Abstract
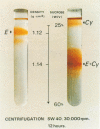
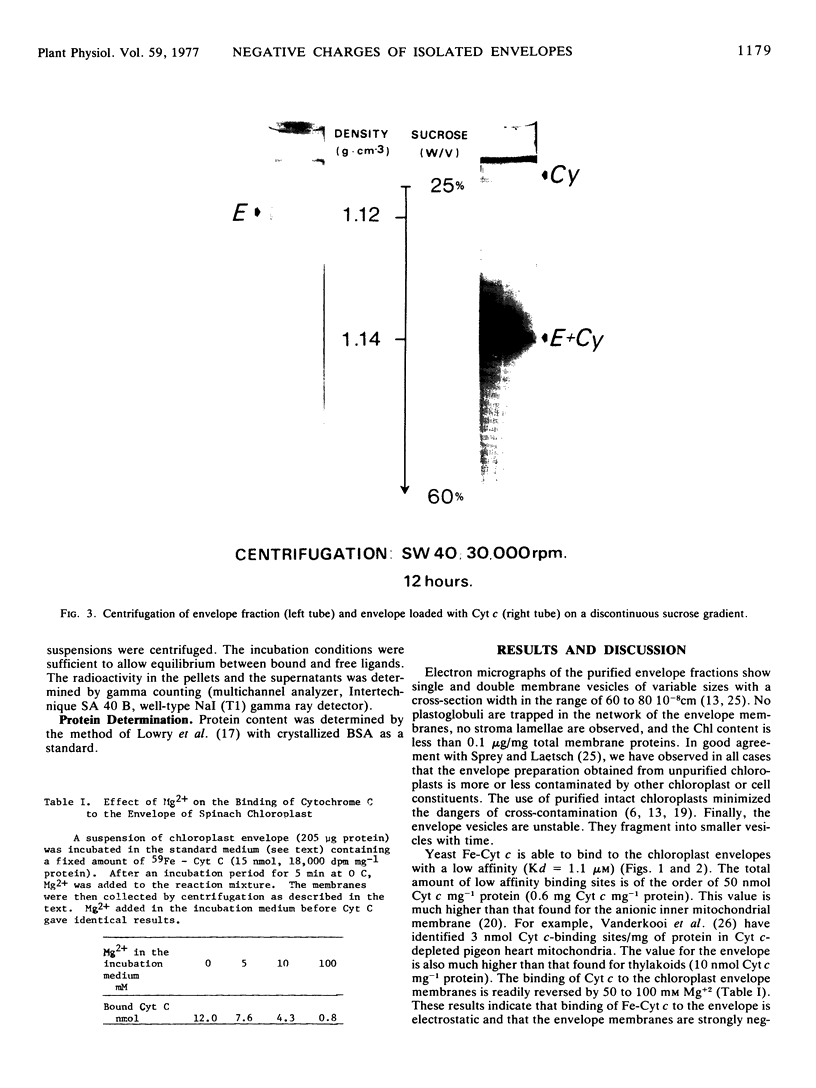
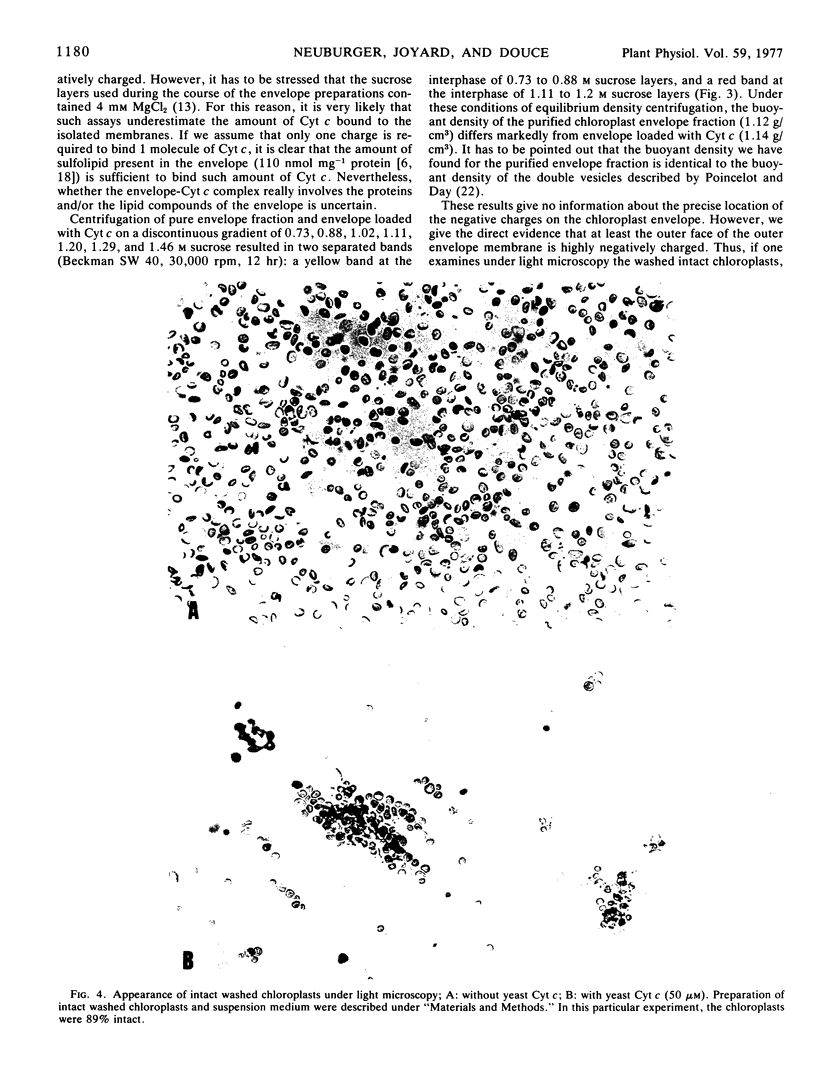
Yeast cationic ferricytochrome c was able to bind to the spinach (Spinacia oleracea) chloroplast envelope with a low affinity (Kd = 1.1 μm). The total amount of low affinity binding sites was of the order of 50 nmol cytochrome c mg−1 protein. We gave the evidence that binding of ferricytochrome c to the envelope was electrostatic and that the envelope membranes were strongly negatively charged. Addition of yeast ferricytochrome c to a preparation of intact washed chloroplasts (class I) induced a strong agglutination of chloroplasts.
Full text
PDF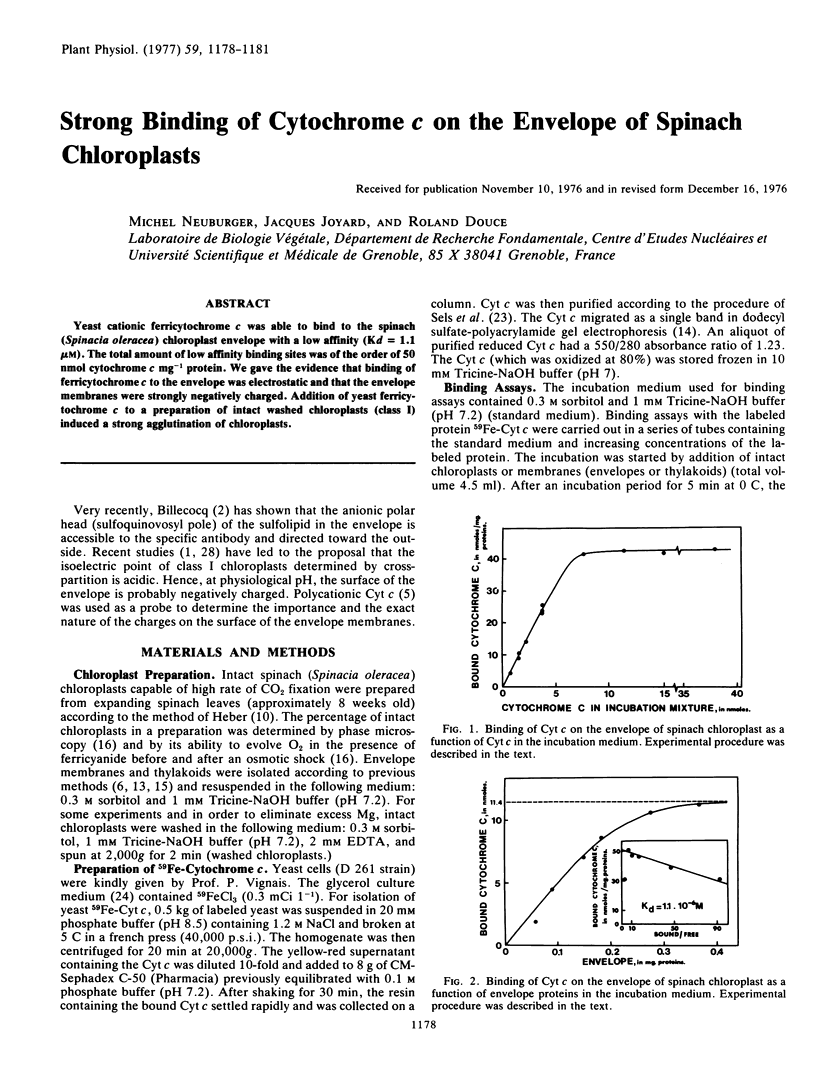

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billecocq A. Structure des membranes biologiques: localisation du sulfoquinovosyldiglycéride dans les diverses membranes des chloroplastes au moyen des anticorps spécifiques. Ann Immunol (Paris) 1975 Apr;126(3):337–352. [PubMed] [Google Scholar]
- Danon D., Goldstein L., Marikovsky Y., Skutelsky E. Use of cationized ferritin as a label of negative charges on cell surfaces. J Ultrastruct Res. 1972 Mar;38(5):500–510. doi: 10.1016/0022-5320(72)90087-1. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E. The structure and history of an ancient protein. Sci Am. 1972 Apr;226(4):58–passim. doi: 10.1038/scientificamerican0472-58. [DOI] [PubMed] [Google Scholar]
- Douce R., Holtz R. B., Benson A. A. Isolation and properties of the envelope of spinach chloroplasts. J Biol Chem. 1973 Oct 25;248(20):7215–7222. [PubMed] [Google Scholar]
