult neurogenesis, whether during migration, differentiation, or integration into existing networks.2 While the distance that neural precursor cells (NPCs) migrate in the SGZ is modest, SVZ-born cells travel comparably long distances via the rostral migratory stream (RMS) to the olfactory bulb (OB) in rodents. Among the numerous CAMs expressed in the brain, the latest on the neurogenic track is CAR (coxsackievirus and adenovirus receptor), a single-pass transmembrane protein belonging to the Ig superfamily.3 Its functions are best described in epithelia where homodimeric interactions in trans help regulate and maintain tight junction homeostasis.4,5 There are a few membrane-bound and soluble CAR isoforms created by alternative splicing, but the predominant isoform in the brain is the ∼46 kDa membrane bound protein. CAR's extracellular domain contains 2 Ig-like domains (D1 and D2) followed by a single-pass trans-membrane domain. The ∼100 aa intracellular domain contains a class 1 PDZ domain located at the extremity of the C terminus, a peptide recognition domain for the clathrin adaptor protein AP2 and a sorting motif.6
CAR—a driver in adult neuronal plasticity
Our recent study supports the conclusion that CAR is expressed exclusively by NPCs and neurons (i.e. not by microglia, macroglia or oligodendrocytes) in the adult mammalian brain parenchyma.7 In the mouse brain, CAR staining is notable in the posterior corpus callosum, layers IV and V of the cerebral cortex, as well as cortex layer I, which contains primarily axons from other cortical areas and apical dendrites of local neurons. In the hippocampus, CAR staining is striking on the axons projecting from the entorhinal cortex, as well as in the stratum lucidum on the mossy fiber axons of newly generated DG neurons. Despite strong axon labeling, almost all cell bodies are devoid of CAR. The exception is in a small population of cells in the neurogenic niche of the SGZ, and in the SVZ where the subcellular location of CAR differs notably (Fig. 1).3,7 In the mouse DG, CAR covers the cell body of immature neurons, as determined by its location and overlap with PSA-NCAM (polysialylated neural cell adhesion molecule), and incorporation of a thymidine analog in the adult brain.7
Figure 1.
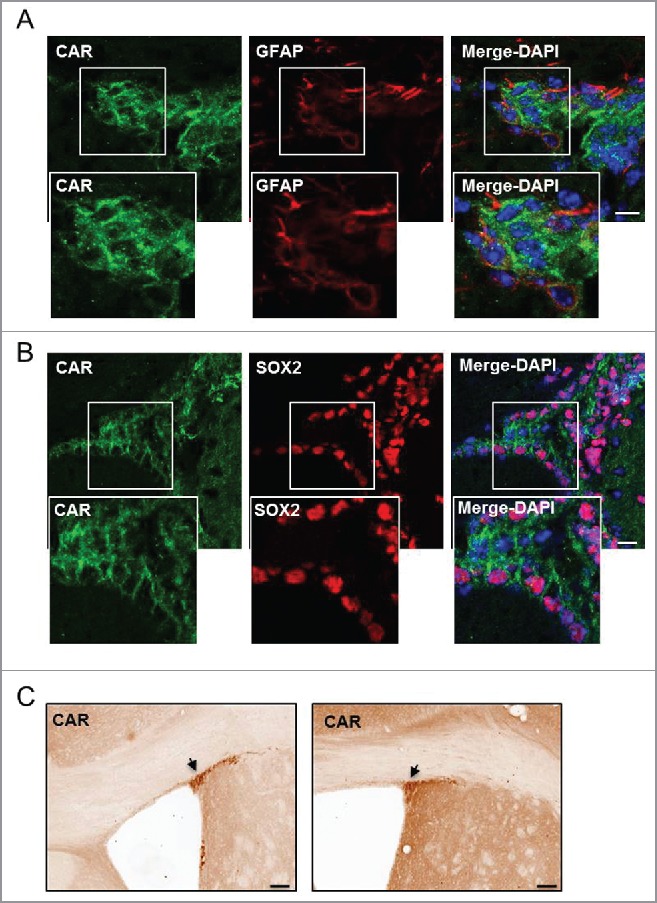
CAR in the SVZ. (A and B) Healthy mouse brains were stained with anti-CAR, anti-GFAP or anti-SOX2 and DAPI. (A) CAR (in green) was found in few radial glial cells of the SVZ identified by GFAP (in red) (white box refers to zoomed in panels). (B) The majority of CAR+ cells in the SVZ express SOX2 (in red), which correspond to proliferating NPCs (white box refers to zoomed in panels). (C) Representative image of anti-CAR immunohistochemistry in the murine SVZ. Black arrows show areas of intense CAR staining in the SVZ. Scale Bars (A and B) 20 μm (C) 200 μm.
