Abstract
Purification of O-acetylserine sulfhydrylase (OASS) from seedlings of two species of Phaseolus reveals the presence in both species of two forms of this enzyme. The isolation and purification procedure gives purification of 7- to 160-fold for individual isoenzymes with specific activities ranging from 33 IU mg−1 to 775 IU mg−1 protein.
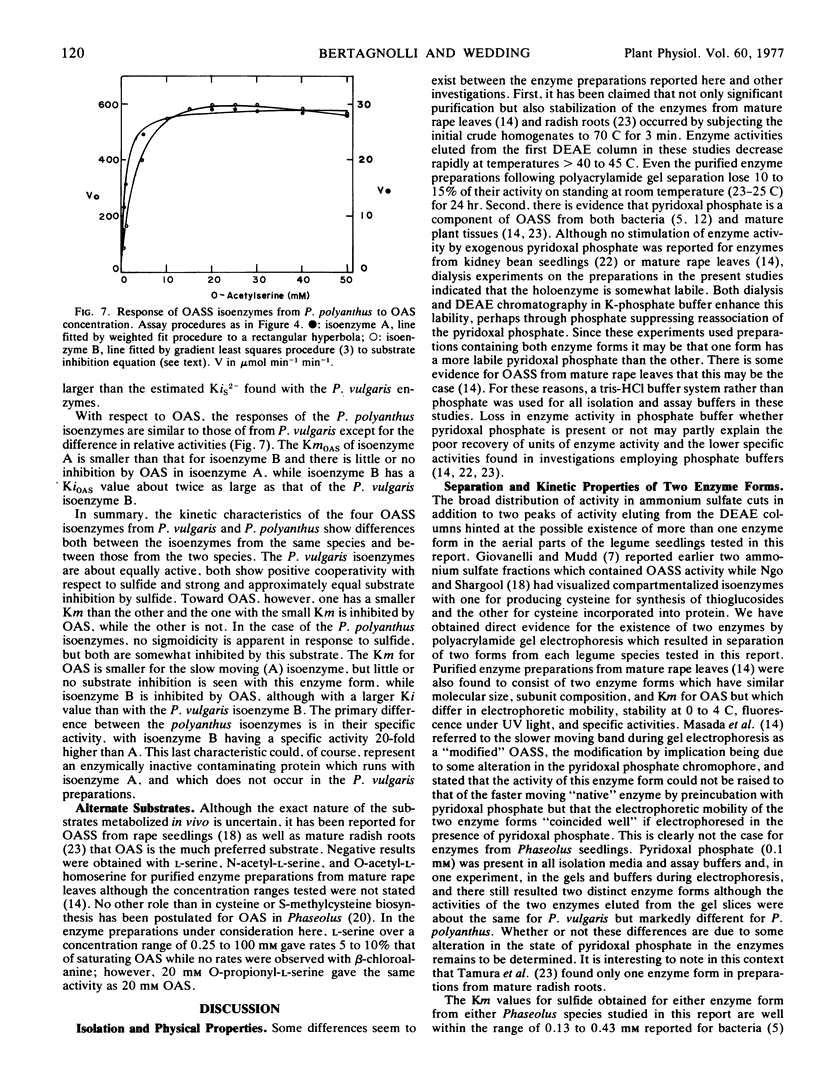
Detailed study of the basic kinetic parameters of the OASS isoenzymes indicates that both forms from Phaseolus vulgaris (which are of about equal specific activity) display substrate inhibition by S2− above 1 mm and positive cooperativity at lower concentrations of S2−. With respect to O-acetylserine (OAS), the second substrate of the reaction, one P. vulgaris isoenzyme shows substrate inhibition by OAS concentrations above 10 mm, while the second is unaffected by OAS concentrations up to 50 mm. The isoenzymes from Phaseolus polyanthus (one of which has a specific activity 24 times higher than the other) are slightly and approximately equally inhibited by both S2− and OAS.
