Abstract
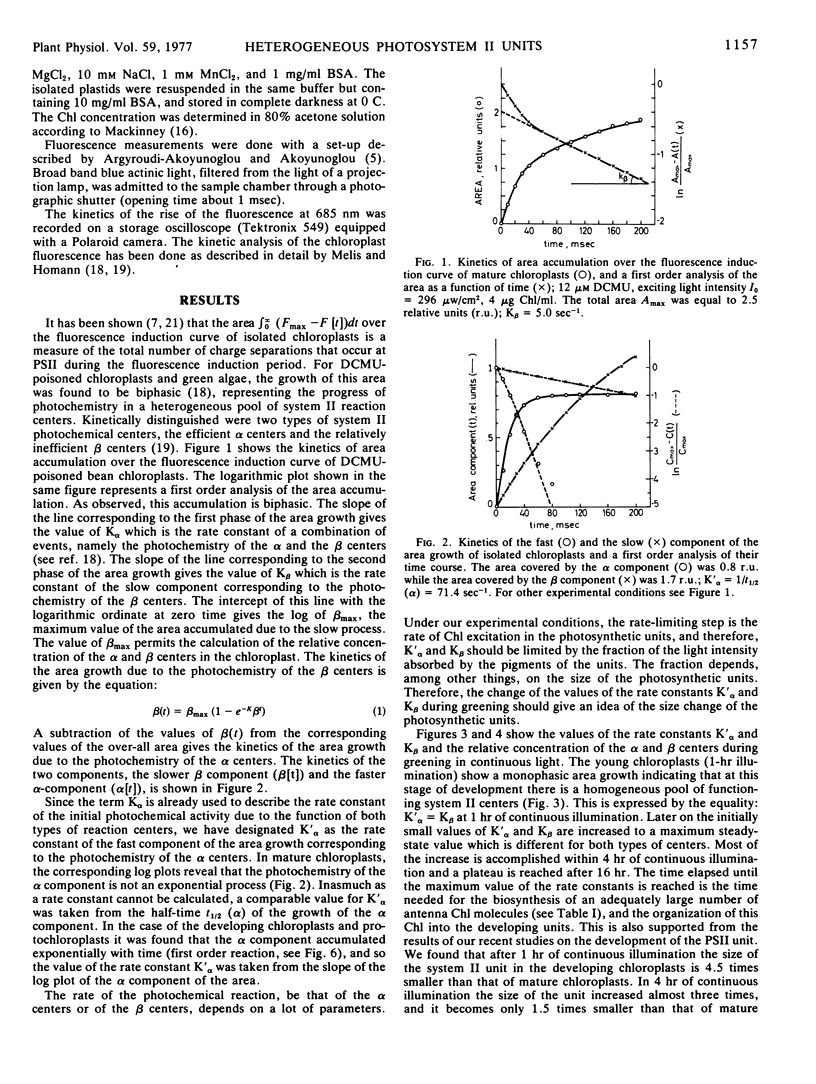
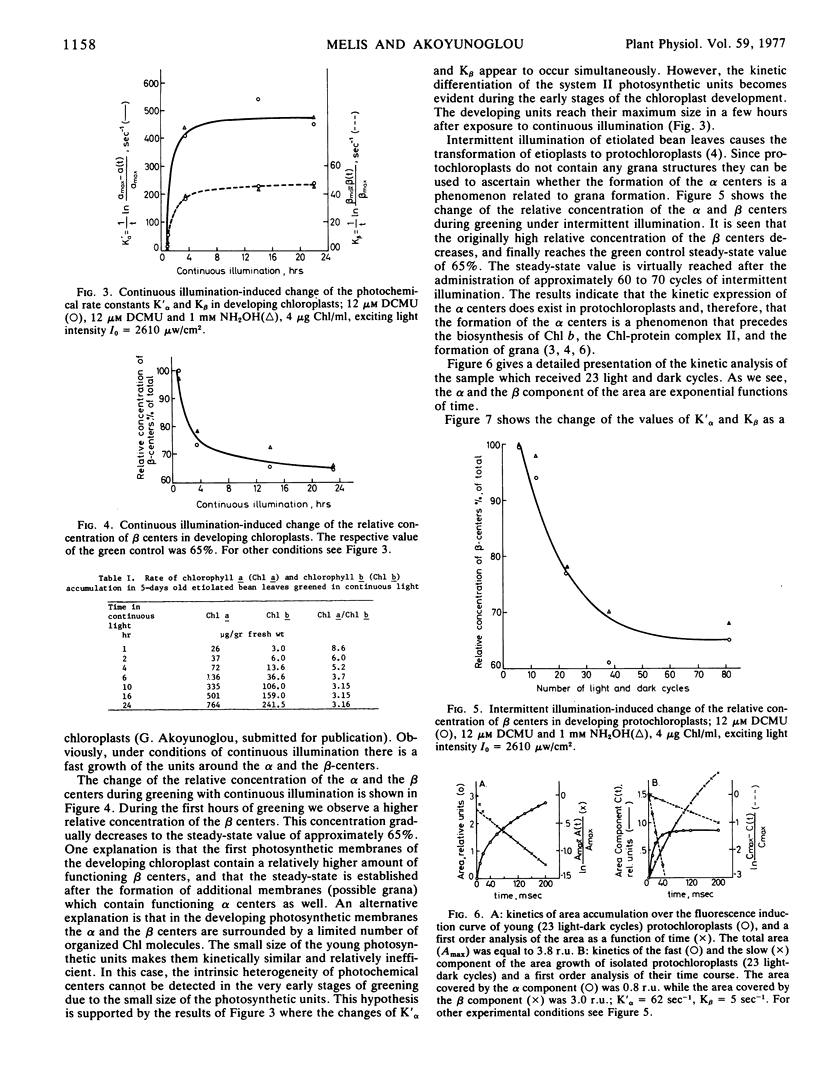
The development of the photosystem II units in relation to the heterogeneity of their photochemical centers was studied in etiolated bean leaves (Phaseolus vulgaris var. red kidney) greened under continuous or intermittent light. The study was done in order to see whether grana are the loci of the units with the efficient photosystem II activity (α units), while the stroma thylakoids are the loci of the units with the less efficient photosystem II activity (β units), as it has been proposed. In addition, the interrelations between α and β centers have been investigated. It was found that the α and the β centers of photosystem II were present in the first photosynthetic membranes irrespective of the mode of greening of the leaves. The magnitude of their respective photochemical rate constants, K′α and Kβ, increased with time in continuous light and it reached the steady-state values of the mature chloroplasts within 16 hours, while in intermittent light it remained smaller. The differentiation of the system II units in α and β centers containing units is more evident under conditions of intermittent illumination, i.e. when the rate of chlorophyll biosynthesis is the limiting step for chloroplast development.
It is concluded that the heterogeneity of the photochemical centers in system II is an endogenous property of the chloroplast lamellae. The α centers and the β centers develop independently of each other from the beginning of the light-induced greening. They do not share the same pigment beds. The presence of grana, chlorophyll b, and the chlorophyll-protein complex II is not a prerequisite for the formation or development of the α centers. The formation of these centers precedes grana formation in greening plastids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Boardman N. K. Fractionation of the photochemical systems of photosynthesis. I. Chlorophyll contents and photochemical activities of particles isolated from spinach chloroplasts. Bibl Laeger. 1966 Mar 14;112(3):403–421. doi: 10.1016/0926-6585(66)90244-5. [DOI] [PubMed] [Google Scholar]
