Abstract
Intact wheat seedlings (Triticum aestivum L.) were induced to senesce by placing them in the dark and at various stages of senescence were placed back in the light and their recoverability observed. Seedlings demonstrated complete recovery of chlorophyll, protein, and rate of photosynthesis after 2 days in the dark, but were unable to recover fully after 4 days. This suggests the onset of an irreversible stage in senescence by day 4. Foliar applied cytokinins delayed senescence, and zeatin at 0.1 mm delayed the onset of the irreversible stage for 6 days. In addition to delaying the loss of total soluble protein, zeatin maintained the net protein recovery capacity of the tissue. Control seedlings, however, lost their potential for net protein recovery at a rate similar to their loss of total soluble protein. Treatment with zeatin had no apparent effect on dark respiration during senescence, and although treatment did delay the loss of membrane permeability to substrate, the change in permeability occurred too late to have a causal role in senescence.
Full text
PDF
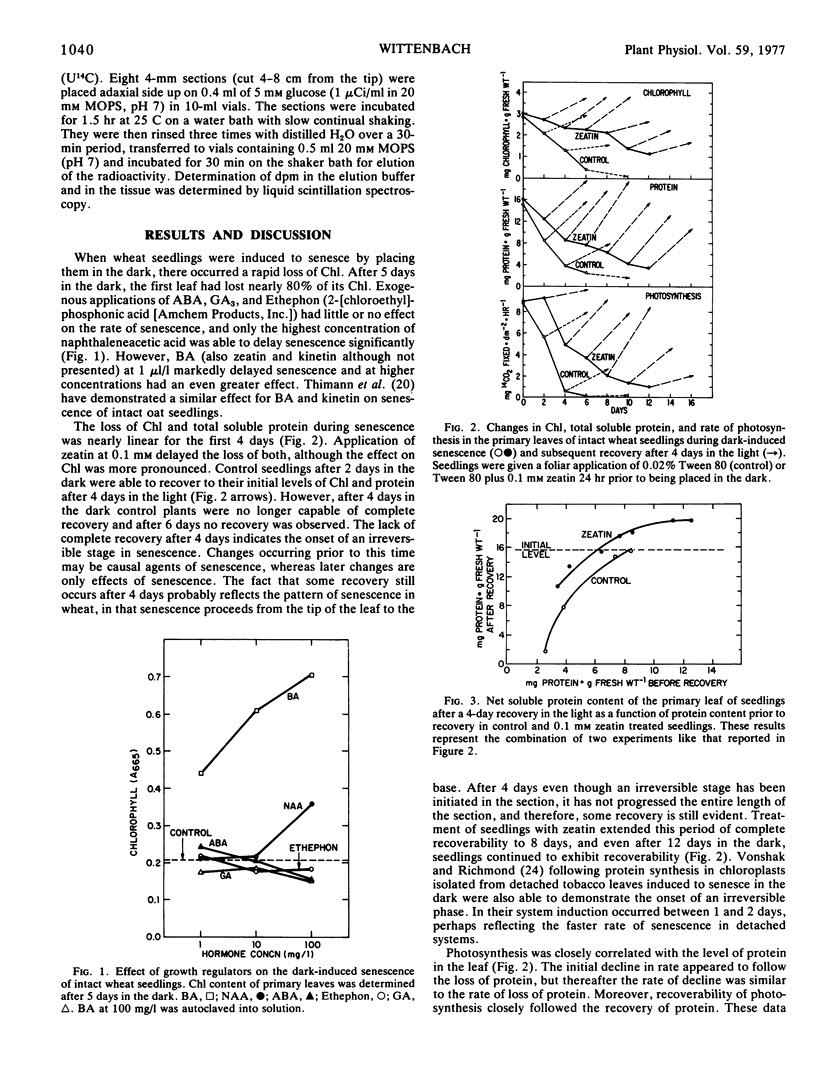
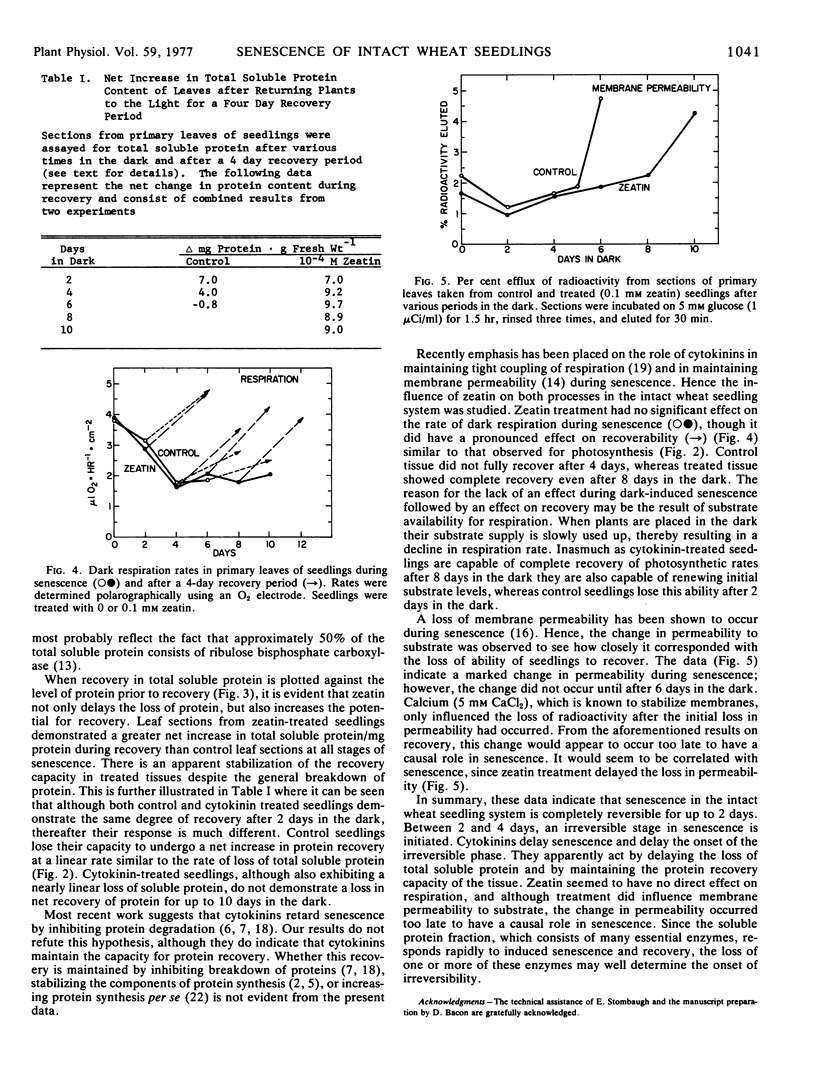

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRELIN E. S. Mitosis in adult cartilage. Science. 1957 Apr 5;125(3249):650–650. doi: 10.1126/science.125.3249.650. [DOI] [PubMed] [Google Scholar]
- Fox J. E., Erion J. L. A cytokinin binding protein from higher plant ribosomes. Biochem Biophys Res Commun. 1975 May 19;64(2):694–700. doi: 10.1016/0006-291x(75)90376-9. [DOI] [PubMed] [Google Scholar]
- Kende H. The cytokinins. Int Rev Cytol. 1971;31:301–338. doi: 10.1016/s0074-7696(08)60061-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Macnicol P. K. Rapid Metabolic Changes in the Wounding Response of Leaf Discs following Excision. Plant Physiol. 1976 Jan;57(1):80–84. doi: 10.1104/pp.57.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C., Thimann K. V. The role of protein synthesis in the senescence of leaves: I. The formation of protease. Plant Physiol. 