Abstract
Objective
Endoplasmic reticulum (ER) aminopeptidase 1 (ERAP1) variants contribute to the risk of ankylosing spondylitis in HLA-B27 positive individuals, implying a disease-related interaction between these gene products. The aim of this study was to determine whether reduced ERAP1 expression would alter the cell surface expression of HLA-B27 and the formation of aberrant disulfide-linked forms that have been implicated in the pathogenesis of spondyloarthritis.
Methods
ERAP1 expression was knocked down in monocytic U937 cells expressing HLA-B27 and endogenous HLA class I. The effect of ERAP1 knockdown on the accumulation HLA-B alleles (B18, B51, and B27) was assessed using immunoprecipitation, isoelectric focusing, and immunoblotting, as well as flow cytometry with antibodies specific for different forms of HLA-B27. Cell surface expression of aberrant disulfide-linked HLA-B27 dimers was assessed by immunoprecipitation and electrophoresis on non-reducing polyacrylamide gels.
Results
ERAP1 knockdown increased the accumulation of HLA-B27 on the cell surface including disulfide-linked dimers, but had no effect on levels of HLA-B18 or -B51. Antibodies with unique specificity for HLA-B27 confirmed increased cell surface expression of complexes shown previously to contain long peptides. IFN-γ treatment resulted in striking increases in the expression of disulfide-linked HLA-B27 heavy chains, even in cells with normal ERAP1 expression.
Conclusions
Our results suggest that normal levels of ERAP1 reduce the accumulation of aberrant and disulfide-linked forms of HLA-B27 in monocytes, and thus help to maintain the integrity of cell surface HLA-B27 complexes.
Keywords: epistasis, major histocompatibility complex, misfolding, ankylosing spondylitis
Introduction
HLA-B27 promotes the development of spondyloarthritis through a mechanism, or mechanisms, that remain incompletely understood (Bowness, 2015). The disease has been hypothesized to be linked to the presentation of arthritogenic peptides to CD8+ T cells by canonical HLA-B27 class I complexes. However, CD8+ T cells are not required for spondyloarthritis in HLA-B27 transgenic rats (Taurog et al., 2009), and direct evidence for arthritogenic peptides in humans is lacking, yet their existence has not been ruled out (Sorrentino et al., 2014). Alternative mechanisms have been proposed based on aberrant features of HLA-B27, including its tendency to misfold and generate endoplasmic reticulum (ER) stress (Colbert et al., 2014), and to form disulfide-linked homodimers on the cell surface that trigger other immune cells (Shaw et al., 2014). Genetic studies, patient-derived material, animal models, and early results from therapeutic trials implicate the IL-23/IL-17 axis in spondyloarthritis pathogenesis (Smith and Colbert, 2014). The aberrant properties of HLA-B27 have been linked to activation of the IL-23/IL-17 axis through ER stress-mediated IL-23 overproduction from myeloid cells in rats (DeLay et al., 2009), and homodimer interaction with the killer immunoglobulin receptor (KIR)-3DL2 on CD4+ Th17 cells in humans, which promotes IL-17 production (Bowness et al., 2011). HLA-B27 also affects the survival and function of CD103+ dendritic cells in HLA-B27 transgenic rats (Utriainen et al., 2012), which may lead to a loss of immunologic tolerance and promote Th17 development (Glatigny et al., 2012), although the mechanism is unclear.
Endoplasmic reticulum (ER) aminopeptidase-1 (ERAP1) plays an important role in the N-terminal processing of ER peptides that are ultimately displayed by MHC class I complexes on the cell surface. ERAP1 has also been implicated in cytokine receptor shedding (then known as ARTS1) (Cui et al., 2002), and can be secreted from stimulated macrophages resulting in enhanced phagocytic activity (Goto et al., 2011; Goto et al., 2014). Non-synonymous coding variants of the ERAP1 gene are associated with ankylosing spondylitis and other HLA class I-associated immune-mediated inflammatory diseases including Behçet’s (HLA-B51) and psoriasis (HLA-Cw6) (Burton et al., 2007; Genetic Analysis of Psoriasis et al., 2010; Kirino et al., 2013). Epistasis between ERAP1 and HLA class I risk alleles suggests that the peptide editing function of ERAP1 is important in these immune-mediated inflammatory diseases, and underscores the need to better understand how this aminopeptidase affects the biology of HLA class I molecules.
