Abstract
Protein misfolding disorders (PMDs) refer to a group of diseases related to the misfolding of particular proteins that aggregate and deposit in the cells and tissues of humans and other mammals. The mechanisms that trigger protein misfolding and aggregation are still not fully understood. Increasing experimental evidence indicates that abnormal interactions between PMD-related proteins and nucleic acids (NAs) can induce conformational changes. Here, we discuss these protein–NA interactions and address the role of deoxyribonucleic (DNA) and ribonucleic (RNA) acid molecules in the conformational conversion of different proteins that aggregate in PMDs, such as Alzheimer’s, Parkinson’s, and prion diseases. Studies on the affinity, stability, and specificity of proteins involved in neurodegenerative diseases and NAs are specifically addressed. A landscape of reciprocal effects resulting from the binding of prion proteins, amyloid-β peptides, tau proteins, huntingtin, and α-synuclein are presented here to clarify the possible role of NAs, not only as encoders of genetic information but also in triggering PMDs.
Keywords: Protein aggregation, Protein misfolding, Protein–nucleic acid interaction, Degenerative diseases, Conformational conversion
Introduction
Protein folding and misfolding are critical processes responsible for regulating either a protein’s biological activity or its cellular location. A great number of biologically active proteins must form oligomers to become active. Structural proteins such as actin and tubulin form sophisticated supramolecular complexes that execute important physiological roles. The formation of these oligomers and functional supramolecular complexes is tightly controlled and important for many cellular functions. On the other hand, misfolded proteins can oligomerize to an uncontrolled and undesired form and are considered potential driving forces in the development of many human diseases.
When several naturally expressed proteins in vivo are incapable of folding correctly into their native structures, they can cause typically age-related diseases termed protein misfolding disorders (PMDs), which include Alzheimer’s (AD), Parkinson’s (PD), and prion diseases, or transmissible spongiform encephalopathies (TSEs) (reviewed in Chiti and Dobson 2006). The common pathogenic hallmark of each of these disorders is the misfolding and aggregation of a particular protein, i.e., the β-amyloid peptide (Aβ) for AD, α-synuclein (α-syn) for PD, and prion protein (PrP) for TSEs (Table 1).
Table 1.
Proteins and neurodegenerative diseases
| Protein | Aggregation site | Associated disease | Fibril characteristic | Acquired or hereditary |
|---|---|---|---|---|
| PrP | Central nervous system, extracellular | Transmissible spongiform encephalopathies | Amyloid, amorphous aggregate, organized aggregates | A/H |
| Aβ | Central nervous system, extracellular | Alzheimer’s disease | Amyloid | A |
| α-syn | Neurons, intracytoplasmic | Parkinson’s disease | Amyloid | A/H |
| Tau | Neurons, intracytoplasmic | Alzheimer’s diseases and other cerebral conditions | Amyloid | A |
| Huntingtin | Neurons, intranuclear | Huntington’s Disease | Amyloid | H |
Adapted from Sipe et al. (2012)
Protein aggregation can proceed in a well-organized fashion to form amyloid fibrils, which are different than amorphous protein aggregates (Fig. 1). The Nomenclature Committee of the International Society of Amyloidosis (ISA) defines amyloid fibrils as proteins that are deposited in human/animal tissues and bind Congo red with high affinity, resulting in green birefringence under polarized light (Sipe et al. 2012). Although amyloid fibrils are a hallmark of the neurodegenerative diseases discussed in this review, less organized or amorphous aggregates can also be associated with PMDs (Ecroyd and Carver 2008). In general, both amyloid and amorphous aggregates are more stable and less hydrated than native, intermediate, and unfolded states (Fig. 1) (Silva et al. 2010a).
Fig. 1.
Free energy and hydration landscape of the protein-folding funnel. Unfolded proteins are highly flexible and hydrated. As the proteins begin to fold, more ordered intermediates that are less hydrated start to populate more stable parts of the funnel. In some cases, these intermediates can escape from the native folding pathway into metastable conformations, leading to the formation of aggregated species (ordered or amyloid) that are generally less hydrated. Modified from Silva et al. (2010a)
The mechanisms that drive a biologically active and soluble protein to adopt an alternative structure with high potential for aggregation depend on the different intermediates formed during the folding process, the energy states of these intermediates, the energy barriers separating these states, and the hydrophobic surface exposed to an aqueous medium (Silva et al. 2010a). The aging process reduces protein quality control mechanisms in some tissues, and these tissues gradually lose their capacity to prevent the accumulation of abnormally formed proteins (Dobson 2001; Bonini 2002; Brignull et al. 2007). This phenomenon might explain the high incidence of PMDs among the elderly. Mutations or abnormal post-translational modifications, as well as exposure to environmental variations or external agents, could also trigger protein misfolding and aggregation (Uversky 2010). Exposure to external agents could facilitate protein interactions with molecules that are not their native partners, such as nucleic acids (NAs), other proteins, lipids, glycosaminoglycans, or even metallic ions; all these molecules have been previously shown to trigger changes in protein conformation leading to aggregation (Atwood et al. 1998; Uversky et al. 2001; Silva et al. 2010b; Liu and Zhang 2011; Ma 2012).
For the last 20 years, nucleic acids (NAs) have been described as possible cofactors that bind amyloidogenic-prone proteins and facilitate the aggregation process (Cordeiro et al. 2001; Deleault et al. 2003; Yin et al. 2009; Jiménez 2010; Di Domizio et al. 2012). Protein interactions with NAs are based on a series of molecular contacts, including hydrogen bonding mediated by water molecules, nonpolar contacts, and hydrophobic interactions. Although some amino acid residues interact preferentially with NAs (Luscombe et al. 2001), the various structural motifs in proteins and NAs, as well as nucleotide variations, make it difficult to establish a single model for protein–NA interactions (reviewed in Rohs et al. 2010). As discussed, a number of research groups have reported nonnative interactions between proteins and NAs in the past few years. These interactions cause, in many cases, protein misfolding and aggregation in vitro and are related to early degenerative disease events that are not completely understood (Nandi and Leclerc 1999; Cordeiro et al. 2001; Deleault et al. 2003; Gomes et al. 2008a; Di Domizio et al. 2012; Camero et al. 2013a). Interestingly, NAs can prevent protein aggregation in some cases and have also been used as tools for developing diagnostic strategies (Cordeiro et al. 2001; Keefe et al. 2010). Prion-like aggregation of mutants of tumor suppressor p53, a DNA-binding protein, has also been implicated in the pathophysiology of cancer (Ishimaru et al. 2003; Ano Bom et al. 2012; Silva et al. 2013). Interestingly, binding of cognate-DNA prevents aggregation of wild-type p53 (Ishimaru et al. 2009).
In this review, we will focus on the abnormal interactions of PMD-linked proteins (Table 1) with nucleic acid molecules that do not participate as coding agents but as cofactors in conformational conversion reactions. Specifically, abnormal interactions with NAs will be discussed for prion proteins, Aβ, tau, huntingtin (htt), and α-syn. Different biophysical characteristics of protein–ribonucleic acids and protein–deoxyribonucleic acids will also be addressed.
Interactions of proteins involved in PMDs with NAs: misfolding, aggregation and therapeutic strategies
Proteins involved in PMDs, such as Aβ and tau protein (in AD), α-syn (in PD), PrPs (in TSEs), or even superoxide dismutase (in amyotrophic lateral sclerosis), form amyloid and other organized aggregates rich in β-sheet secondary structures (Ross and Poirier 2004; Sipe et al. 2012). Interestingly, almost all of these proteins have been shown to interact with nucleic acids (reviewed in Silva et al. 2008; Yin et al. 2009; Jiménez 2010); this interaction may modulate misfolding and aggregation, alter transcription patterns and result in toxic effects (Jiménez 2010; Silva et al. 2010b). Thus, many groups have hypothesized that some neurodegenerative diseases develop partly as the result of an aberrant interaction between a disease-related protein and a NA (Yin et al. 2009; Jiménez 2010; Silva et al. 2008; Silva et al. 2010b). An interesting report showed that interaction of a bacterial protein with specific DNA sequences promoted protein assembly into amyloid fibrils (Giraldo 2007), indicating a general mechanism for DNA-induced protein aggregation.
Protein–DNA complex formation occurs mainly through hydrogen bonding between specific amino acid side chains and nucleic acid bases. Nevertheless, both electrostatic and van der Waals interactions between a number of amino acid residues and the DNA phosphate and deoxyribose groups bring the two molecules together to form an interaction. DNA-binding proteins primarily combine specific and non-specific interactions (Luscombe et al. 2001), and proteins can bind DNA duplexes (double-stranded, dsDNA) either at the major or the minor grooves. Specific protein–DNA interactions depend not only on the nucleotide sequence of the DNA binding site but also on the particular three-dimensional structure of both the protein and DNA (Rohs et al. 2010). Therefore, a mixture of direct and indirect (i.e., through water molecules) interactions is expected for most DNA-binding proteins.
RNAs are more structurally diverse than DNA, although the latter can also be found in different conformations, such as hairpins, quadruplexes, and i-motifs (Fig. 2) (Chastain and Tinoco 1991). RNAs are flexible molecules, and this structural property allows them to perform different functions in the cell (Dinger et al. 2008) and to interact with different partners. During the last decade, a number of RNA molecules unable to encode proteins (ncRNAs) was identified, such as microRNAs and other small RNAs. Recent evidence suggests a variety of roles for these molecules in eukaryotic cells, suggesting that ncRNAs have specific and important cellular functions (Costa 2007). It is not surprising that these molecules interact with amyloid proteins and participate in protein misfolding and aggregation (Deleault et al. 2003; Gomes et al. 2008b; Yin et al. 2009). However, the amount of information regarding RNA interactions with proteins responsible for PMDs is scarce. Most reported interactions between amyloidogenic proteins and NAs involve DNA molecules, which are also discussed in this review.