Of note for those using viral vectors (e.g. canine adenovirus type 2 or “CAV-2”) that engage CAR as an attachment molecule, circumstantial evidence suggests that CAR is not expressed by all neuron subtypes. We have previously shown that CAV-2 vectors infect motor, cholinergic, dopamine, melanin-concentrating hormone, noradrenergic neurons,8,9 and all available evidence suggest that CAV-2 vector infection depends on CAR expression.10-12 In contrast to the trans-cellular interaction in epithelial cells, we showed that when CAR is at the synapse of mature neurons, it is exclusively pre- but not post-synaptic.7 However, not all neurons appear to be targeted by CAV-2 vectors.8,9 For example, we have routinely had poor transgene expression when trying to transduce Purkinje cells in the mouse brain with CAV-2 vectors. Similar limited efficacy has been seen with striatal interneurons in rats. To complicate the identification of CAR-expressing neuron subtypes, CAV-2 vector tropism appears to varies between species: injections in the primate cerebellum or striatum readily transduce Purkinje cells and interneurons, respectively. This leads to the obvious question of whether CAR is expressed at variable levels in neuron subtypes (which could influence CAV-2 vector tropism) and/or if CAR has divergent roles in a given subtype via posttranscriptional regulation. Clearly, a comprehensive mapping of CAR expressing neurons could provide insight into these possibilities. Yet, identifying the neurons that express CAR and quantify the levels in the axons and at the presynapse would be challenging.
An unexpected finding in our study was that the absence of CAR in neuron-specific knockout mice (CAR-CNSKO) led to a decrease in the generation of newborn mature neurons. In CAR-CNSKO mice global hippocampal neurotransmission was impaired, and the mice developed cognitive deficits such as compromised memory (short and long-term) and anxiety7 as early as 2 months old. One important piece of the map regarding the role of CAR in the modulation of newborn neuron integration would be to measure neurotransmission in the CA3 region of CAR-CNSKO animals. Indeed, in our study we measured LTP in the CA1 region of the hippocampus, to have a general view of hippocampal synaptic transmission since this region is in the last part of the network. Because newborn neurons generated in the DG project toward the CA3 region and integrate after synaptic genesis and maturation in the existing network, directly measuring LTP in this area will directly address the role of CAR in the modulation of adult neurogenesis and synaptic transmission.
CAR in the neurogenic niche of the SVZ
The second site of adult neurogenesis differs between rodents and humans: in humans, C14 dating demonstrates that considerable amounts of new GABAergic neurons are added in the striatum.13,14 By contrast, in rodents the second major site of postnatal neuronal differentiation is the OB. In the SVZ, pre-determined neuronal stem cells (NSCs) generate large amounts of NPCs. After amplification, NPCs undergo long distance chain migration via the RMS. Once they arrive in the OB, they migrate individually into their target layers in the granule and glomerular layers where they differentiate into interneurons that use GABA, dopamine and glutamate as their neurotransmitters.15,16 In the SVZ, immunofluorescent staining shows that CAR and SOX2 overlap, suggesting an expression of CAR in an early stage of neurogenesis (Fig. 1). Accordingly, CAV-2 injection in the ventricles leads to efficient transduction of NSCs or NPCs that will undergo migration in the RMS and integration after differentiation in the OB (Fig. 2). The impact of CAR loss on OB-specific neurogenesis in rodents has not yet been determined and will be the subject of future studies.
Figure 2.