1972 Jan;49(1):64–71. doi: 10.1104/pp.49.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne D. J. Effect of Kinetin on Protein & Nucleic Acid Metabolism in Xanthium Leaves During Senescence. Plant Physiol. 1962 Sep;37(5):595–602. doi: 10.1104/pp.37.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L. W., Kleinkopf G. E., Huffaker R. C. Evidence for lack of turnover of ribulose 1,5-diphosphate carboxylase in barley leaves. Plant Physiol. 1973 Jun;51(6):1042–1045. doi: 10.1104/pp.51.6.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah B. W., Leopold A. C. Deferral of leaf senescence with calcium. Plant Physiol. 1973 Sep;52(3):236–239. doi: 10.1104/pp.52.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J. A. Hormonal Control of Senescence of Bean Endocarp: Auxin-suppression of RNase. Plant Physiol. 1969 Feb;44(2):313–314. doi: 10.1104/pp.44.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J. A. Relationship between Auxin and Membrane-Integrity in Tissue Senescence and Abscission. Science. 1957 Jun 14;125(3259):1199–1200. doi: 10.1126/science.125.3259.1199. [DOI] [PubMed] [Google Scholar]
- Tetley R. M., Thimann K. V. The Metabolism of Oat Leaves during Senescence: I. Respiration, Carbohydrate Metabolism, and the Action of Cytokinins. Plant Physiol. 1974 Sep;54(3):294–303. doi: 10.1104/pp.54.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann K. V., Tetley R. R., Van Thanh T. The Metabolism of Oat Leaves during Senescence: II. Senescence in Leaves Attached to the Plant. Plant Physiol. 1974 Dec;54(6):859–862. doi: 10.1104/pp.54.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas A. Control of the Protein Turnover Rates in Lemna minor. Plant Physiol. 1972 Jan;49(1):47–51. doi: 10.1104/pp.49.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonshak A., Richmond A. E. Initial stages in the onset of senescence in tobacco leaves. Plant Physiol. 1975 Apr;55(4):786–790. doi: 10.1104/pp.55.4.786. [DOI] [PMC free article] [PubMed] [Google Scholar]