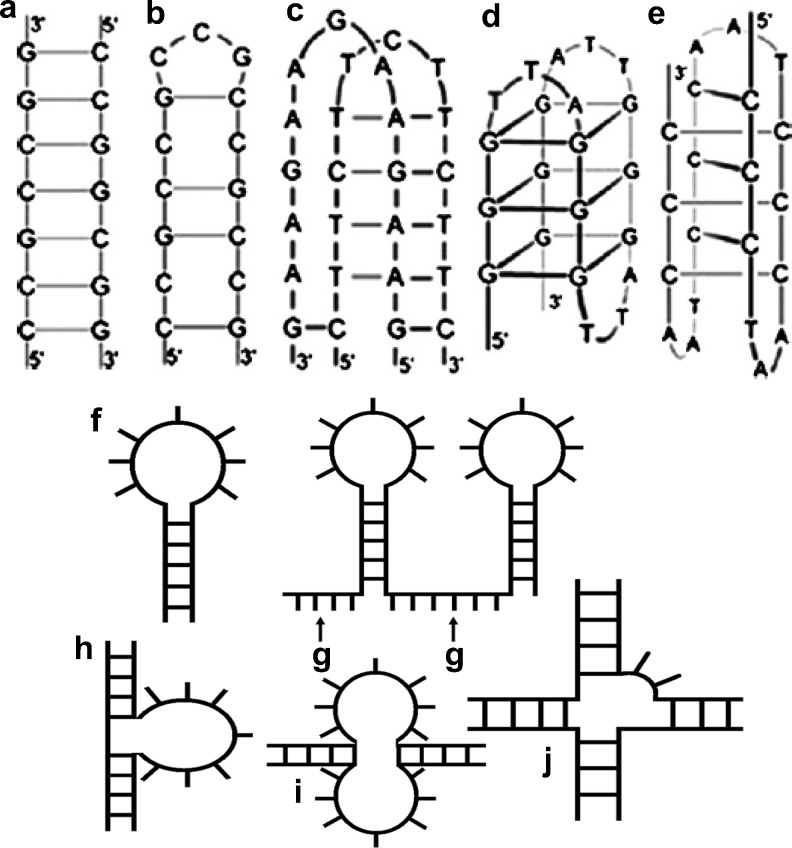
Fig. 2.
Nucleic acid secondary structure motifs. Top Types of DNA structures. a duplex DNA (antiparallel, intermolecular); b hairpin (antiparallel, intramolecular); c triplex (parallel, intermolecular); d G-quadruplex (antiparallel, intramolecular); e i-motif (intramolecular). Adapted with permission (license number: 3197650337266) from Jaumot et al. (2009). Bottom RNA secondary structure elements: f hairpin; g single stranded regions; h bulge loop; i internal loop; j multi-branched loop or junction. Modified with permission (license number: 3197650632969) from Chastain and Tinoco (1991)
It is still debatable whether NA interactions with proteins related to PMDs are specific for a particular sequence, size, or NA conformation. Different effects on protein aggregation, aggregate morphology, and cytotoxicity in the formed aggregated species have been reported, depending on the binding partners in question (for both protein and NAs) (Hegde et al. 2003; Bera and Nandi 2007; Hegde and Rao 2007; Maloney and Lahiri 2011; Vasudevaraju et al. 2012; Macedo et al. 2012). Experimental evidence is converging to the hypothesis that the NA conformation is indeed crucial for binding PMD-related proteins, resulting in reciprocal conformational changes (Macedo et al. 2012; Cavaliere et al. 2013). Although most work has been carried out with DNA duplexes adopting the classical B-DNA conformation, both hairpins and G-quadruplexes (Fig. 2) have been shown to interact with PrP (Mashima et al. 2009, 2013; Cavaliere et al. 2013), and both tau and α-syn interact with conformation-specific GC-rich DNA (Vasudevaraju et al. 2012).
Prion protein (PrP)
Transmissible spongiform encephalopathies (TSEs) form a group of rare neurodegenerative and fatal conformational diseases that affect humans and other mammals (Prusiner 1998). Clinical manifestations of TSEs in humans include dementia, ataxia, insomnia, involuntary muscle contraction (myoclonus), and altered behavior (Prusiner 1998). All TSEs have been assigned to the same infectious agent with an abnormal conformation derived from a constituent protein, namely, the prion protein (PrP). TSEs, or prion diseases, are associated with an isoform of the innocuous cellular form of PrP (PrPC), which is known as the scrapie PrP (PrPSc).
Mature PrPC is composed of 209 amino acid residues, contains two glycosylation sites, and is normally attached to the outer portion of the plasma membrane by a GPI anchor (Prusiner 1998). The first NMR solution structure of PrP was determined by Riek et al. (1996). A comparative analysis of various PrP structures from different organisms demonstrates that all PrPs have a similar three-dimensional structure made of two structurally distinct domains, a flexible N-terminal region and a globular C-terminal domain that contains three α-helices and a small antiparallel β-sheet. A sulfur bridge connects the two cysteine residues located in the second and third helices (reviewed in Gomes et al. 2012).
In contrast to PrPC, PrPSc is an insoluble protein, and it is partially resistant to proteolysis (therefore also named PrPRes), and has a tendency to aggregate and form amorphous aggregates and/or amyloid-like structures (Prusiner 1998). Changes in secondary structure appear to be the fundamental basis for the biochemical differences between PrPC and PrPSc; however, spontaneous conversion between the two isoforms is prevented by a high energetic barrier (Cohen and Prusiner 1998; Cordeiro and Silva 2005).
PrP can interact with both DNA and RNA molecules, and most studies were carried out in vitro with recombinant PrP (rPrP). This protein is perhaps the richest model for addressing interactions between NA and amyloidogenic proteins. A great challenge to the prion field is to understand the PrPC to PrPSc conversion reaction. In this context, our group proposed that NA molecules act as catalysts, lowering the energy barrier between these two PrP conformations (Cordeiro and Silva 2005) (Fig. 3).
Fig. 3.
PrPC➪PrPSc conversion energy diagram. Misfolding of PrPC (circle) into PrPSc (star) seems to be the central event in TSEs. The two PrP forms can be recognized by the dramatic changes in their secondary structure content; PrPC is mostly α-helical whereas PrPSc has a higher β-sheet content. They are separated by a large energetic barrier that is associated with PrP unfolding/refolding. I and U represent the intermediate and unfolded PrP states, respectively. The existence of this high-energy barrier raises the possibility of another biomolecule’s (such as NAs) involvement in this conversion reaction, which would act as an adjuvant factor by lowering the energy barrier between PrPC and PrPSc. Adapted from Silva et al. (2010b)
Spectroscopic studies from other groups, including ours, showed that DNA molecules bind both PrP isolated domains and the recombinant full-length PrP (rPrP) to induce the formation of amorphous aggregates and amyloid fibrils (Nandi and Leclerc 1999; Cordeiro et al. 2001; Lima et al. 2006; Macedo et al. 2012).
The first description of a DNA–PrP interaction revealed binding of the neurotoxic PrP peptide (PrP106–126) to a fluorescently labeled linearized plasmidial dsDNA derived from bovine papilloma virus, as reported by Nandi (1997). Subsequent publications, also based on spectroscopic techniques, have presented data on the formation of amyloid structures by PrP106–126 and the full-length rPrP induced by plasmids and synthetic dsDNA interactions (Nandi 1998; Nandi and Leclerc 1999).
Our group was the first to show that DNA molecules have a dual effect on PrP conformation and aggregation. Light scattering, intrinsic fluorescence, and circular dichroism spectroscopy experiments revealed that dsDNA sequences (both small oligonucleotides, from 18 to 36 bp, to plasmids) were able to completely inhibit aggregation of hydrophobic PrP domains (the domains comprise PrP residues 109–149 and 109–141) (Cordeiro et al. 2001). In contrast to PrP hydrophobic domains, DNA binding to full-length rPrP induced aggregation and conversion into a β-sheet-rich species. We have therefore proposed that DNA is directly involved in PrP structural conversion, modulating the equilibrium between PrPC and PrPSc by reducing protein mobility and facilitating protein–protein interactions (Cordeiro et al. 2001; Cordeiro and Silva 2005).
In a subsequent study, the rPrP:DNA complex was characterized using different rPrP constructions and a 21-bp DNA sequence. Nuclear magnetic resonance (NMR) and small angle x-ray spectroscopy (SAXS) measurements indicated that the globular C-terminal domain of rPrP directly binds to DNA, which was also observed by other groups. Nandi et al. (2002) observed that 950-bp dsDNAs were able to induce partial unfolding and aggregation into amyloid species in a PrP construction containing residues 121–231; King et al. (2007) identified single-stranded DNA thioaptamers (thiophosphate modified aptamers) capable of binding Syrian hamster PrP domain 90–231. However, DNA-binding by rPrP also caused conformational changes in the N-terminal domain (Lima et al. 2006).
More recently, we showed that different small dsDNA sequences bind to rPrP, and the resulting rPrP:DNA complexes aggregate into supramolecular structures that lack the classical amyloid fibril morphology (Macedo et al. 2012). The GC content of the DNA molecule seems to be important for binding, affinity, stability, aggregation patterns and toxic species generation. Various dsDNA sequences were able to induce different changes in rPrP tertiary structure, as observed by a fluorescence-quenching assay (Macedo et al. 2012).
Aside from its base content, the DNA secondary structure also seems to contribute to PrP–DNA complex formation, as well as the physical and chemical characteristics of the complex. quadruplex NA structures (Fig. 2) in both DNA and RNA are able to recognize and bind to different PrP forms with high affinity (at the nanomolar range) (Cavaliere et al. 2013; Mashima et al. 2013). This interaction induces a loss of secondary structure content in both PrP and the NA molecule (Gomes et al. 2008a; Macedo et al. 2012; Cavaliere et al. 2013), indicating that there is a reciprocal conformational change upon PrP binding to NA.
Interactions between PrP and RNA have been frequently reported in the past few years. RNA molecules can trigger PrP aggregation and conversion into a proteinase K (PK)-resistant isoform (PrPres) in vitro, depending on the RNA source (Adler et al. 2003; Deleault et al. 2003; Liu et al. 2007; Gomes et al. 2008a). Single-stranded RNA (ssRNA) molecules isolated from hamster brains were reported as necessary to produce PrPres (the misfolded form of PrP resistant to PK digestion) in a cell-free conversion assay in the presence of scrapie brain homogenates used as seed (Deleault et al. 2003). Other groups have reported that highly structured RNA molecules could participate in prion conversion in vitro, generating PK-resistant species (Adler et al. 2003).
Using a spectroscopic approach, we demonstrated that murine rPrP aggregates in vitro when incubated with RNA molecules. This interaction depends on the RNA source (bacteria, yeast, and mammalian cells) and can trigger aggregation with different intensities as observed by light scattering measurements (Gomes et al. 2008a). The efficiency of rPrP conversion induced by different sources of RNA was also observed using the PMCA technique (Deleault et al. 2003).