Full text
PDF

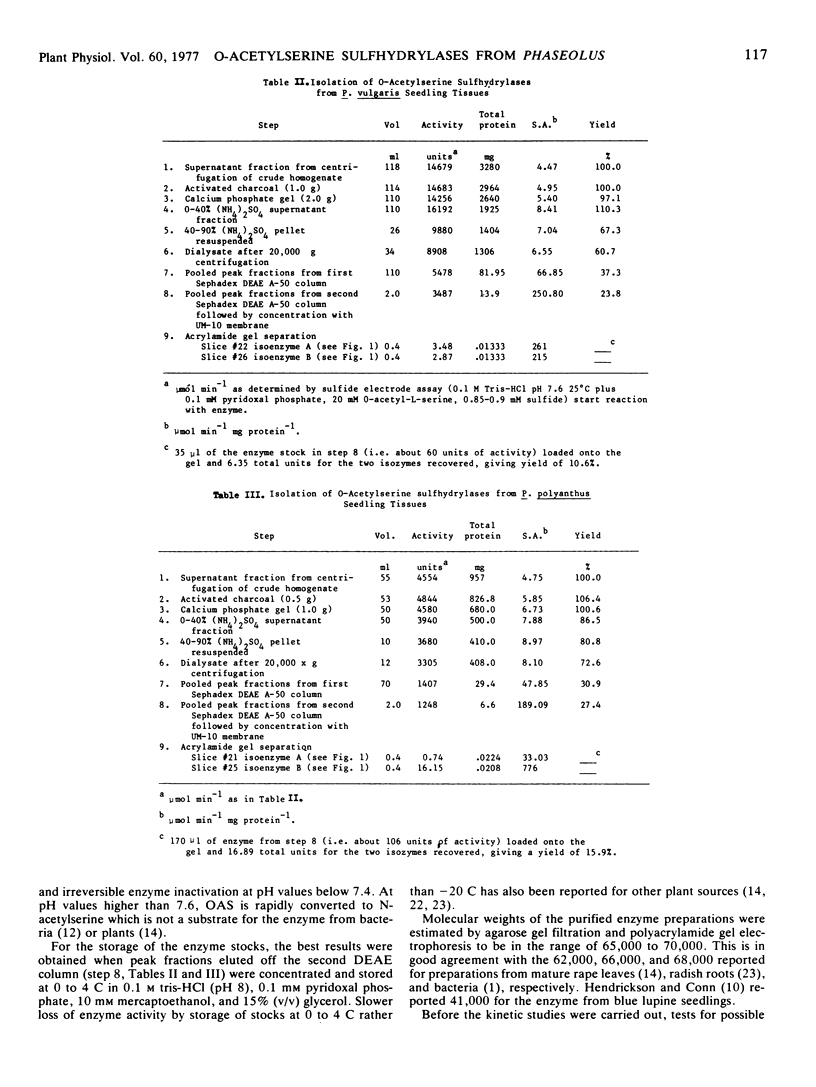
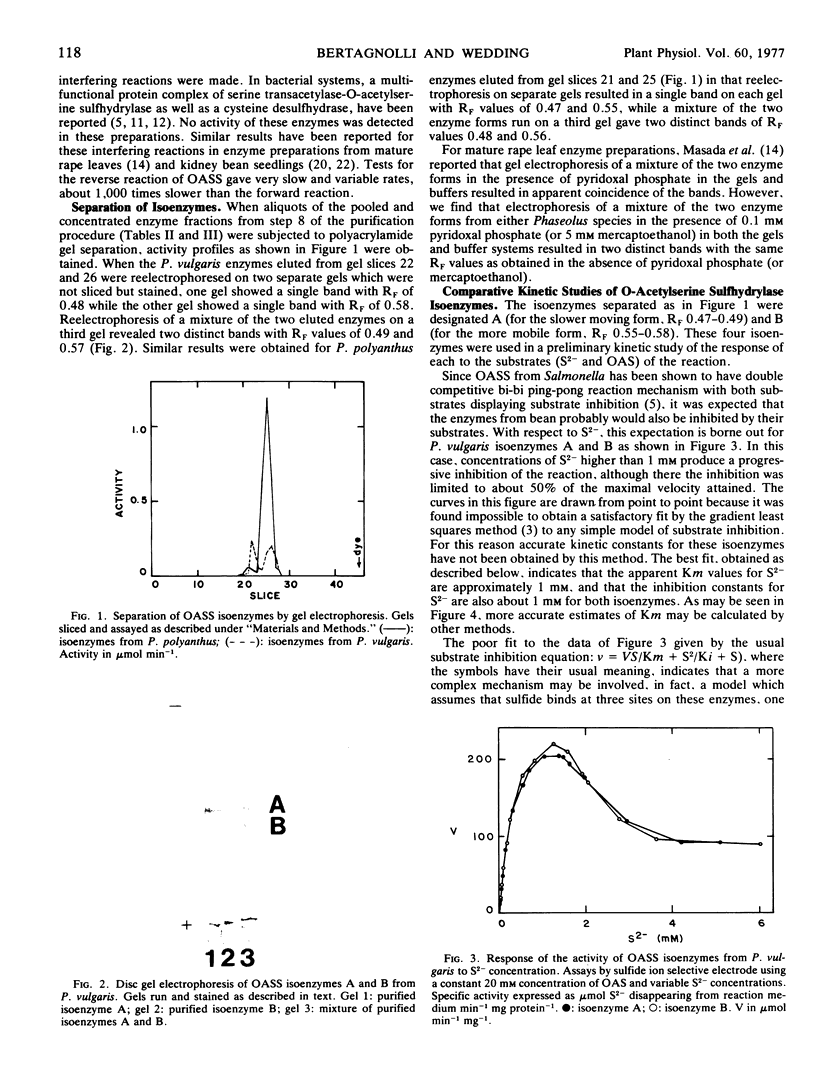
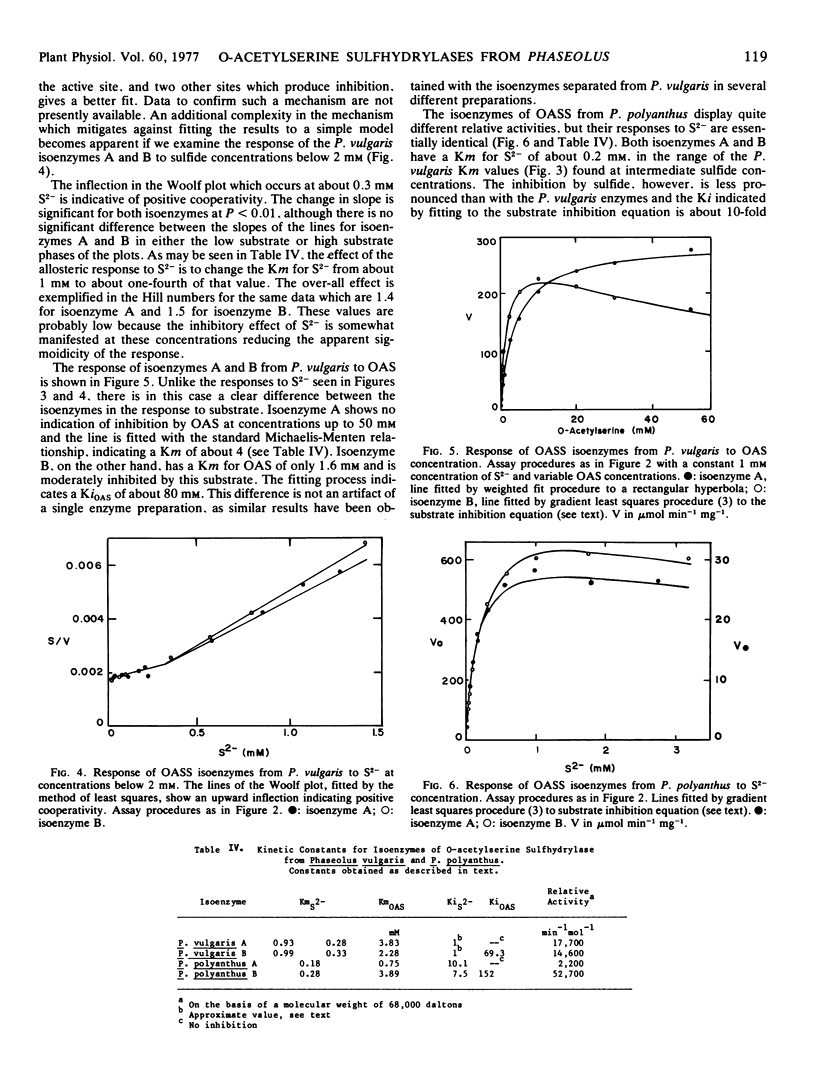

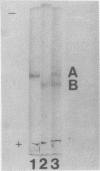
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker M. A., Kredich N. M., Tomkins G. M. The purification and characterization of O-acetylserine sulfhydrylase-A from Salmonella typhimurium. J Biol Chem. 1969 May 10;244(9):2418–2427. [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Cook P. F., Wedding R. T. A reaction mechanism from steady state kinetic studies for O-acetylserine sulfhydrylase from Salmonella typhimurium LT-2. J Biol Chem. 1976 Apr 10;251(7):2023–2029. [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H. Sulfuration of O-acetylhomoserine and O-acetylserine by two enzyme fractions from spinach. Biochem Biophys Res Commun. 1968 Apr 19;31(2):275–280. doi: 10.1016/0006-291x(68)90742-0. [DOI] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H. Synthesis of homocysteine and cysteine by enzyme extracts of spinach. Biochem Biophys Res Commun. 1967 Apr 20;27(2):150–156. doi: 10.1016/s0006-291x(67)80054-8. [DOI] [PubMed] [Google Scholar]