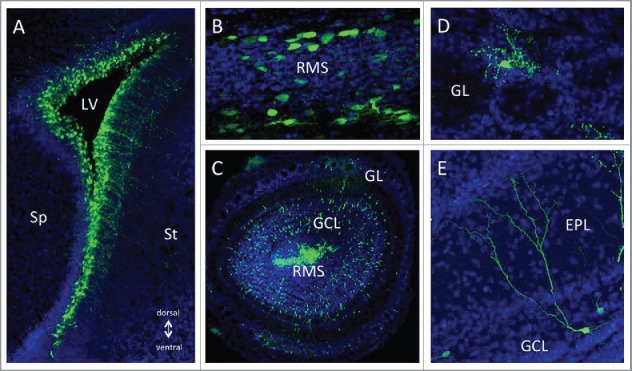
GFP expression from CAV-GFP infected cells in the postnatal forebrain neurogenic system. 1 × 109 physical particles of CAVGFP33 was injected in the lateral ventricle (LV) of P1 mice. (A) 1 day post-injection (dpi) exclusively radial glia type neural stem cells located in the ventricular zone surrounding the lateral ventricle (LV) expressed GFP. Over the following days, tangentially migrating neuroblasts in the rostral migratory stream (B) and radially migrating neuroblasts in the olfactory bulb (OB, C) were GFP+. After arrival in the olfactory bulb (at 20 dpi) newly generated neurons in the glomerular layer (GL, D) and the granule cell layer (GCL, E) showed strong and long-lasting GFP expression. EPL; External Plexiform Layer, Sp; septum, St; striatum.
Canonical CAM functions during adult neurogenesis?
Proteins like Ig-CAMs, cadherins, ephrins and neurexins play critical roles in general brain homeostasis by influencing adult neurogenesis, dendritic spine development, axon fasciculation, axon guidance, neurite outgrowth, and synapse remodelling2,17 - which combined form the bases of neuronal plasticity. What needs to be addressed in future studies is how CAR functions compare and contrast to PSA-NCAM: both are expressed in the main regions of ongoing neurogenesis as well as during migration and axon fasciculation: PSA-NCAM was the first molecular factor to be implicated in the control of postnatal and adult neurogenesis. PSA-NCAM-deficient mice show a severe size reduction of the OB,18 which is due to loss of chain migration of neuronal precursors.19 One question to address is whether the loss of CAR, like the loss of PSA-NCAM20,21 impacts axon outgrowth and synaptic plasticity in the hippocampal mossy fiber system. In addition, does “somal” CAR have a yet unidentified post-translational modification that imparts a specific function? Of note, CAR interacts with numerous intracellular and extracellular proteins and can induce different signaling pathways.22 Moreover, its intracellular domain can be cleaved off, translocated to the nucleus, and appears to act as transcriptional regulator (our unpublished data). Finally, while CAR expression in the SGZ is almost exclusively in PSA-NCAM+ cells, in the SVZ, we found CAR in both SOX-2+ and PSA-NCAM+ cells (Fig. 1 and data not shown), suggesting that CAR could have different roles in both neurogenic niches. Clearly, both niches share common, but not identical, characteristics.23 It is possible that CAR plays divergent roles in the SVZ versus the SGZ because it is expressed from early stages of neurogenesis in the SVZ (SOX2+ cells) until the OB bulb. Migration of NPCs in the SVZ-RMS-OB neurogenic route is much more important in term of distance than in the SGZ, where newborn cells barely move from the granular layer, but have to integrate into a large complex network. One could imagine that in the SGZ, CAR regulates network development and integration, whereas in the SVZ, it could play a role in NPCs migration along the RMS and possibly also during differentiation and integration. This aspect will need to be addressed in future studies. Together, the expression, structure, interactions and subcellular distribution confirm that CAR is an important regulator of neurogenic processes.