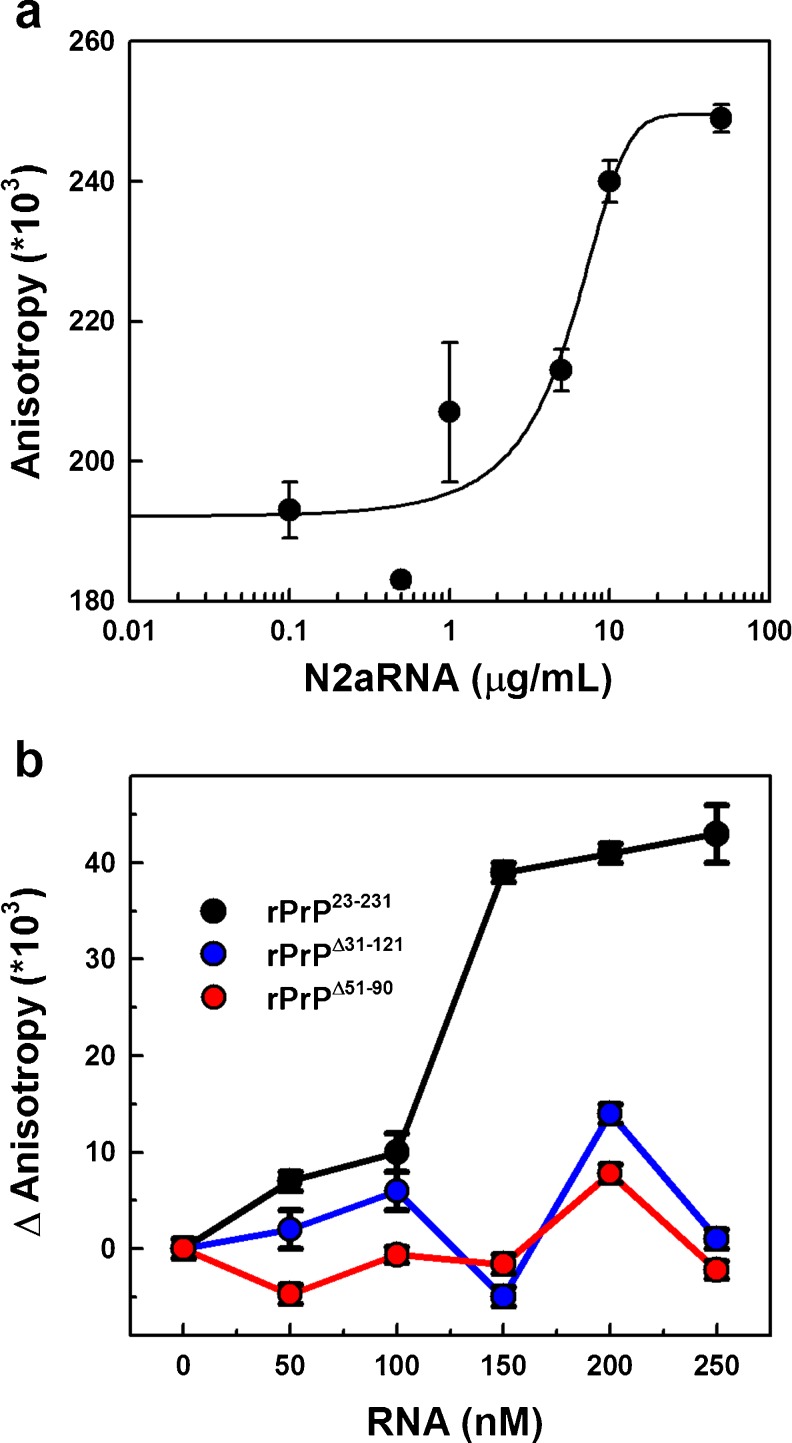
RNA interactions can also change PrP morphology as observed by transmission electron microscopy (TEM) (Gomes et al. 2008a). Aggregated PrP species were induced by incubation with RNA. The ordered aggregates formed do not present classical amyloid fibril characteristics. Changes in both the RNA and PrP secondary structure were observed by circular dichroism (Gomes et al. 2008a). Fluorescence anisotropy experiments suggested direct RNA binding by PrP, mediated by the N-terminal flexible region, most likely between residues 51 and 90 (Fig. 4) (Gomes et al. 2008a). NMR spectroscopy measurements suggested that the N-terminal region of PrP (which is highly disordered and essential to RNA binding) loses its flexibility in the presence of RNA.
Fig. 4.
RNA binding to rPrP as observed by fluorescence anisotropy. a N2aRNA binds rPrP23–231 with high affinity. N2aRNA was titrated into a 1.15 μg/ml (50 nM) FITC-rPrP23–231 solution. b The amino-terminal region is essential to RNA binding with rPrP. opRNA was titrated into 50 nM FITC-labeled rPrP23–231 (black), rPrPΔ51–90 (red), or rPrPΔ32–121 (blue). Excitation was set at 490 nm in (a) and (b). Modified from Gomes et al. (2008a)
Another interesting observation is that the aggregated form protects both rPrP and RNA from PK and RNase A digestion, respectively (Gomes et al. 2008a). Finally, rPrP–RNA aggregates are highly toxic to cultured neuroblastoma (N2a) cells when the RNA extracted from N2a cells is used to produce the aggregates. RNA or rPrP alone were not able to induce toxicity (Gomes et al. 2008a). Other groups have also observed toxic species formation resulting from PrP–RNA interactions. Liu et al. (2007) characterized an ovine rPrP–RNA complex by circular dichroism (CD) spectroscopy, revealing high β-sheet content, and the same group reported the neurotoxicity of this complex (Liu et al. 2011).
As observed with DNA, RNA binding to PrP also alters both protein and RNA secondary structures (Gomes et al. 2008a; Cavaliere et al. 2013), but a specific binding sequence for this interaction has not been revealed to date, suggesting a non-specific affinity for RNA, which is characteristic of an RNA chaperone (Gomes et al. 2012). It has also been reported that cytoplasmic PrP can induce the formation of a large ribonucleoprotein particle that could be involved in posttranscriptional gene regulation (Beaudoin et al. 2009).
Some groups have reported that RNA aptamers (nucleic acids isolated in vitro for their ability to bind a molecule of interest) selected against specific PrP isoforms have diagnostic and therapeutic potential (Rhie et al. 2003; Sayer et al. 2004). Recently, NMR measurements showed that a 12-mer RNA aptamer forms a quadruplex structure capable of binding PrP peptides from the PrP N-terminal region and blocks PrP structural conversion (Mashima et al. 2009, 2013) (Fig. 5). Interestingly, binding of low molecular weight heparin (LMWHep) to recombinant murine PrP does not lead to PrP conversion and prevents aggregation induced by RNA (Vieira et al. 2011).
Fig. 5.
Structural information on the interaction between bovine PrP and an RNA aptamer. Overall organization of the complex between bovine PrP with a 12-mer RNA aptamer (R12) from NMR data obtained with N-terminal PrP peptides (P1 residues 25–35; P16 residues 108–119). PrP Lys and R12 guanosine residues involved in electrostatic interactions are shown in orange. Trp and guanosine residues involved in stacking interactions are shown in blue. The C-terminal domain of bovine PrP was drawn from PBD file 1DX0. Figure reproduced with permission (license number: 3197641505803) from Mashima et al. (2013)
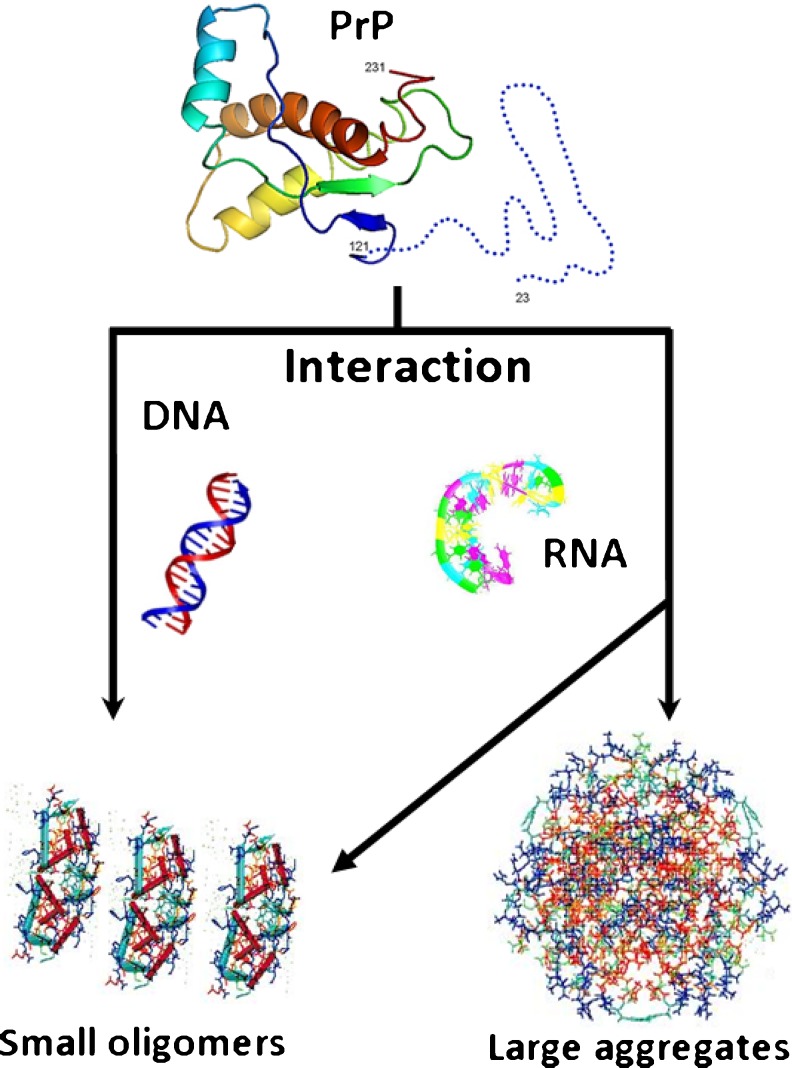
Based on the data discussed above, we propose that NAs are likely to be involved in the pathology of prion diseases. PrP binds directly to DNA and RNA, and this interaction induces PrP oligomerization/aggregation. Spectroscopic studies indicate that these interactions are different for each kind of NA: DNA-binding mediated mainly by the C-terminal globular domain produces small oligomers; and RNA-binding mediated by the unstructured N-terminal domain can produce both small oligomers and large aggregates (Fig. 6) (Gomes et al. 2008b). The NA-binding capacity of PrP can also be used as a tool to investigate the misfolding/aggregation process and to develop diagnostic methodologies and therapies.
Fig. 6.
Schematic representation of NA effects on rPrP misfolding/aggregation. Interaction with DNA molecules (left) leads to the formation of small toxic oligomers; interaction with RNA molecules (right) can generate large toxic aggregates or small non-toxic oligomers depending on the RNA source. Modified from Gomes et al. (2008b)
Aβ peptide
Alzheimer’s disease (AD) is the most studied PMD because of its dramatically increased prevalence in the elderly. AD is the sixth-leading cause of death in the United States, and it is estimated that one in eight older Americans has Alzheimer’s disease, which is invariably fatal (Alzheimer’s Association, 2012). Post-mortem analysis of the brain of diseased patients has revealed two different types of aberrant protein aggregates, namely the extracellular senile plaques, mainly composed of aggregated Aβ peptide, and the intracellular neurofibrillar tangles of the tau protein (Grundke-Iqbal et al. 1986; Selkoe 1997). Aβ peptide is found in two different forms comprised of either 40 (Aβ1-40) or 42 (Aβ1-42) amino acid residues. These peptides result from the cleavage of the integral membrane protein amyloid-beta precursor protein (APP), which is expressed in different tissues but is concentrated in neuronal synapses (Citron et al. 1996). Aβ1-40 is the most abundant peptide in amyloid plaques, but it has a slower aggregation rate than Aβ1-42 (Harper and Lansbury 1997). Tau protein is a microtubule-stabilizing protein mostly expressed in neurons, and it participates in tubulin assembly and cytoskeleton formation (Weingarten et al. 1975; Binder et al. 1985). Either extracellular deposition of Aβ or intracellular accumulation of tau protein can lead to synaptic dysfunction, neuronal death, and clinical dementia (Cummings 2004). However, genetic and biochemical evidence indicates that Aβ production is the central event in AD pathogenesis. Although still under discussion, it is becoming increasingly evident that the soluble Aβ oligomers are the main AD toxic species (Lambert et al. 1998; Bayer et al. 2001; De Felice et al. 2004). These oligomeric species might diffuse easily through the brain parenchyma and alter synaptic structure and function, ultimately leading to neuronal death (Haass and Selkoe 2007).