- Granroth B., Sarnesto A. Synthesis of S-substituted cysteine derivatives by the cysteine synthase (O-acetylserine sulfhydrylase) of onion (Allium cepa) and Escherichia coli. Acta Chem Scand B. 1974;28(7):814–814. doi: 10.3891/acta.chem.scand.28b-0814. [DOI] [PubMed] [Google Scholar]
- Hendrickson H. R., Conn E. E. Cyanide metabolism in higher plants. IV. Purification and properties of the beta-cyanolanine synthase of blue lupine. J Biol Chem. 1969 May 25;244(10):2632–2640. [PubMed] [Google Scholar]
- Kredich N. M., Becker M. A., Tomkins G. M. Purification and characterization of cysteine synthetase, a bifunctional protein complex, from Salmonella typhimurium. J Biol Chem. 1969 May 10;244(9):2428–2439. [PubMed] [Google Scholar]
- Kredich N. M., Tomkins G. M. The enzymic synthesis of L-cysteine in Escherichia coli and Salmonella typhimurium. J Biol Chem. 1966 Nov 10;241(21):4955–4965. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Masada M., Fukushima K., Tamura G. Cysteine synthase from rape leaves. J Biochem. 1975 May;77(5):1107–1115. doi: 10.1093/oxfordjournals.jbchem.a130811. [DOI] [PubMed] [Google Scholar]
- Ngo T. T., Shargool P. D. The enzymatic synthesis of L-cysteine in higher plant tissues. Can J Biochem. 1974 Jun;52(6):435–440. doi: 10.1139/o74-066. [DOI] [PubMed] [Google Scholar]