ERAP1 trims peptides that are longer than 8–9 amino acids (Saric et al., 2002), reducing their length and optimizing their suitability to bind MHC class I proteins. Reducing ERAP1 expression through targeted deletion or knockdown approaches can reduce MHC class I expression on the cell surface (Hammer et al., 2006; Saveanu et al., 2005), although increased expression or no change have also been noted (York et al., 2002). In ERAP1-deficient mice where MHC class I expression was reduced, N-terminally extended peptides were overrepresented in pools of peptides eluted from MHC class I (Blanchard et al., 2010). However, examination of individual epitopes recognized by CD8+ T cells has also revealed that while ERAP1 is necessary for generating certain epitopes, others are destroyed (York et al., 2006). These studies suggest that while the general rules governing the role of ERAP1 in antigen processing may be clear, effects on individual MHC class I alleles and epitopes may differ.
Several effects of reduced ERAP1 expression on HLA-B27 have been reported. Folded forms of HLA-B27 were found to be unchanged (Chen et al., 2015; Haroon et al., 2012), increased (Seregin et al., 2013; Zervoudi et al., 2013), or decreased (Akram et al., 2014). Intracellular β2m-free HLA-B27 heavy chains and a subset of cell surface complexes that contain longer peptides (MARB4-reactive) were both increased in ERAP1 knockdown C1R cells transfected with HLA-B27 (Haroon et al., 2012). ERAP1 knockdown was also shown to increase the abundance of longer peptides (11–13 amino acids) presented by HLA-B27 in C1R and HeLa cells at the expense of 9 amino acid ligands (Chen et al., 2014), but to decrease free heavy chain expression in these cells (Chen et al., 2015). Previous studies have not determined whether ERAP1 alters the formation of aberrant forms of HLA-B27, nor have effects on other B alleles been compared under the same conditions. To address these questions, we compared folded and unfolded forms of HLA-B27 with HLA-B18 and HLA-B51 in human monocytic U937 cells where ERAP1 expression had been knocked down, and examined effects of IFN-γ. We show that several forms of HLA-B27 in monocytes are increased by reduced ERAP1 expression, and demonstrate for the first time ERAP1 knockdown leads to accumulation of aberrant disulfide-linked forms of HLA-B27. Interestingly, reduced ERAP1 expression had a differential effect on HLA-B27 compared to HLA-B51 and HLA-B18, suggesting that the ERAP1-HLA-B27 interaction may have unique immunobiological consequences.
Materials and Methods
Antibodies and reagents
Antibodies used in this study were HC10 (mouse IgG2a), which recognizes HLA-B and –C, β2m-free unfolded heavy chains (Stam et al., 1986); 3B10.7 (rat IgG2a), which recognizes HLA-B in immunoblots (Dangoria et al., 2002; Lutz and Cresswell, 1987); W6/32 (mouse IgG2a), which recognizes folded (conformational epitope) HLA class I (Barnstable et al., 1978); ME1 (mouse IgG1), which recognizes HLA-B27, -B7, -B42, -B67, and -Bw22 (Chen et al., 2015; Ellis et al., 1982); and MARB4 (mouse IgG2a), which recognizes a subset of HLA-B27 molecules that includes complexes containing long peptides and β2m-free heavy chains (Malik et al., 2002; Urban et al., 1994). These antibodies were isolated from hybridoma cultures and affinity purified on protein A-sepharose. Antibodies against ERAP1 (Novus Biologicals, Littleton, CO) and GAPDH (Santa Cruz Biotechnology, Dallas, TX) for immunoblots were purchased and used as recommended by the suppliers. W6/32 conjugated to Pacific Blue (Biolegend, San Diego, CA) and HLA.ABC.m3 conjugated to FITC (Millipore, Billerica, MA) were used in flow cytometry studies. HLA.ABC.m3 recognizes a conformational epitope on HLA-B27. HC10 was conjugated to APC using a kit (Columbia BioSciences, Frederick, MD) and following the manufacturer’s instructions. ME1 and MARB4 were conjugated to Pacific Blue (Life Technologies, Grand Island, NY) per manufacturer’s instructions. Methyl methanethiosulfonate (MMTS) was obtained from ThermoScientific (Rockford, IL), and N-glycanase from Prozyme (Hayward, CA).
Cell lines and ERAP1 knockdown
The human monocytic cell line U937 (HLA-A*03:01, A*31:01, B*18:01, B*51:01, C*01:02 and C*07:02) (Gebreselassie et al., 2006) stably expressing HLA-B27 (B*27:05) (U937.B27) was created by co-transfection with genomic HLA-B27 DNA and pSV2Neo and selection for G418 resistance and HLA-B27 expression (Penttinen et al., 2004). U937 cells transfected with pSV2Neo alone (U937.pSV2) were used as controls. U937.B27 and U937.pSV2 lines were maintained in RPMI supplemented with 10% FBS, penicillin-streptomycin (100 U/ml), and 600 μg/ml G418. U937.B27 cells were infected with retrovirus encoding ERAP1 shRNA or scrambled control shRNA (Origene, Rockville, MD), and selected with puromycin and for loss of ERAP1 expression by immunoblotting. Cells were maintained in the same medium (described above) supplemented with 1.5 ug/ml puromycin. Cells cultured in medium without antibiotics for 2–3 days prior to performing experiments. Cells were treated with recombinant human IFN-γ (Peprotech, Rocky Hill, NJ) at 100 ng/ml or PBS for 24 hours before harvesting.