Both nuclear and cytoplasmic localization of the Aβ peptide were reported by Johnstone et al. (1995). This finding might explain the occurrence of Aβ-dependent toxicity before significant extracellular deposition of aggregated peptides. Some reports have identified the presence of Aβ aggregates in different cellular compartments, including the nucleus (Buckig et al. 2002; Hegde et al. 2003; Ohyagi et al. 2005). The earliest observation that short nucleotides bind to senile plaques and cerebral vessels in AD was reported in 1991. The authors suggested that Aβ could bind extra chromosomal oligonucleotides in continuous cell destruction situations (Syrjanen et al. 1991). In fact, Aβ peptides can interact with DNA in vitro, as demonstrated by electrophoretic mobility shift assay (EMSA) (Ahn et al. 2000), and ex vivo, in which soluble Aβ aggregates were found in the nucleus of AD brain samples (Yu et al. 2007; Barrantes et al. 2007). In an interesting study, Camero et al. (2013b) used surface plasmon resonance (SPR) studies to show that amyloid fibrils formed by Aβ(25-35), a highly cytotoxic amyloid peptide, and Aβ(1-40) strongly interact with DNA (similar to DNA-histones interactions), in contrast to other non-amyloidogenic aggregated proteins, such as albumin, myoglobin, casein, and β-lactoglobulin (Camero et al. 2013b). Additionally, a scrambled sequence of Aβ(25-35) abolished protein susceptibility to aggregation, neurotoxicity, and DNA-binding (Barrantes et al. 2012). One must conclude that a tendency to aggregate, which is inherent in the primary sequence of amyloid peptides, is crucial for DNA binding and toxicity. Moreover, these amyloid structures display greater affinity for DNA molecules than for other polyanions, such as heparin and polyglutamic acid (Camero et al. 2013a). Similar results were also described for the Aβ1-42 form (Barrantes et al. 2007). Because Aβ monomers are negatively charged at neutral pH, the driving force for Aβ binding to DNA cannot be explained by non-specific electrostatic interactions with the DNA phosphate backbone but rather by particular DNA characteristics, such as the sequence and conformation. This idea has already been underlined by two recent reports demonstrating that amyloid–DNA interactions were dependent on the DNA sequence (Yu et al. 2007; Maloney and Lahiri 2011). The DNA conformation changed in the presence of Aβ, and Aβ induced DNA condensation in a time-dependent manner (Yu et al. 2007). The Aβ–DNA interaction was shown to be dependent on the DNA sequence, as indicated by EMSA (Maloney and Lahiri 2011). A G-rich decamer was proposed as a consensus matrix for Aβ–DNA interactions. The single-base substitution G↔A on the DNA sequence diminishes the peptide-interaction, and most double-stranded oligonucleotides that were tested did not display significant interactions with Aβ as indicated by EMSA (Maloney and Lahiri 2011). It is believed that Aβ initiates the aggregation process when segments of its backbone, particularly its N–H groups and C = O groups, are exposed (Eisenberg and Jucker 2012). This conformational change would allow the peptide to form hydrogen bonds with DNA. Altogether, these results suggest that when Aβ translocates to the nucleus (Johnstone et al. 1996) and cross-talks with DNA, the protein remains inside the nucleus because of its tendency to aggregate. This localization might be an important factor responsible for altering gene expression and regulation, leading to neuronal toxicity that could contribute to the onset of AD.
There are very few data available about the interactions between Aβ and RNA molecules. The first evidence of this event was provided by a Korean group in 2000. They found that both monomers and oligomers formed by Aβ1-40 and Aβ25-35 could shift RNA mobility in agarose gels, suggesting a high affinity interaction. This explanation could be caused by the capacity of a single RNA molecule to interact with more than one Aβ molecule, forming high molecular weight species or inducing Aβ aggregation that would retain the bound RNA (Ahn et al. 2000).
It has also been reported that RNA can inhibit Aβ oligomerization. Using the SELEX (Systematic Evolution of Ligands by Exponential Enrichment) tool to obtain NA aptamers that can bind a broad range of target molecules with high affinities, Takahashi et al. (2009) were able to select two RNA aptamers against Aβ1-40 that blocked Aβ fibrillation. Based on these findings, RNA aptamers could be used as a diagnostic tool to identify Aβ oligomers to establish an early diagnostic strategy or, in a more ambitious goal, to develop a therapy to prevent the formation of toxic Aβ oligomers. In both cases, these proposals require further studies.
Tau protein
In addition to its participation in AD, tau protein is also involved in other neurodegenerative disorders, named tauopathies, in which tau aggregates are present. Tau was identified as a core protein in the neurofibrillary tangles (NFTs), also referred to as the paired helical filament (PHF) of AD, which constitutes the most characteristic cytoskeleton alteration in affected neurons (Grundke-Iqbal et al. 1986). There are six brain tau isoforms that are expressed from a single gene by alternative mRNA splicing, each of which is full-length and hyperphosphorylated (Goedert et al. 2006). This protein was found in the isolated nuclei of human brains and was covalently cross-linked to DNA (Brady et al. 1995; Greenwood and Johnson 1995). In fact, multiple isoforms can bind the nucleolus of cells in the interphase and the nucleolar organizing regions (NORs) of acrocentric chromosomes from cultured human neuronal cells (Thurston et al. 1996). Tau interactions with DNA were investigated using EMSA. Tau was found to bind to dsDNA but not to ssDNA (Hua and He 2003). Tau protein was able to improve DNA annealing and prevent dsDNA thermal denaturation, thereby stabilizing the DNA structure (Hua and He 2003). DNA conformational changes induced by tau were also observed by atomic force microscopy (Qu et al. 2004). Tau was recently shown to protect DNA from peroxidation damage, and it binds preferentially to oligonucleotides ≥13 bp in length, with significant affinity-loss for interactions with shorter dsDNAs. The use of specific minor and major groove ligands revealed that tau binds the minor groove of the DNA double helix (Wei et al. 2008). The association seems to occur with no apparent sequence-specificity because previous studies showed that tau binds different DNA sequences of eukaryotic (bovine thymus) or prokaryotic (plasmid) origins (Hua and He 2003). In fact, some DNA-associated proteins, such as the histones H2b and H3, interact with the minor groove of the DNA helix without nucleotide sequence specificity (Adams and Kamakaka 1999; Rohs et al. 2010).
Molecular analysis revealed that tau hyper-phosphorylation might be one of the most important events in the process leading to its aggregation (Buee et al. 2000). In fact, even when phosphorylated by neuronal cdc2-like kinase, tau maintains its capacity to associate with DNA (plasmid or polynucleotide), as shown by EMSA, and increases the melting temperature of calf thymus DNA (Hua and He 2002). However, when tau was aggregated, neither native tau nor phosphorylated tau interacted with DNA (Hua and He 2002). It was recently shown that tau binds with conformation and sequence specificity to polyGC DNA oligonucleotides (CGCGCGCG)2, which form the major component sequences of promoter regions involved in gene expression (Antequera 2003). The interaction again increases DNA thermal stability and alters its structure as revealed by circular dichroism (CD) spectroscopy, where a shift in the positive peak from 280 to 275 nm and a reduced intensity of the negative peaks at 235 and 210 nm characterize an altered B-DNA conformation (Vasudevaraju et al. 2012). The B-DNA form is predominant in normal brains, and altered conformational changes have been reported in the genomic DNA of both moderate and severe AD-affected brains (Suram et al. 2002).
In summary, tau-DNA binding stabilizes the DNA structure and leads to nucleosome rearrangements along chromatin fibers, suggesting that tau carries a DNA chaperone activity that would participate in neuronal cell dysfunction in AD.
Regarding RNA interactions with tau protein, it has been reported that tau aggregation is largely enhanced by RNA molecules in vitro (Kampers et al. 1996). As with PrP (Silva et al. 2008), tau interactions with RNA may lower the energy barrier between non-aggregated and aggregated isoforms (Fig. 3).
α-synuclein
Alpha-synuclein (α-syn) is a small intracellular protein that localizes in the nucleus and presynaptic nerves and is centrally implicated in Parkinson’s disease (PD) (Maroteaux et al. 1988; Goedert 2001). It is estimated that PD afflicts approximately 1 % of Americans older than 60 years of age and 4-5 % of individuals older than 85 years of age (Alkhuja 2013). Although the etiology of PD is not yet fully understood, it can be caused by familial and sporadic factors, with prevalent sporadic forms (∼90 % of PD cases). Genetic information on the familial forms and immunohistochemical analysis led to the identification of α-syn protein deposition. Aggregates of α-syn found in the cytosol of dopaminergic cells constitute the major component of Lewy bodies (cytoplasmic inclusion bodies) and Lewy neurites (dystrophic neurites) associated with the synucleinopathies, a neuropathology of progressive dementia and neuronal loss in the substantia nigra of the brain (Bisaglia et al. 2009). Movement disability and general motor dysfunction are the most common clinical signs of this disease. The correlation between protein inclusion formation and neurotoxicity is still not consistent, as discussed for other PMDs. Biochemical studies and high-resolution microscopic techniques suggest that soluble oligomers may be the primary culprit in PD neurodegeneration (Kalia et al. 2013). Many structural studies have revealed that α-syn has a native unfolded tertiary structure, like tau protein, although it was shown that α-syn may adopt a helical tetrameric form in the brain and red blood cells (Wang et al. 2011; Bartels et al. 2011). Little information about the exact mechanism of its accumulation and fibrillization in the cell is available. The kinetics of α-syn oligomer formation and morphology coincide with those observed in vitro for Aβ oligomers, indicating a common aggregation process (Harper et al. 1997). The physiological role of α-syn is poorly understood, but it may protect neurons from injuries and maintain synaptic function (Kaplan et al. 2003; Chandra et al. 2005), promoting the assembly of the SNARE machinery (Thayanidhi et al. 2010) as well as neurotransmitter release (Bartels et al. 2011). Several factors, such as metals, oxidative stress, protein degradation, and mutations, as well as processes such as phosphorylation, glycation, and DNA-binding, are linked to the α-syn aggregation process (Vasudevaraju et al. 2012).