- Argyroudi-Akoyunoglou J. H., Akoyunoglou G. Photoinduced changes in the chlorophyll a to chlorophyll B ratio in young bean plants. Plant Physiol. 1970 Aug;46(2):247–249. doi: 10.1104/pp.46.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyroudi-Akoyunoglou J. H., Feleki Z., Akoyunoglou G. Formation of two chlorophyll-protein complexes during greening of etiolated bean leaves. Biochem Biophys Res Commun. 1971 Nov 5;45(3):606–614. doi: 10.1016/0006-291x(71)90460-8. [DOI] [PubMed] [Google Scholar]
- Bennoun P., Li Y. New results on the mode of action of 3,-(3,4-Dichlorophenyl)-1,1-dimethylurea in spinach chloroplasts. Biochim Biophys Acta. 1973 Jan 18;292(1):162–168. doi: 10.1016/0005-2728(73)90260-0. [DOI] [PubMed] [Google Scholar]
- Briantais J. M., Vernotte C., Moya I. Intersystem excition transfer in isolated chloroplasts. Biochim Biophys Acta. 1973 Dec 14;325(3):530–538. doi: 10.1016/0005-2728(73)90212-0. [DOI] [PubMed] [Google Scholar]
- Doschek W. W., Kok B. Photon trapping in photosystem II of photosynthesis. The fluorescence rise curve in the presence of 3-(3,4-dichlorophenyl)-1,1-dimetnhylurea. Biophys J. 1972 Jul;12(7):832–838. doi: 10.1016/s0006-3495(72)86126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M., Butler W. L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim Biophys Acta. 1975 Jan 31;376(1):105–115. doi: 10.1016/0005-2728(75)90209-1. [DOI] [PubMed] [Google Scholar]
- Lavorel J., Joliot P. A connected model of the photosynthetic unit. Biophys J. 1972 Jul;12(7):815–831. doi: 10.1016/S0006-3495(72)86125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A., Homann P. H. Heterogeneity of the photochemical centers in system II of chloroplasts. Photochem Photobiol. 1976 May;23(5):343–350. doi: 10.1111/j.1751-1097.1976.tb07259.x. [DOI] [PubMed] [Google Scholar]
- Murata N., Nishimura M., Takamiya A. Fluorescene of chlorophyll in photosynthetic systems. II. Induction of fluorescence in isolated spinach chloroplasts. Biochim Biophys Acta. 1966 May 12;120(1):23–33. doi: 10.1016/0926-6585(66)90273-1. [DOI] [PubMed] [Google Scholar]
- Sane P. V., Goodchild D. J., Park R. B. Characterization of chloroplast photosystems 1 and 2 separated by a non-detergent method. Biochim Biophys Acta. 1970 Aug 4;216(1):162–178. doi: 10.1016/0005-2728(70)90168-4. [DOI] [PubMed] [Google Scholar]