- Flügge U. I., Heldt H. W. Identification of a protein involved in phosphate transport of chloroplasts. FEBS Lett. 1976 Oct 1;68(2):259–262. doi: 10.1016/0014-5793(76)80449-8. [DOI] [PubMed] [Google Scholar]
- Hackenbrock C. R., Miller K. J. The distribution of anionic sites on the surfaces of mitochondrial membranes. Visual probing with polycationic ferritin. J Cell Biol. 1975 Jun;65(3):615–630. doi: 10.1083/jcb.65.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U. Stoichiometry of reduction and phosphorylation during illumination of intact chloroplasts. Biochim Biophys Acta. 1973 Apr 27;305(1):140–152. doi: 10.1016/0005-2728(73)90239-9. [DOI] [PubMed] [Google Scholar]
- Joy K. W., Ellis R. J. Protein synthesis in chloroplasts. IV. Polypeptides of the chloroplast envelope. Biochim Biophys Acta. 1975 Jan 6;378(1):143–151. doi: 10.1016/0005-2787(75)90145-8. [DOI] [PubMed] [Google Scholar]
- LEECH R. M. THE ISOLATION OF STRUCTURALLY INTACT CHLOROPLASTS. Biochim Biophys Acta. 1964 May 25;79:637–639. doi: 10.1016/0926-6577(64)90235-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mackender R. O., Leech R. M. The Galactolipid, Phospholipid, and Fatty Acid Composition of the Chloroplast Envelope Membranes of Vicia faba. L. Plant Physiol. 1974 Mar;53(3):496–502. doi: 10.1104/pp.53.3.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenthaler J. J., Mendiola-Morgenthaler L. Synthesis of soluble, thylakoid, and envelope membrane proteins by spinach chloroplasts purified from gradients. Arch Biochem Biophys. 1976 Jan;172(1):51–58. doi: 10.1016/0003-9861(76)90046-1. [DOI] [PubMed] [Google Scholar]