Inflammation-induced CAR loss
In combination with genetic triggers, a compounding and unifying factor in the physiopathology of many brain diseases is an inflammatory environment that perturbs synapse homeostasis and adult neurogenesis.24 A pro-inflammatory environment, whether due directly to infection or damage to the brain, or indirectly via effects systemically, is responsible for impaired cognition in the healthy brain and amplifies cognitive defects in many neurodegenerative diseases. Pro-inflammatory cytokine-induced cognitive defects have been recapitulated in mice,25 highlighting the global repercussions of cytokines in brain homeostasis. Yet, the mechanistic link is poorly understood. In epithelial cells, CAR levels can be indirectly reduced by the pro-inflammatory cytokines TNF (tumor necrosis factor) and INF-γ (interferon–gamma).26 These observations led us to assay CAR levels in primary murine hippocampal neurons and in adult murine NPCs after TNF and INF-γ treatment. We found that CAR levels significantly decrease here in a dose-dependent response.7 We then asked if there was a connection in vivo by investigating if CAR levels in the brain are affected by systemic inflammation. Injection of lipopolysaccharides (LPS) into the peritoneal cavity did not affect global CAR levels. By contrast, CAR was strikingly reduced in DG immature neurons, and their axons, whereas PSA-NCAM staining was unaffected. This demonstrated that during acute systemic induction of inflammatory cytokines, CAR levels are affected on immature neurons. The post-translational link between systemic inflammation and CAR loss on newborn neurons is likely far from linear. Intraperitoneal LPS typically induces about half of its systemic (and brain) cytokine response through C5a receptors with the other half is through toll-like receptor 4 (TLR-4). While others showed that pro-inflammatory cytokines are acting through glial cells to perturb neuron homeostasis,27 our ex vivo results demonstrated that the loss of CAR in neurons can be via a direct effect of TNF and IFN-γ. It will be critical to determine whether TNF or IFN-γ play a role in the reduction of CAR levels in the DG. In addition, quantifying the time it takes for CAR levels to return to normal in newborn neurons in the DG—in young and old primates—will help us determine the impact of systemic and brain inflammation on CAR during neurogenesis.
The sex biased cognitive effect
One of the worst kept secrets in biology is that the male and female brains are not the same. Numerous neurologic disorders, including depression, anxiety and AD show a gender bias.28 The general consensus is that the more active female immune system, due to the impact of sex hormones, leads to higher response level, diseases-associated autoimmunity, and excessive inflammation. Consistent with this, AD is also associated with impaired adult neurogenesis.14,29-32 In hippocampal extracts from AD patients, we detected a significant decrease in CAR expression (on newborn neurons and in axons projecting from the entorhinal cortex, a region particularly affected in AD), suggesting that CAR is affected/involved in disorders where chronic inflammation, impaired adult neurogenesis, and synapse homeostasis are found.7 Yet, it remains unclear as to why the genetic ablation of CAR expression (early in development) impacted female mice greater than male mice. The impact of CAR loss could be more readily understood in a “normal” environment (i.e., not in a transgenic mouse that is depleted in CAR during development). In the normal environment the estrogen response element in the Cxadr (and CXADR) promoter should be (paradoxically) more active in the male brain because of higher local estrogen levels. Whether CAR loss is a phenomenon that impacts other diseases associated with neuroinflammation (e.g., Down syndrome) needs to be explored. It also remains to be determined whether CAR has a particular function in a subset of neurons.
Conclusion
While a lot of effort was undertaken to understand CAR's role in epithelia and endothelia, very little was known regarding its function in the brain. Interestingly, despite a proposed role during neuronal development, CAR-CNSKO mice had no striking phenotype. By contrast, we showed that CAR impacts neuronal plasticity in the adult brain. Both synaptic biology and adult neurogenesis are affected by CAR loss, but whether it is through its cell adhesion functions, or others pathways, remain to be demonstrated.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank EKL members and collaborators involved in the original work referred here.
Funding
This work was supported, in part, by France Parkinson, BrainCAV (EU-FP7 contract #292222), BrainVectors (EU-FP7 contract #286071), Université de Montpellier, IGMM, LabEx/EpiGenMed, Vaincre les Maladies Lysosomales, and Association pour la Recherche sur la Sclérose latérale amyotrophique.