We also determined the common ERAP1 variants present in the U937 cells by sequencing. They are homozygous for rs72773968 C (encodes Thr at position 12; Thr12), rs3734016 G (Glu56), rs26653 C (Pro127), rs26618 A (Ile276), rs27895 G (Gly346), rs2287987 A (Met349), rs10050860 G (Asp575), and rs17482078 G (Arg725). U937 cells are heterozygous at rs30187 A/G (Lys528 and Arg528) and rs27044 C/G (Gln730 and Glu730).
Immunoprecipitations
Cells were harvested and treated with MMTS (10 mM) in ice-cold PBS for 15 min to prevent spontaneous sulfhydryl bond formation and loss during and after cell lysis. Immunoprecipitations were performed as previously described (Dangoria et al., 2002) with some modifications. All steps were carried out at 4°C. Briefly, cells were lysed in lysis buffer (20 mM Tris-HCl pH 7.5, 100 mM NaCl, 1% Triton X-100, 10 mM EDTA, 1X Protease inhibitor complete tablet (Roche, Indianapolis, IN), 0.04% NaN3, 0.5 mM PMSF, 10 mM MMTS). Cell lysates were centrifuged at 16,000 × g for 5 min. Supernatants were collected and pre-cleared with protein A-sepharose and normal mouse serum for 1 hour. Following removal of protein A-sepharose by centrifugation, supernatants were incubated with antibody or mouse IgG2a (control) together with protein A-sepharose overnight. Immuoprecipitates were then washed once each with the following: Buffer 1 (20 mM Tris HCl pH 7.5, 100 mM NaCl, 10 mM EDTA, 1% Triton X-100, 0.1% SDS, 1% BSA), Buffer 2 (2 mM Tris Cl pH 7.5, 90 mM NACl, 1 mM EDTA, 0.1% Triton X-100), Buffer 3 (20 mM Tri HCl pH 7.5, 100 mM NaCl, 10 mM EDTA, 1% Triton X-100), and then subjected to further analysis.
For immunoprecipitation of cell surface HLA class I molecules, cells were treated with MMTS, washed and then harvested, resuspended in PBS, and incubated with HC10, W6/32, or mouse IgG2a (control) for 1 hour. Cells were washed 3 times with PBS to remove non-binding antibody, and then lysed as described above for 1 hour. Cell lysates were centrifuged at 16,000 × g for 5 min and then antibody-HLA class I complexes were collected with protein A-sepharose and washed exactly as described above, and then subjected to further analysis.
Protein separation and immunoblotting
Immunoprecipitates for isoelectric focusing (IEF) were treated with N-glycanase (1 mU for material from 1×105 cells) overnight at 37°C (Dangoria et al., 2002) to remove sialylated carbohydrate side chains. HLA class I heavy chains were separated on IEF gels (Hoeffer) (Ploegh, 1995). Gels were then pre-soaked in 50% methanol with 1% (w/v) SDS, 5 mM Tris-HCl (pH 8.0) before immunoblotting. Samples for SDS-polyacrylamide gel electrophoresis (SDS-PAGE) were dissolved in Laemmli sample buffer (without reducing agents), boiled for 90 sec, and then applied to 4–20% gradient gels (Bio-RAD, Hercules, CA) under non-reducing conditions (Dangoria et al., 2002). Proteins on IEF and polyacrylamide gels were transferred to PVDF membranes (Bio-RAD), and HLA-B alleles were visualized using 3B10.7 as the primary antibody and goat anti-rat IgG2a-horseradish peroxidase (Southern Biotech, Birmingham, AL) as the secondary antibody. The signal was developed with SuperSignal West Femto Maximum Sensitivity substrates (ThermoScientific, Waltham, MA) and ECL reagents.
Image analysis and quantitation
Immunblot images were captured using a ChemiDoc Imager (BioRad, Hercules, CA) and quantified by Imagelab software program (BioRad). Exposures were adjusted to enable band quantitation in the linear response range, and avoid saturated bands. All replicates for a given experiment were imaged from the same gel.
Flow cytometry
Cells were harvested, washed and incubated in PBS with 5% normal mouse serum for 1 hour at 4°C to block non-specific antibody binding, then washed and incubated with fluorescence probe-conjugated specific antibodies or IgG controls for 1 hour at 4°C. Cells were then washed 4 times before analysis (BD FACS CANTO II, BD Biosciences, San Jose, CA).
Statistical analysis
Statistical analyses were performed using Student’s t-test.