The structural flexibility of α-syn, as well as other intrinsically unfolded proteins (IUP), increases their binding power to other macromolecules; therefore, many different ligands can modulate α-syn transition to an aggregated state upon binding, which normally accelerates the reaction. Glycosaminoglycans, polyamines, metal cations, fatty acids, anionic detergents, and nucleic acids are the most common examples (Cohlberg et al. 2002; Antony et al. 2003; Necula et al. 2003; Cherny et al. 2004). Nuclear localization of α-syn allows it to interact with histones, forming a stable and tight complex (stoichiometry of 2:1 α-syn to histones) that stimulates α-syn fibrillation in vitro (Goers et al. 2003). The authors observed that the intensity of the effect depends on the nature of the polymer, its length, and concentration. In fact, Cherny et al. (2004) first reported α-syn interactions with another chromatin component, dsDNA (linear or supercoiled). DNA association with either wild-type α-syn or two disease-related mutants (A30P and A53T) stimulate α-syn assembly into mature fibrils. Electron microscopy and electrophoresis clearly demonstrate α-syn–DNA complex formation, protecting DNA from endonuclease digestion (Cherny et al. 2004). Similarly, it was shown that different DNAs induced different conformational changes in α-syn (Hegde and Rao 2007). Circular ssDNA induced gain in alpha helices for α-syn and greatly delayed its fibrillation, whereas smaller oligonucleotides (8-mer) and linear dsDNA, both rich in GC content, induced partial α-syn folding, which then becomes prone to aggregation. In contrast, polyAT dsDNA could bind α-syn but did not cause conformational transition or modulate protein aggregation, thereby indicating a particular DNA-binding effect on the α-syn fibrillation pathway (Hegde and Rao 2007).
From the above considerations, we can conclude that nuclear-translocated α-syn can interact with histone-free, transcriptionally active DNA segments and, hence, may alter gene expression regulation. Moreover, this interaction may modulate α-syn folding with relevant implications for its biological function and disease progression.
Huntingtin
Huntington’s disease (HD) belongs to a family of neurodegenerative hereditary diseases caused by mutations that expand a CAG repeat tract in DNA, leading to abnormal repetitions of glutamine residues (polyQ) in the encoded protein (Sugars and Rubinsztein 2003); thus, HD is also known as a polyglutamine disease. HD is caused by a CAG tract with more than 35 repeats in exon 1 of the gene encoding a protein called huntingtin (htt) (The Huntington’s Disease Collaboration Research Group 1993). This disease is characterized by a significant loss of brain mass associated with a decrease in motor ability, dementia and emotional changes, culminating in death approximately 20 years following disease onset (The Huntington’s Disease Collaboration Research Group 1993). Wild-type htt, a protein with an estimated molecular mass of 350 kDa, is mainly localized in the cytoplasm of neurons, although it has been found in other subcellular compartments, including the nucleus (Ho et al. 2001; Kegel et al. 2002). The polyQ expansion in htt influences the localization and frequency of intraneuronal aggregates of mutant htt in vitro and in vivo (Martindale et al. 1998). The primary hypothesis suggests that neurotoxicity arises from the cleavage and accumulation of the N-terminal fragments containing the polyQ tract, but the relevance of this expansion to HD pathogenesis is still under debate (Dyer and McMurray 2001; Sugars and Rubinsztein 2003). In fact, htt cleavage promotes nuclear localization, with the polyQ length influencing the extent of its accumulation (DiFiglia et al. 1997). Although wild-type htt can be found in the nucleus, the polyQ-expanded htt displays a higher frequency of nuclear localization than the non-expanded protein (Dorsman et al. 1999; Kegel et al. 2002). In the nucleus, this mutant might bind several cellular transcription factors and is highly detrimental in vitro and in vivo (Kegel et al. 2002; Schilling et al. 2004; Benn et al. 2005). Kegel et al. (2002) have shown that the polyQ N-terminal fragments of htt can repress transcription only when targeted to DNA by interfering directly in the transcription machinery rather than by sequestering essential transcription components into htt aggregates (Kegel et al. 2002). This evidence is in agreement with Steffan et al. (2000), who suggested that transcriptional repression may occur regardless of aggregate formation. It was also shown that p231HBP/HYPB, a protein that interacts with the N-terminus of htt, is a DNA-binding factor, further suggesting that huntingtin is in close proximity to DNA in situ (Rega et al. 2001). More recently, it was reported that either the wild-type or the mutant htt can interact directly with DNA in the absence of other proteins, and they can differentially alter DNA conformation (Benn et al. 2008). It was also shown that polyQ expansions increase htt–DNA interactions as estimated by DNA immunoprecipitation using htt-specific antibodies. Moreover, many transcription factor activities were affected in response to mutant htt (Benn et al. 2008).
In summary, these findings suggest several roles for htt in the nucleus that can be affected by the polyQ tract. The abnormal interaction of mutant htt with DNA could alter the DNA conformation, leading to a transcriptional deregulation that seems to be the central pathogenic mechanism in HD (reviewed in Luthi-Carter and Cha 2003).
Final considerations
Throughout this review, we discussed the role of protein-nucleic acid interactions for a set of proteins involved in human degenerative diseases. In summary, aberrant nucleic acid-protein interactions may result in protein aggregation and the formation of toxic species depending on the nucleic acid sequence and/or conformation, leading to reciprocal structural changes. The main effects observed upon nucleic acid binding by PrP, tau, Aβ, α-syn, and huntingtin are summarized in Table 2.
Table 2.
Proteins involved in PMDs, and their interactions with RNA and/or DNA molecules
| Protein | DNA | Effect | Refs. | RNA | Effect | Refs. |
|---|---|---|---|---|---|---|
| PrP | + | Aggregation inhibition or induction; conversion into β-sheet-rich species that may be cytotoxic. | Cordeiro et al. 2001; King et al. 2007; Macedo et al. 2012; Cavaliere et al. 2013 | + | Aggregation inhibition or induction; conversion into β-sheet-rich species; generation of cytotoxic species. | Deleault et al. 2003; Gomes et al. 2008a, b; Mashima et al. 2009 |
| tau | + | Binding stabilizes the dsDNA molecule. | Vasudevaraju et al. 2012; Hua and He 2003; Qu et al. 2004 | + | Aggregation is enhanced in vitro by RNA molecules. | Kampers et al. 1996 |
| Aβ | + | Aβ fibrils bind DNA; interactions alter DNA conformation. | Yu et al. 2007; Maloney and Lahiri 2011; Camero et al. 2013b | + | Modulation of peptide aggregation. | Ahn et al. 2000; Takahashi et al. 2009 |
| α-syn | + | Induction of α-syn assembly into mature fibrils; folding of α-syn. | Cherny et al. 2004; Hegde and Rao 2007 | - | Not reported. | |
| Htt | + | Alters DNA conformation. | Benn et al. 2008 | - | Not reported. |
There is a substantial amount of information regarding abnormal interactions of prion proteins, tau, α-syn, and Aβ with DNA molecules. Such interactions lead to increased β-sheet contents for these proteins, and, in some cases, it has been reported that a dual conformational change takes place, which also affects the DNA molecule. It remains to be established whether protein––DNA binding depends on protein translocation to the nucleus or whether exogenous DNA participates in binding and further structural conversion. The specificity of such interactions is still under debate, but recent data support the idea that both DNA and the target protein conformation are relevant for this process. We should also remark that all of these neurodegenerative diseases lead to cell death and the release of nucleic acids, which can cause further protein–nucleic acid interactions and subsequent aggregation, likely perpetuating a prion-like effect.
In the case of RNA molecules, it is evident that protein–RNA interactions frequently occur inside the cell. For most RNA molecules to perform their cellular functions, they must first bind to a protein. RNA secondary structure is usually important for this phenomenon to occur (Nagai 1996). In proteins, there are two main types of domains that are able to recognize RNA molecules; in the first domain, RNA binding occurs through an alpha-helix or loop; in the second, some amino acids involved in a β-sheet structure interact with unpaired RNA bases (Draper and Reynaldo 1999). Therefore, it is expected that the protein that binds the RNA maintains at least part of its secondary structure. It has also been hypothesized that PrP, for instance, is involved in RNA metabolism. PrP can interact with RNA from HIV and form structures similar to those obtained with capsidic retroviral proteins (Gabus et al. 2001). Additionally, PrP is thought to act as an RNA chaperone (Gabus et al. 2001; Ivanyi-Nagy et al. 2005; Silva et al. 2008) because nucleic acid chaperone activity is based on specific and non-specific NA recognition and binding. These proteins play an important role in DNA and RNA processing and are important to RNA translation and transport (Gomes et al. 2012).
Acknowledgments
The laboratory work of Y.C. and J.L.S. was supported by grants from the Conselho Nacional de Desenvolvimento Cientifíco e Tecnológico (CNPq), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Financiadora de Estudos e Projetos (FINEP) of Brazil.
Conflict of interest
Authors Yraima Cordeiro, Bruno Macedo, Jerson L. Silva and Mariana P. B. Gomes declare that they have no conflict of interest.