References
- [1].Jessberger S, Gage FH. Adult neurogenesis: Bridging the gap between mice and humans. Trends Cell Biol 2014; 24(10):558-63; PMID:25124338; http://dx.doi.org/ 10.1016/j.tcb.2014.07.003 [DOI] [PubMed] [Google Scholar]
- [2].Togashi H, Sakisaka T, Takai Y. Cell adhesion molecules in the central nervous system. Cell Adh Migr 2009; 3:29-35; PMID:19372758; http://dx.doi.org/ 10.4161/cam.3.1.6773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Loustalot F, Kremer EJ, Salinas S. Membrane dynamics and signaling of coxsackievirus and adenovirus receptor. Int Rev Cell Mol Bio 2015; 322:331-62. [DOI] [PubMed] [Google Scholar]
- [4].Coyne CB, Bergelson JM. CAR: a virus receptor within the tight junction. Adv Drug Deliv Rev 2005; 57:869-82; PMID:15820557; http://dx.doi.org/ 10.1016/j.addr.2005.01.007 [DOI] [PubMed] [Google Scholar]
- [5].Freimuth P, Philipson L, Carson SD. The coxsackievirus and adenovirus receptor. Curr Top Microbiol Immunol 2008; 323:67-87. Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd = Retrieve&db=PubMed&dopt=Citation&list_uids=18357766; PMID:18357766 [DOI] [PubMed] [Google Scholar]
- [6].Cohen CJ, Gaetz J, Ohman T, Bergelson JM. Multiple regions within the coxsackievirus and adenovirus receptor cytoplasmic domain are required for basolateral sorting. J Biol Chem 2001; 276:25392-8; PMID:11316797; http://dx.doi.org/ 10.1074/jbc.M009531200 [DOI] [PubMed] [Google Scholar]
- [7].Zussy C, Loustalot F, Junyent F, Gardoni F, Bories C, Valero J, Desarménien MG, Bernex F, Henaff D, Bayo-Puxan N, et al.. Coxsackievirus adenovirus receptor loss impairs adult neurogenesis, synapse content and hippocampus plasticity. J Neurosci 2016; 36:9558-71; PMID:27629708; http://dx.doi.org/ 10.1523/JNEUROSCI.0132-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Junyent F, Kremer EJ. CAV-2-why a canine virus is a neurobiologist's best friend. Curr Opin Pharmacol 2015; 24:86-93; PMID:26298516; http://dx.doi.org/ 10.1016/j.coph.2015.08.004 [DOI] [PubMed] [Google Scholar]
- [9].Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE, Ren J, Ibanes S, Malenka RC, Kremer EJ, et al.. Viral-genetic tracing of the input–output organization of a central noradrenaline circuit. Nature 2015; 6:88-92; http://dx.doi.org/ 10.1038/nature14600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Salinas S, Bilsland LG, Henaff D, Weston AE, Keriel A, Schiavo G, Kremer EJ. CAR-associated vesicular transport of an adenovirus in motor neuron axons. PLoS Pathog 2009; 5:e1000442; PMID:19461877; http://dx.doi.org/ 10.1371/journal.ppat.1000442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Salinas S, Zussy C, Loustalot F, Henaff D, Menendez G, Morton PE, Parsons M, Schiavo G, Kremer EJ. Disruption of the coxsackievirus and adenovirus receptor-homodimeric interaction triggers lipid microdomain- and dynamin-dependent endocytosis and lysosomal targeting. J Biol Chem 2014; 289:68095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Soudais C, Boutin S, Hong SS, Chillon M, Danos O, Bergelson JM, Boulanger P, Kremer EJ, et al.. Canine adenovirus type 2 attachment and receptor, alternative receptors, and an RGD-independent pathway. J Virol 2000; 74:10639-49; PMID:11044108; http://dx.doi.org/ 10.1128/JVI.74.22.10639-10649.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Paredes MF, James D, Gil-Perotin S, Kim H, Cotter JA, Ng C, Sandoval K, Rowitch DH, Xu D, McQuillen PS, et al.. Extensive migration of young neurons into the infant human frontal lobe. Science (80- ) 2016; 354:7073-77; http://dx.doi.org/ 10.1126/science.aaf7073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ernst AA, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisén J. Neurogenesis in the striatum of the adult human brain. Cell 2014; 156:1072-83; PMID:24561062; http://dx.doi.org/ 10.1016/j.cell.2014.01.044 [DOI] [PubMed] [Google Scholar]
- [15].Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci 2002; 22:629-34; PMID:11826091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lledo PM, Merkle FT, Alvarez-Buylla A. Origin and function of olfactory bulb interneuron diversity. Trends Neurosci 2008; 31:392-00; PMID:18603310; http://dx.doi.org/ 10.1016/j.tins.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sakisaka T, Takai Y. Cell adhesion molecules in the CNS. J Cell Sci 2005; 118:5407-10; PMID:16306219; http://dx.doi.org/ 10.1242/jcs.02672 [DOI] [PubMed] [Google Scholar]