- Ngo T. T., Shargool P. D. The formation of sulphur-containing amino acids in germinating seeds of rape (Brassica napus L.). Biochem J. 1972 Feb;126(4):985–991. doi: 10.1042/bj1260985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. K. Studies of l-Cysteine Biosynthetic Enzymes in Phaseolus vulgaris L. Plant Physiol. 1972 Oct;50(4):477–479. doi: 10.1104/pp.50.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. K., Thompson J. F. Purification and characterization of L-serine transacetylase and O-acetyl-L-serine sulfhydrylase from kidney bean seedlings (Phaseolus vulgaris). Biochim Biophys Acta. 1971 Feb 10;227(2):288–295. doi: 10.1016/0005-2744(71)90061-1. [DOI] [PubMed] [Google Scholar]
- Smith I. K., Thompson J. F. The synthesis of O-acetylserine by extracts prepared from higher plants. Biochem Biophys Res Commun. 1969 Jun 27;35(6):939–945. doi: 10.1016/0006-291x(69)90715-3. [DOI] [PubMed] [Google Scholar]
- That-Tjien-Ngo, Shargool P. D. The use of a sulfide ion selective electrode to study O-acetylserine sulfhydrylase from germinating rapeseed. Anal Biochem. 1973 Jul;54(1):247–261. doi: 10.1016/0003-2697(73)90269-8. [DOI] [PubMed] [Google Scholar]