Human and Animal Studies
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Abbreviations
- AD
Alzheimer’s disease
- α-syn
alpha-synuclein
- HD
Huntington’s disease
- Htt
huntingtin
- PD
Parkinson’s disease
- PrP
prion protein
- PK
proteinase K
- rPrP
recombinant prion protein
- TSE
transmissible spongiform encephalopathy
Footnotes
Special Issue Advances in Biophysics in Latin America
References
- Adams CR, Kamakaka RT. Chromatin assembly: biochemical identities and genetic redundancy. Curr Opin Genet Dev. 1999;9:185–190. doi: 10.1016/S0959-437X(99)80028-8. [DOI] [PubMed] [Google Scholar]
- Adler V, Zeiler B, Kryukov V, Kascsak R, Rubenstein R, Grossman A. Small, highly structured RNAs participate in the conversion of human recombinant PrP(Sen) to PrP(Res) in vitro. J Mol Biol. 2003;332:47–57. doi: 10.1016/s0022-2836(03)00919-7. [DOI] [PubMed] [Google Scholar]
- Ahn BW, Song DU, Jung YD, Chay KO, Chung MA, Yang SY, Shin BA. Detection of beta-amyloid peptide aggregation using DNA electrophoresis. Anal Biochem. 2000;284:401–405. doi: 10.1006/abio.2000.4719. [DOI] [PubMed] [Google Scholar]
- Alkhuja S. Parkinson disease: research update and clinical management. South Med J. 2013;106(5):334. doi: 10.1097/SMJ.0b013e318290f72a. [DOI] [PubMed] [Google Scholar]
- Ano Bom AP, Rangel LP, Costa DC, de Oliveira GA, Sanches D, Braga CA, Gava LM, Ramos CH, Cepeda AO, Stumbo AC, De Moura Gallo CV, Cordeiro Y, Silva JL. Mutant p53 aggregates into prion-like amyloid oligomers and fibrils: implications for cancer. J Biol Chem. 2012;287:28152–28162. doi: 10.1074/jbc.M112.340638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antequera F. Structure, function and evolution of CpG island promoters. Cell Mol Life Sci. 2003;60:1647–1658. doi: 10.1007/s00018-003-3088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony T, Hoyer W, Cherny D, Heim G, Jovin TM, Subramaniam V. Cellular polyamines promote the aggregation of a-synuclein. J Biol Chem. 2003;278:3235–3240. doi: 10.1074/jbc.M208249200. [DOI] [PubMed] [Google Scholar]
- Atwood CS, Moir RD, Huang X, Scarpa RC, Bacarra NM, Romano DM, Hartshorn MA, Tanzi RE, Bush AI. Dramatic aggregation of Alzheimer abeta by Cu(II) is induced by conditions representing physiological acidosis. J Biol Chem. 1998;273:12817–12826. doi: 10.1074/jbc.273.21.12817. [DOI] [PubMed] [Google Scholar]
- Barrantes A, Rejas MT, Benıtez MJ, Jimenez JS. Interaction between Alzheimer’s Aβ1-42 peptide and DNA detected by surface plasmon resonance. J Alzheimers Dis. 2007;12:345–355. doi: 10.3233/jad-2007-12408. [DOI] [PubMed] [Google Scholar]
- Barrantes A, Camero S, Garcia-Lucas A, Navarro PJ, Benitez MJ, Jiménez JS. Alzheimer’s disease amyloid peptides interact with DNA, as proved by surface plasmon resonance. J Curr Alzheimer Res. 2012;9:924–934. doi: 10.2174/156720512803251101. [DOI] [PubMed] [Google Scholar]
- Bartels T, Choi JG, Selkoe DJ. alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer TA, Wirths O, Majtenyi K, Hartmann T, Multhaup G, Beyreuther K, Czech C. Key factors in Alzheimer’s disease: beta-amyloid precursor protein processing, metabolism and intraneuronal transport. Brain Pathol. 2001;11:1–11. doi: 10.1111/j.1750-3639.2001.tb00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin S, Vanderperre B, Grenier C, Tremblay I, Leduc F, Roucou X. A large ribonucleoprotein particle induced by cytoplasmic PrP shares striking similarities with the chromatoid body, an RNA granule predicted to function in posttranscriptional gene regulation. Biochim Biophys Acta. 2009;1793:335–345. doi: 10.1016/j.bbamcr.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Benn CL, Landles C, Li H, Strand AD, Woodman B, Sathasivam K, Li SH, Ghazi-Noori S, Hockly E, Faruque SM, Cha JH, Sharpe PT, Olson JM, Li XJ, Bates GP. Contribution of nuclear and extranuclear polyQ to neurological phenotypes in mouse models of Huntington’s disease. Hum Mol Genet. 2005;14:3065–3078. doi: 10.1093/hmg/ddi340. [DOI] [PubMed] [Google Scholar]
- Benn CL, Sun T, SadriVakili G, McFarland KN, DiRocco DP, Yohrling GJ, Clark TW, Bouzou B, Cha JJ. Huntingtin modulates transcription, occupies gene promoters In Vivo, and binds directly to DNA in a polyglutamine dependent manner. J Neurosci. 2008;28:10720–10733. doi: 10.1523/JNEUROSCI.2126-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bera A, Nandi PK. Biological polyamines inhibit nucleic-acid-induced polymerisation of prion protein. Arch Virol. 2007;152:655–668. doi: 10.1007/s00705-006-0907-8. [DOI] [PubMed] [Google Scholar]
- Binder LI, Frankfurter A, Rebhun LI. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985;101:1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaglia M, Mammi S, Bubacco L. Structural insights on physiological functions and pathological effects of alphasynuclein. FASEB J. 2009;23:329–340. doi: 10.1096/fj.08-119784. [DOI] [PubMed] [Google Scholar]
- Bonini NM. Chaperoning brain degeneration. Proc Natl Acad Sci USA. 2002;99:16407–16411. doi: 10.1073/pnas.152330499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady RM, Zinkowski RP, Binder LI. Presence of tau in isolated nuclei from human brain. Neurobiol Aging. 1995;16:479–486. doi: 10.1016/0197-4580(95)00023-8. [DOI] [PubMed] [Google Scholar]
- Brignull HR, Morley JF, Morimoto RI. The stress of misfolded proteins: C. elegans models for neurodegenerative disease and aging. Adv Exp Med Biol. 2007;594:167–189. doi: 10.1007/978-0-387-39975-1_15. [DOI] [PubMed] [Google Scholar]
- Buckig A, Tikkanen R, Herzog V, Schmitz A. Cytosolic and nuclear aggregation of the amyloid-peptide following its expression in the endoplasmic reticulum. Histochem Cell Biol. 2002;118:353–360. doi: 10.1007/s00418-002-0459-2. [DOI] [PubMed] [Google Scholar]
- Buee L, Bussiere T, Buée-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- Camero S, Benítez MJ, Jiménez JS. Anomalous Protein-DNA Interactions Behind Neurological Disorders. Adv Protein Chem Struct Biol. 2013;91:37–63. doi: 10.1016/B978-0-12-411637-5.00002-0. [DOI] [PubMed] [Google Scholar]
- Camero S, Ayuso JM, Barrantes A, Benítez MJ, Jiménez JS. Specific binding of DNA to aggregated forms of Alzheimer’s disease amyloid peptides. Int J Biol Macromol. 2013;55:201–206. doi: 10.1016/j.ijbiomac.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Cavaliere P, Pagano B, Granata V, Prigent S, Rezaei H, Giancola C, Zagari A. Cross-talk between prion protein and quadruplex-forming nucleic acids: a dynamic complex formation. Nucleic Acids Res. 2013;41:327–339. doi: 10.1093/nar/gks970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Gallardo G, Fernandez-Chacon R, Schlüter OM, Südhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Chastain M, Tinoco I., Jr Structural elements in RNA. Prog Nucleic Acid Res Mol Biol. 1991;41:131–177. doi: 10.1016/S0079-6603(08)60008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherny D, Hoyer W, Subramaniam V, Jovin TM. Double-stranded DNA stimulates the fibrillation of alpha-synuclein in vitro and is associated with the mature fibrils: an electron microscopy study. J Mol Biol. 2004;344:929–938. doi: 10.1016/j.jmb.2004.09.096. [DOI] [PubMed] [Google Scholar]
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- Citron M, Diehl TS, Gordon G, Biere AL, Seubert P, Selkoe DJ. Evidence that the 42- and 40-amino acid forms of amyloid beta protein are generated from the beta-amyloid precursor protein by different protease activities. Proc Natl Acad Sci USA. 1996;93:13170–13175. doi: 10.1073/pnas.93.23.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen FE, Prusiner SB. Pathologic conformations of prion proteins. Annu Rev Biochem. 1998;67:793–819. doi: 10.1146/annurev.biochem.67.1.793. [DOI] [PubMed] [Google Scholar]
- Cohlberg JA, Li J, Uversky VN, Fink AL. Heparin and other glycosaminoglycans stimulate the formation of amyloid fibrils from a-synuclein in vitro. Biochemistry. 2002;41:1502–1511. doi: 10.1021/bi011711s. [DOI] [PubMed] [Google Scholar]
- Cordeiro Y, Silva JL. The hypothesis of the catalytic action of nucleic acid on the conversion of prion protein. Protein Pept Lett. 2005;12:251–255. doi: 10.2174/0929866053587138. [DOI] [PubMed] [Google Scholar]
- Cordeiro Y, Machado F, Juliano L, Juliano MA, Brentani RR, Foguel D, Silva JL. DNA converts cellular prion protein into the beta-sheet conformation and inhibits prion peptide aggregation. J Biol Chem. 2001;276:49400–49409. doi: 10.1074/jbc.M106707200. [DOI] [PubMed] [Google Scholar]
- Costa FF. Non-coding RNAs: lost in translation? Gene. 2007;386:1–10. doi: 10.1016/j.gene.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Alzheimer’s disease. N Engl J Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- De Felice FG, Vieira MN, Saraiva LM, Figueroa-Villar JD, Garcia-Abreu J, Liu R, Chang L, Klein WL, Ferreira ST. Targeting the neurotoxic species in Alzheimer’s disease: inhibitors of Abeta oligomerization. FASEB J. 2004;18:1366–1372. doi: 10.1096/fj.04-1764com. [DOI] [PubMed] [Google Scholar]
- Deleault NR, Lucassen RW, Supattapone S. RNA molecules stimulate prion protein conversion. Nature. 2003;425:717–720. doi: 10.1038/nature01979. [DOI] [PubMed] [Google Scholar]
- Di Domizio J, Zhang R, Stagg LJ, Gagea M, Zhuo M, Ladbury JE, Cao W. Binding with nucleic acids or glycosaminoglycans converts soluble protein oligomers to amyloid. J Biol Chem. 2012;287:736–747. doi: 10.1074/jbc.M111.238477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP, Aronin N. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- Dinger ME, Mercer TR, Mattick JS. RNAs as extracellular signaling molecules. J Mol Endocrinol. 2008;40:151–159. doi: 10.1677/JME-07-0160. [DOI] [PubMed] [Google Scholar]
- Dobson CM. Protein folding and its links with human disease. Biochem Soc Symp. 2001;68:1–26. [PubMed] [Google Scholar]