- Nicholls P. Cytochrome c binding to enzymes and membranes. Biochim Biophys Acta. 1974 Dec 30;346(3-4):261–310. doi: 10.1016/0304-4173(74)90003-2. [DOI] [PubMed] [Google Scholar]
- Pineau B., Douce R. Analyse électrophoretique des protéines de l'enveloppe des chloroplastes d'epinard. FEBS Lett. 1974 Oct 15;47(2):255–259. doi: 10.1016/0014-5793(74)81024-0. [DOI] [PubMed] [Google Scholar]
- Poincelot R. P., Day P. R. An improved method for the isolation of spinach chloroplast envelope membranes. Plant Physiol. 1974 Nov;54(5):780–783. doi: 10.1104/pp.54.5.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELS A. A., FUKUHARA H., PERE G., SLONIMSKI P. P. CIN'ETIQUE DE LA BIOSYNTH'ESE INDUITE DE L'ISO-1-CYTOCHROME C ET DE L'ISO-2-CYTOCHROME C AU COURS DE L'ADAPTATION 'A L'OXYG'ENE. Biochim Biophys Acta. 1965 Mar 15;95:486–502. [PubMed] [Google Scholar]
- Sherman F., Taber H., Campbell W. Genetic determination of iso-cytochromes c in yeast. J Mol Biol. 1965 Aug;13(1):21–39. doi: 10.1016/s0022-2836(65)80077-8. [DOI] [PubMed] [Google Scholar]
- Vanderkooi J., Erecińska M., Chance B. Cytochrome c interaction with membranes. II. Comparative study of the interaction of c cytochromes with the mitochondrial membrane. Arch Biochem Biophys. 1973 Aug;157(2):531–540. doi: 10.1016/0003-9861(73)90672-3. [DOI] [PubMed] [Google Scholar]
- Westrin H., Albertsson P. A., Johansson G. Hydrophobic affinity partition of spinach chloroplasts in aqueous two-phase systems. Biochim Biophys Acta. 1976 Jul 1;436(3):696–706. doi: 10.1016/0005-2736(76)90451-x. [DOI] [PubMed] [Google Scholar]