Abstract
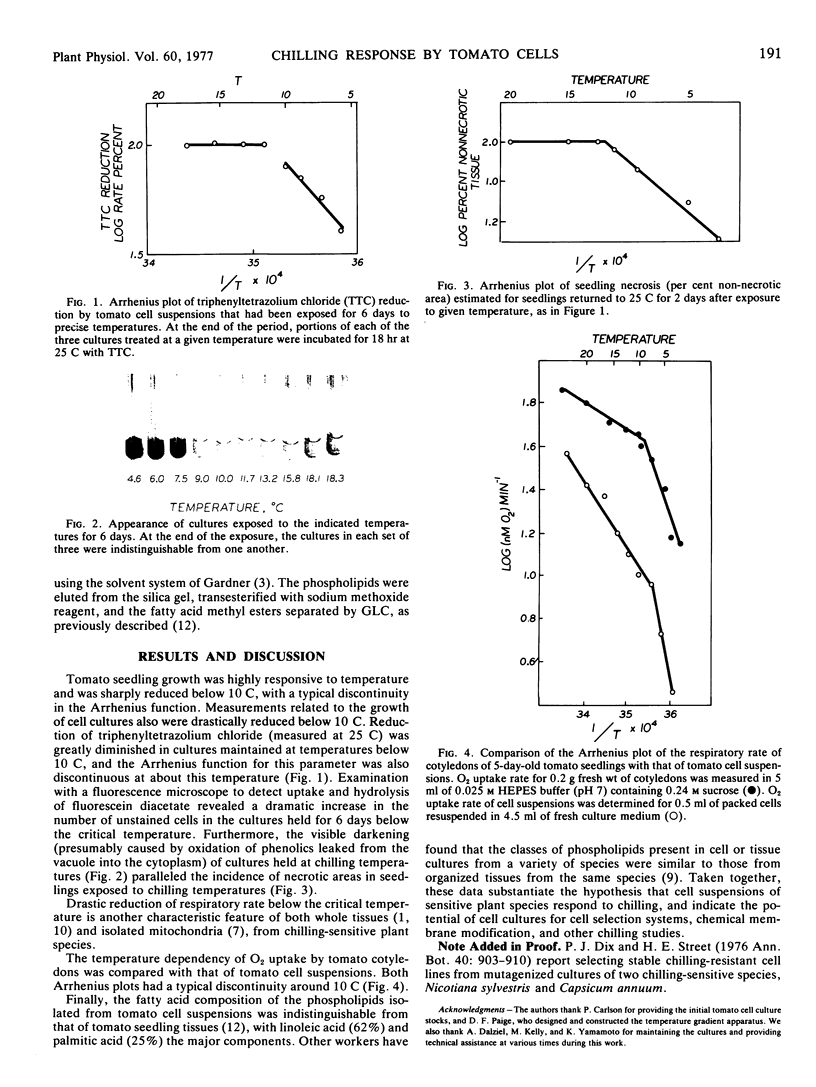
Tomato cell suspensions and seedlings (Lycopersicon esculentum) responded comparably when exposed to chilling temperatures (10 C or below). Seedling growth and cellular activities related to cell viability and culture growth (triphenyltetrazolium chloride reduction, fluoroscein diacetate uptake, and hydrolysis) were sharply diminished below 10 C. Arrhenius plots of the respiratory O2 consumption by both seedlings and cell suspensions had a break at 10 C, as is characteristic for chilling-sensitive species. The acyl chains that were found in the phospholipids of both cell cultures and seedlings were similar. These results indicate the potential usefulness of plant suspension cultures for studies of chilling injury.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breidenbach R. W., Wade N. L., Lyons J. M. Effect of chilling temperatures on the activities of glyoxysomal and mitochondrial enzymes from castor bean seedlings. Plant Physiol. 1974 Sep;54(3):324–327. doi: 10.1104/pp.54.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gardner H. W. Preparative isolation of monogalactosyl and digalactosyl diglycerides by thin-layer chromatography. J Lipid Res. 1968 Jan;9(1):139–141. [PubMed] [Google Scholar]
- Lyons J. M., Raison J. K. Oxidative activity of mitochondria isolated from plant tissues sensitive and resistant to chilling injury. Plant Physiol. 1970 Apr;45(4):386–389. doi: 10.1104/pp.45.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwan S. S., Spener F., Mangold H. K. Lipids in plant tissue cultures. IV. The characteristic patterns of lipid classes in callus cultures and suspension cultures. Chem Phys Lipids. 1975 Feb;14(1):72–78. doi: 10.1016/0009-3084(75)90017-1. [DOI] [PubMed] [Google Scholar]
- Waring A. J., Breidenbach R. W., Lyons J. M. In vivo modification of plant membrane phospholipid composition. Biochim Biophys Acta. 1976 Aug 16;443(2):157–168. doi: 10.1016/0005-2736(76)90499-5. [DOI] [PubMed] [Google Scholar]
- Widholm J. M. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 1972 Jul;47(4):189–194. doi: 10.3109/10520297209116483. [DOI] [PubMed] [Google Scholar]