- Dorsman JC, Smoor MA, Maat-Schieman ML, Bout M, Siesling S, van Duinen SG, Verschuuren JJ, den Dunnen JT, Roos RA, van Ommen GJ. Analysis of the subcellular localization of huntingtin with a set of rabbit polyclonal antibodies in cultured mammalian cells of neuronal origin: comparison with the distribution of huntingtin in Huntington’s disease autopsy brain. Philos Trans R Soc Lond B. 1999;354:1061–1067. doi: 10.1098/rstb.1999.0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper DE, Reynaldo LP. RNA binding strategies of ribosomal proteins. Nucleic Acids Res. 1999;27:381–388. doi: 10.1093/nar/27.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer RB, McMurray CT. Mutant protein in Huntington disease is resistant to proteolysis in affected brain. Nat Genet. 2001;29:270–278. doi: 10.1038/ng745. [DOI] [PubMed] [Google Scholar]
- Ecroyd H, Carver JA. Unraveling the mysteries of protein folding and misfolding. IUBMB Life. 2008;60:769–774. doi: 10.1002/iub.117. [DOI] [PubMed] [Google Scholar]
- Eisenberg D, Jucker M. The amyloid state of proteins in human diseases. Cell. 2012;148:1188–1203. doi: 10.1016/j.cell.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabus C, Derrington E, Leblanc P, Chnaiderman J, Dormont D, Swietnicki W, Morillas M, Surewicz WK, Marc D, Nandi P, Darlix JL. The prion protein has RNA binding and chaperoning properties characteristic of nucleocapsid protein NCP7 of HIV-1. J Biol Chem. 2001;276:19301–19309. doi: 10.1074/jbc.M009754200. [DOI] [PubMed] [Google Scholar]
- Giraldo R. Defined DNA sequences promote the assembly of a bacterial protein into distinct amyloid nanostructures. Proc Natl Acad Sci USA. 2007;104:17388–17393. doi: 10.1073/pnas.0702006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M. a-synuclein and neurodegenerative diseases. Nature Rev Neurosci. 2001;2:492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- Goedert M, Klug A, Crowther RA. Tau protein, the paired helical filament and Alzheimer’s disease. J Alzheimers Dis. 2006;9:195–207. doi: 10.3233/jad-2006-9s323. [DOI] [PubMed] [Google Scholar]
- Goers J, Manning-Bog AB, McCormack AL, Millett IS, Doniach S, Di Monte DA, Uversky VN, Fink AL. Nuclear localization of a-synuclein and its interaction with histones. Biochemistry. 2003;42:8465–8471. doi: 10.1021/bi0341152. [DOI] [PubMed] [Google Scholar]
- Gomes MP, Millen TA, Ferreira PS, e Silva NL, Vieira TC, Almeida MS, Silva JL, Cordeiro Y. Prion protein complexed to N2a cellular RNAs through its N-terminal domain forms aggregates and is toxic to murine neuroblastoma cells. J Biol Chem. 2008a;283:19616–19625. doi: 10.1074/jbc.M802102200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes MP, Cordeiro Y, Silva JL. The peculiar interaction between mammalian prion protein and RNA. Prion. 2008;2:64–66. doi: 10.4161/pri.2.2.6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes MP, Vieira TC, Cordeiro Y, Silva JL. The role of RNA in mammalian prion protein conversion. WIREs RNA. 2012;3:415–428. doi: 10.1002/wrna.118. [DOI] [PubMed] [Google Scholar]
- Greenwood JA, Johnson GV. Localization and in situ phosphorylation state of nuclear tau. Exp Cell Res. 1995;220:332–337. doi: 10.1006/excr.1995.1323. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261:6084–6089. [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Harper JD, Lansbury PT., Jr Models of amyloid seeding in Alzheimer’s disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu Rev Biochem. 1997;66:385–407. doi: 10.1146/annurev.biochem.66.1.385. [DOI] [PubMed] [Google Scholar]
- Harper JD, Lieber CM, Lansbury PT Jr. (1997) Atomic force microscopic imaging of seeded fibril formation and fibril branching by the Alzheimer’s disease amyloid-beta protein. Chem Biol 4951-4959 [DOI] [PubMed]
- Hegde ML, Rao KS. DNA induces folding in synuclein: Understanding the mechanism using chaperone properties of osmolites. Arch Biochem Biophys. 2007;464:57–69. doi: 10.1016/j.abb.2007.03.042. [DOI] [PubMed] [Google Scholar]
- Hegde ML, Anitha S, Latha KS, Mustak MS, Stein R, Ravid R, Rao KS. First evidence for helical transitions in supercoiled DNA by amyloid-peptide (1-42) and aluminium. J Mol Neurosci. 2003;22:19–31. doi: 10.1385/jmn:22:1-2:19. [DOI] [PubMed] [Google Scholar]
- Ho LW, Carmichael J, Swartz J, Wyttenbach A, Rankin J, Rubinsztein DC. The molecular biology of huntington’s disease. Psychol Med. 2001;31:3–14. doi: 10.1017/s0033291799002871. [DOI] [PubMed] [Google Scholar]
- Hua Q, He RQ. Effect of phosphorylation and aggregation on tau binding to DNA Protein. Pept Lett. 2002;9:349–357. doi: 10.2174/0929866023408652. [DOI] [PubMed] [Google Scholar]
- Hua Q, He RQ. Tau could protect DNA double helix structure. Biochim Biophys Acta. 2003;1645:205–211. doi: 10.1016/s1570-9639(02)00538-1. [DOI] [PubMed] [Google Scholar]
- Ishimaru D, Andrade LR, Teixeira LS, Quesado PA, Maiolino LM, Lopez PM, Cordeiro Y, Costa LT, Heckl WM, Weissmüller G, Foguel D, Silva JL. Fibrillar aggregates of the tumor suppressor p53 core domain. Biochemistry. 2003;42:9022–9027. doi: 10.1021/bi034218k. [DOI] [PubMed] [Google Scholar]
- Ishimaru D, Ano Bom AP, Lima LM, Quesado PA, Oyama MF, de Moura Gallo CV, Cordeiro Y, Silva JL. Cognate DNA stabilizes the tumor suppressor p53 and prevents misfolding and aggregation. Biochemistry. 2009;48:6126–6135. doi: 10.1021/bi9003028. [DOI] [PubMed] [Google Scholar]
- Ivanyi-Nagy R, Davidovic L, Khandjian EW, Darlix JL. Disordered RNA chaperone proteins: from functions to disease. Cell Mol Life Sci. 2005;62:1409–1417. doi: 10.1007/s00018-005-5100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaumot J, Eritja R, Navea S, Gargallo R. Classification of nucleic acids structures by means of the chemometric analysis of circular dichroism spectra. Anal Chim Acta. 2009;642:117–126. doi: 10.1016/j.aca.2008.12.052. [DOI] [PubMed] [Google Scholar]
- Jiménez JS. Protein-DNA interaction at the origin of neurological diseases: a hypothesis. J Alzheimers Dis. 2010;22:375–391. doi: 10.3233/JAD-2010-100189. [DOI] [PubMed] [Google Scholar]
- Johnstone EM, Bebbey LE, Stephenson D, Paul DC, Santerre RF, Clemens JA, Williams DC, Little SP. Nuclear and cytoplasmic localization of the beta-amyloid peptide (1-43) in transfected 293 cells. Biochem Biophys Res Commun. 1996;220:710–718. doi: 10.1006/bbrc.1996.0469. [DOI] [PubMed] [Google Scholar]
- Kalia LV, Kalia SK, McLean PJ, Lozano AM, Lang AE. α-Synuclein oligomers and clinical implications for Parkinson disease. Ann Neurol. 2013;73:155–169. doi: 10.1002/ana.23746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampers T, Friedhoff P, Biernat J, Mandelkow EM, Mandelkow E. RNA stimulates aggregation of microtubule-associated protein tau into Alzheimer-like paired helical filaments. FEBS Lett. 1996;399:344–349. doi: 10.1016/s0014-5793(96)01386-5. [DOI] [PubMed] [Google Scholar]
- Kaplan B, Ratner V, Haas E. Alpha-synuclein: its biological function and role in neurodegenerative diseases. J Mol Neurosci. 2003;20:83–92. doi: 10.1385/JMN:20:2:83. [DOI] [PubMed] [Google Scholar]
- Keefe AD, Pai S, Ellington A. Aptamers as therapeutics. Nat Rev Drug Discov. 2010;9:537–550. doi: 10.1038/nrd3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegel KB, Meloni AR, Yi Y, Kim YJ, Doyle E, Cuiffo BG, Sapp E, Wang Y, Qin Z, Chen JD, Nevins JR, Aronin N, Figlia M. Huntingtin is present in the nucleus, interacts with the transcriptional corepressor C-terminal binding protein, and represses transcription. J Biol Chem. 2002;277:7466–7476. doi: 10.1074/jbc.M103946200. [DOI] [PubMed] [Google Scholar]
- King DJ, Safar JG, Legname G, Prusiner SB. Thioaptamer interactions with prion proteins: sequence-specific and non-specific binding sites. J Mol Biol. 2007;369:1001–1014. doi: 10.1016/j.jmb.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima LM, Cordeiro Y, Tinoco LW, Marques AF, Oliveira CL, Sampath S, Kodali R, Choi G, Foguel D, Torriani I, Caughey B, Silva JL. Structural insights into the interaction between prion protein and nucleic acid. Biochemistry. 2006;45:9180–9187. doi: 10.1021/bi060532d. [DOI] [PubMed] [Google Scholar]
- Liu C, Zhang Y. Nucleic acid-mediated protein aggregation and assembly. Adv Protein Chem Struct Biol. 2011;84:1–40. doi: 10.1016/B978-0-12-386483-3.00005-7. [DOI] [PubMed] [Google Scholar]
- Liu ML, Yu S, Yang J, Yin X, Zhao D. RNA and CuCl2 induced conformational changes of the recombinant ovine prion protein. Mol Cell Biochem. 2007;294:197–203. doi: 10.1007/s11010-006-9260-1. [DOI] [PubMed] [Google Scholar]
- Liu ML, Wen JJ, Xu XF, Zhao DM. Neurotoxic effect of the complex of the ovine prion protein (OvPrP(C)) and RNA on the cultured rat cortical neurons. Neurochem Res. 2011;36:1863–1869. doi: 10.1007/s11064-011-0506-2. [DOI] [PubMed] [Google Scholar]
- Luscombe NM, Laskowski RA, Thornton JM. Amino acid-base interactions: a three-dimensional analysis of protein-DNA interactions at an atomic level. Nucleic Acids Res. 2001;29:2860–2874. doi: 10.1093/nar/29.13.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi-Carter R, Cha JH. Mechanisms of transcriptional dysregulation in Huntington’s disease. Clin Neurosci Res. 2003;3:165–177. [Google Scholar]
- Ma J. The role of cofactors in prion propagation and infectivity. PLoS Pathog. 2012;8:e1002589. doi: 10.1371/journal.ppat.1002589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo B, Millen TA, Braga CA, Gomes MP, Ferreira PS, Kraineva J, Winter R, Silva JL, Cordeiro Y. Nonspecific prion protein-nucleic acid interactions lead to different aggregates and cytotoxic species. Biochemistry. 2012;51:5402–5413. doi: 10.1021/bi300440e. [DOI] [PubMed] [Google Scholar]
- Maloney B, Lahiri DK. The Alzheimer’s amyloid β-peptide (Aβ) binds a specific DNA Aβ-interacting domain (AβID) in the APP, BACE1, and APOE promoters in a sequence-specific manner: characterizing a new regulatory motif. Gene. 2011;488:1–12. doi: 10.1016/j.gene.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale D, Hackam A, Wieczorek A, Ellerby L, Wellington C, McCutcheon K, Singaraja R, Kazemi-Esfarjani P, Devon R, Kim SU, Bredesen DE, Tufaro F, Hayden MR. Length of huntingtin and its polyglutamine tract influences localization and frequency of intracellular aggregates. Nat Genet. 1998;18:150–154. doi: 10.1038/ng0298-150. [DOI] [PubMed] [Google Scholar]