- [18].Cremer H, Lange R, Christoph A, Plomann M, Vopper G, Roes J, Brown R, Baldwin S, Kraemer P, Scheff S, et al.. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature 1994; 367:455-9; PMID:8107803; http://dx.doi.org/ 10.1038/367455a0 [DOI] [PubMed] [Google Scholar]
- [19].Chazal G, Durbec P, Jankovski A, Rougon G, Cremer H. Consequences of neural cell adhesion molecule deficiency on cell migration in the rostral migratory stream of the mouse. J Neurosci 2000; 20:1446-57; PMID:10662835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cremer H, Chazal G, Goridis C, Represa A. NCAM is essential for axonal growth and fasciculation in the hippocampus. Mol Cell Neurosci 1997; 8:323-35; PMID:9073395; http://dx.doi.org/ 10.1006/mcne.1996.0588 [DOI] [PubMed] [Google Scholar]
- [21].Cremer H, Chazal G, Carleton A, Goridis C, Vincent JD, Lledo PM. Long-term but not short-term plasticity at mossy fiber synapses is impaired in neural cell adhesion molecule-deficient mice. Proc Natl Acad Sci U S A 1998; 95:13242-7; PMID:9789073; http://dx.doi.org/ 10.1073/pnas.95.22.13242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Loustalot F, Kremer EJ, Salinas S. The intracellular domain of the coxsackievirus and adenovirus receptor differentially influences adenovirus entry. J Virol 2015; 89:9417-26; PMID:26136571; http://dx.doi.org/ 10.1128/JVI.01488-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 2011; 70:687-702; PMID:21609825; http://dx.doi.org/ 10.1016/j.neuron.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol 2006; 147(Suppl):S232-40; PMID:16402109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Valero J, Mastrella G, Neiva I, Sánchez S, Malva JO. Long-term effects of an acute and systemic administration of LPS on adult neurogenesis and spatial memory. Front Neurosci Frontiers 2014; 8:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vincent T, Pettersson RF, Crystal RG, Leopold PL. Cytokine-mediated downregulation of coxsackievirus-adenovirus receptor in endothelial cells. J Virol 2004; 78:8047-58; PMID:15254176; http://dx.doi.org/ 10.1128/JVI.78.15.8047-8058.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Habbas S, Santello M, Becker D, Stubbe H, Zappia G, Liaudet N, Klaus FR, Kollias G, Fontana A, Pryce CR, et al.. Neuroinflammatory TNF?? Impairs Memory via Astrocyte Signaling. Cell 2015; 163:1730-41; PMID:26686654; http://dx.doi.org/ 10.1016/j.cell.2015.11.023 [DOI] [PubMed] [Google Scholar]
- [28].Hanamsagar R, Bilbo SD. Sex differences in neurodevelopmental and neurodegenerative disorders: Focus on microglial function and neuroinflammation during development. J Steroid Biochem Mol Biol 2015; 160:127-33; PMID:26435451; http://dx.doi.org/ 10.1016/j.jsbmb.2015.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fuster-Matanzo A, Llorens-Martín M, Hernández F, Avila J. Role of neuroinflammation in adult neurogenesis and Alzheimer disease: Therapeutic approaches. Mediators Inflamm 2013; 2013:260925; PMID:23690659; http://dx.doi.org/ 10.1155/2013/260925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ben M'Barek K, Pla P, Orvoen S, Benstaali C, Godin JD, Gardier AM, Saudou F, David DJ, Humbert S. Huntingtin Mediates Anxiety/Depression-Related Behaviors and Hippocampal Neurogenesis. J Neurosci 2013; 33:8608-20; PMID:23678106; http://dx.doi.org/ 10.1523/JNEUROSCI.5110-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yun S, Reynolds RP, Masiulis I, Eisch AJ. Re-evaluating the link between neuropsychiatric disorders and dysregulated adult neurogenesis. Nat Med 2016; 22:1239-47; PMID:27783068; http://dx.doi.org/ 10.1038/nm.4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schoenfeld TJ, Cameron HA. Adult neurogenesis and mental illness. Neuropsychopharmacology 2015; 40:113-28; PMID:25178407; http://dx.doi.org/ 10.1038/npp.2014.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kremer EJ, Boutin S, Chillon M, Danos O. Canine adenovirus vectors: an alternative for adenovirus-mediated gene transfer. J Virol 2000; 74:505-12; PMID:10590140; http://dx.doi.org/ 10.1128/JVI.74.1.505-512.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]