- Mashima T, Matsugami A, Nishikawa F, Nishikawa S, Katahira M. Unique quadruplex structure and interaction of an RNA aptamer against bovine prion protein. Nucleic Acids Res. 2009;37:6249–6258. doi: 10.1093/nar/gkp647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashima T, Nishikawa F, Kamatari YO, Fujiwara H, Saimura M, Nagata T, Kodaki T, Nishikawa S, Kuwata K, Katahira M. Anti-prion activity of an RNA aptamer and its structural basis. Nucleic Acids Res. 2013;41:1355–1362. doi: 10.1093/nar/gks1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K. RNA-protein complexes. Curr Opin Struct Biol. 1996;6:53–61. doi: 10.1016/s0959-440x(96)80095-9. [DOI] [PubMed] [Google Scholar]
- Nandi PK. Interaction of prion peptide HuPrP106-126 with nucleic acid. Arch Virol. 1997;142:2537–2545. doi: 10.1007/s007050050261. [DOI] [PubMed] [Google Scholar]
- Nandi PK. Polymerization of human prion peptide HuPrP 106-126 to amyloid in nucleic acid solution. Arch Virol. 1998;143:1251–1263. doi: 10.1007/s007050050373. [DOI] [PubMed] [Google Scholar]
- Nandi PK, Leclerc E. Polymerization of murine recombinant prion protein in nucleic acid solution. Arch Virol. 1999;144:1751–1763. doi: 10.1007/s007050050702. [DOI] [PubMed] [Google Scholar]
- Nandi PK, Leclerc E, Nicole JC, Takahashi M. DNA-induced partial unfolding of prion protein leads to its polymerisation to amyloid. J Mol Biol. 2002;322:153–161. doi: 10.1016/s0022-2836(02)00750-7. [DOI] [PubMed] [Google Scholar]
- Necula M, Chirita CN, Kuret J. Rapid anionic micelle-mediated a-synuclein fibrillization in vitro. J Biol Chem. 2003;278:46674–46680. doi: 10.1074/jbc.M308231200. [DOI] [PubMed] [Google Scholar]
- Ohyagi Y, Asahara H, Chui DH, Tsuruta Y, Sakae N, Miyoshi K, Yamada T, Kikuchi H, Taniwaki T, Murai H, Ikezoe K, Furuya H, Kawarabayashi T, Shoji M, Checler F, Iwaki T, Makifuchi T, Takeda K, Kira J, Tabira T. Intracellular Abeta42 activates p53 promoter: a pathway to neurodegeneration in Alzheimer’s disease. FASEB J. 2005;19:255–257. doi: 10.1096/fj.04-2637fje. [DOI] [PubMed] [Google Scholar]
- Prusiner SB. Prions. Proc Natl Acad Sci USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu MH, Li H, Tian R, Nie CL, Liu Y, Han BS, He RQ. Neuronal tau induces DNA conformational changes observed by atomic force microscopy. Neuroreport. 2004;15:2723–2727. [PubMed] [Google Scholar]
- Rega S, Stiewe T, Chang D-I, Pollmeier B, Esche H, Bardenheuer W, Marquitan G, Putzer BM. Identification of the full-length huntingtin- interacting protein p231HBP/HYPB as a DNA-binding factor. Mol Cell Neurosci. 2001;18:68–79. doi: 10.1006/mcne.2001.1004. [DOI] [PubMed] [Google Scholar]
- Rhie A, Kirby L, Sayer N, Wellesley R, Disterer P, Sylvester I, Gill A, Hope J, James W, Tahiri-Alaoui A. Characterization of 2′-fluoro-RNA aptamers that bind preferentially to disease-associated conformations of prion protein and inhibit conversion. J Biol Chem. 2003;278:39697–39705. doi: 10.1074/jbc.M305297200. [DOI] [PubMed] [Google Scholar]
- Riek R, Hornemann S, Wider G, Billeter M, Glockshuber R, Wutrich K. NMR structure of the mouse prion protein domain PrP(121-321) Nature. 1996;382:180–182. doi: 10.1038/382180a0. [DOI] [PubMed] [Google Scholar]
- Rohs R, Jin X, West SM, Joshi R, Honig B, Mann RS. Origins of specificity in protein-DNA recognition. Annu Rev Biochem. 2010;79:233–269. doi: 10.1146/annurev-biochem-060408-091030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10:S10–17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- Sayer NM, Cubin M, Rhie A, Bullock M, Tahiri-Alaoui A, James W. Structural determinants of conformationally selective, prion-binding aptamers. J Biol Chem. 2004;279:13102–13109. doi: 10.1074/jbc.M310928200. [DOI] [PubMed] [Google Scholar]
- Schilling G, Savonenko AV, Klevytska A, Morton JL, Tucker SM, Poirier M, Gale A, Chan N, Gonzales V, Slunt HH, Coonfield ML, Jenkins NA, Copeland NG, Ross CA, Borchelt DR. Nuclear-targeting of mutant huntingtin fragments produces Huntington’s disease-like phenotypes in transgenic mice. Hum Mol Genet. 2004;13:1599–1610. doi: 10.1093/hmg/ddh175. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: genotypes, phenotypes, and treatments. Science. 1997;275:630–631. doi: 10.1126/science.275.5300.630. [DOI] [PubMed] [Google Scholar]
- Silva JL, Lima LM, Foguel D, Cordeiro Y. Intriguing nucleic-acid-binding features of mammalian prion protein. Trends Biochem Sci. 2008;33:132–140. doi: 10.1016/j.tibs.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Silva JL, Vieira TC, Gomes MP, Bom AP, Lima LM, Freitas MS, Ishimaru D, Cordeiro Y, Foguel D. Ligand Binding and Hydration in Protein Misfolding: Insights from Studies of Prion and p53 Tumor Suppressor Proteins. Acc Chem Res. 2010;43:271–279. doi: 10.1021/ar900179t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JL, Gomes MP, Vieira TC, Cordeiro Y. PrP interactions with nucleic acids and glycosaminoglycans in function and disease. Front Biosci. 2010;15:132–150. doi: 10.2741/3611. [DOI] [PubMed] [Google Scholar]
- Silva JL, Rangel LP, Costa DC, Cordeiro Y, De Moura Gallo CV. Expanding the Prion Concept to Cancer Biology: Dominant-Negative Effect of Aggregates of Mutant p53 Tumor Suppressor. Biosci Rep. 2013 doi: 10.1042/BSR20130065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe JD, Benson MD, Buxbaum JN, Ikeda S, Merlini G, Saraiva MJ, Westermark P. Amyloid fibril protein nomenclature: 2012 recommendations from the Nomenclature Committee of the International Society of Amyloidosis. Amyloid. 2012;19:167–170. doi: 10.3109/13506129.2012.734345. [DOI] [PubMed] [Google Scholar]
- Steffan JS, Kazantsev A, Spasic-Boskovic O, Greenwald M, Zhu Y-Z, Gohler H, Wanker EE, Bates GP, Housman DE, Thompson LM. The Huntington’s disease protein interacts with p53 and CREB-binding protein and represses transcription. Proc Natl Acad Sci USA. 2000;97:6763–6768. doi: 10.1073/pnas.100110097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugars KL, Rubinsztein DC. Transcriptional abnormalities in Huntington’s disease. Trends Genet. 2003;19:233–238. doi: 10.1016/S0168-9525(03)00074-X. [DOI] [PubMed] [Google Scholar]
- Suram A, Rao KS, Latha KS, Viswamitra MA. First evidence to show the topological change of DNA from B-DNA to Z-DNA conformation in the hippocampus of Alzheimer’s brain. Neuromolecular Med. 2002;2:289–297. doi: 10.1385/nmm:2:3:289. [DOI] [PubMed] [Google Scholar]
- Syrjanen S, Heinonen O, Miettinen R, Paljarvi L, Syrjanen K, Riekkinen P. Short biotinylated oligonucleotides bind non-specifically to senile plaques of Alzheimer’s disease. Neurosci Lett. 1991;130:89–91. doi: 10.1016/0304-3940(91)90234-k. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Tada K, Mihara H. RNA aptamers selected against amyloid β-peptide (Aβ) inhibit the aggregation of Aβ. Mol Biosyst. 2009;5:986–991. doi: 10.1039/b903391b. [DOI] [PubMed] [Google Scholar]
- Thayanidhi N, Helm JR, Nycz DC, Bentley M, Liang Y, Hay JC. Alpha-synuclein delays endoplasmic reticulum (ER)-to-Golgi transport in mammalian cells by antagonizing ER/Golgi SNAREs. Mol Biol Cell. 2010;21:1850–1863. doi: 10.1091/mbc.E09-09-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Huntington’s Disease Collaboration Research Group A novel gene containing a trinucleotide repeats that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Thurston VC, Zinkowski RP, Binder LI. Tau as a nucleolar protein in human nonneural cells in vitro and in vivo. Chromosoma. 1996;105:20–30. doi: 10.1007/BF02510035. [DOI] [PubMed] [Google Scholar]
- Uversky VN. Mysterious oligomerization of the amyloidogenic proteins. FEBS J. 2010;277:2940–2953. doi: 10.1111/j.1742-4658.2010.07721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human a-synuclein. A possible molecular link between Parkinson’s disease and heavy metal exposure. J Biol Chem. 2001;276:44284–44296. doi: 10.1074/jbc.M105343200. [DOI] [PubMed] [Google Scholar]
- Vasudevaraju P, Guerrero E, Hegde ML, Collen TB, Britton GB, Rao KS. New evidence on α-synuclein and Tau binding to conformation and sequence specific GC* rich DNA: Relevance to neurological disorders. J Pharm Bioallied Sci. 2012;4:112–117. doi: 10.4103/0975-7406.94811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira TC, Reynaldo DP, Gomes MP, Almeida MS, Cordeiro Y, Silva JL. Heparin binding by murine recombinant prion protein leads to transient aggregation and formation of RNA-resistant species. J Am Chem Soc. 2011;133:334–344. doi: 10.1021/ja106725p. [DOI] [PubMed] [Google Scholar]
- Wang W, Perovic I, Chittuluru J, Kaganovich A, Nguyen LT, Liao J, Auclair JR, Johnson D, Landeru A, Simorellis AK, Ju S, Cookson MR, Asturias FJ, Agar JN, Webb BN, Kang C, Ringe D, Petsko GA, Pochapsky TC, Hoang QQ. A soluble alpha-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci USA. 2011;108:17797–17802. doi: 10.1073/pnas.1113260108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Qu MH, Wang XS, Chen L, Wang DL, Liu Y, Hua Q, He RQ. Binding to the minor groove of the double-strand, tau protein prevents DNA from damage by peroxidation. PLoS ONE. 2008;3:e2600. doi: 10.1371/journal.pone.0002600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci USA. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Chen R, Liu C. Nucleic acid induced protein aggregation and its role in biology and pathology. Front Biosci. 2009;14:5084–5106. doi: 10.2741/3588. [DOI] [PubMed] [Google Scholar]
- Yu H, Ren J, Qu X. Time-dependent DNA condensation induced by amyloid beta-peptide. Biophys J. 2007;92:185–191. doi: 10.1529/biophysj.106.093559. [DOI] [PMC free article] [PubMed] [Google Scholar]