Results
Expression of free and folded HLA class I in U937 cells
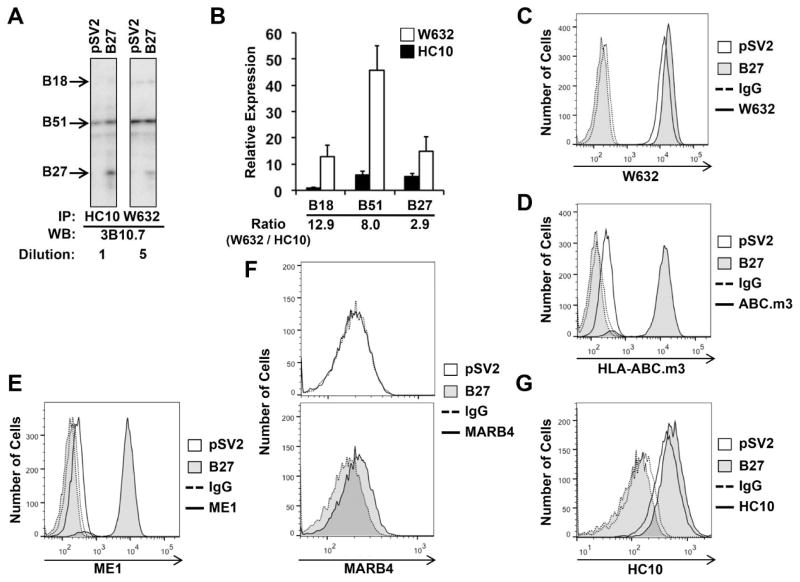
To examine effects of ERAP1 deficiency on HLA-B*27:05 (HLA-B27) in the context of other HLA-B alleles, we used U937 cells stably transfected with HLA-B27 (U937.B27). U937 cells are myeloid in origin, and express HLA-A*03:01 and A*31:01, HLA-B*18:01 (HLA-B18) and B*51:01 (HLA-B51), and HLA-C*01:02, C*07:02 from endogenous loci (Gebreselassie et al., 2006). The relative abundance of unfolded (β2m free) and folded HLA-B heavy chains immunoprecipitated by HC10 and W6/32, respectively, was compared using isoelectric focusing after removal of N-linked carbohydrates with N-glycanase (Ploegh, 1995). Isoelectric focusing distinguishes the different class I alleles that have identical molecular weights, and carbohydrate removal eliminates multiple isoforms of the heavy chain resulting from sialylation, thus enabling identification of specific alleles and facilitating quantitation (Neefjes and Ploegh, 1988). The expression of HLA-B27 is intermediate between HLA-B51 and HLA-B18, and as expected is seen only in the transfected cells (Figure 1A). The ratio of folded to unfolded heavy chain is approximately 3 for HLA-B27, substantially lower than for HLA-B51 and B18 (Figure 1B), consistent with the known inefficient folding of HLA-B27 (Dangoria et al., 2002). HLA-B27-expressing cells exhibit slightly greater staining with W6/32 (Figure 1C), whereas staining with HLA.ABC.m3 or ME1 is dependent on HLA-B27 expression (Figure 1D,E). HLA.ABC.m3 is sold as HLA-B27-specific, and ME1 recognizes several B alleles, none of which are expressed in U937 cells (Gebreselassie et al., 2006). MARB4, which recognizes a subset of HLA-B27 molecules that includes dimers (Dangoria et al., 2002), free heavy chains (Malik et al., 2002), and complexes containing longer peptides (Urban et al., 1994), only reacts with the HLA-B27 transfected cells (Figure 1F). Mock-transfected U937 cells (pSV2) are positive for HC10, and staining is marginally increased on HLA-B27-expressing cells, consistent with the presence of free cell surface HLA-B heavy chains (Figure 1G). These results establish the experimental system and specificity of antibodies used to determine effects of ERAP1 deficiency on various forms of HLA-B27.
Figure 1. Expression of free and folded HLA-B in U937 cells.
(A,B) U937.B27 and U937.pSV2 cells were lysed, and HLA class I immunoprecipitated (IPd) with the HC10 or W6/32. IPs were N-glycanase treated and subjected to IEF. HLA class I heavy chains are detected by Western blotting (WB) with 3B10.7. Visualization and quantitation was performed as described in Materials and Methods. (A) Representative IEF gel of HC10 and W6/32 IPs. W6/32 IPs were diluted 5-fold. Different regions of the same gel are shown. (B) Relative expression of each allele IPd with W6/32 (open bar) and HC10 (closed bar). HLA-B18 HC10 IP was set to 1. Means are the average of duplicate samples with the range (bar). (C–G) Cell surface staining of U937.B27 and U937.pSV2 cells with various antibodies compared to isotype (IgG) control. Two panels are used in (F) to facilitate comparison.
Reduced ERAP1 expression promotes HLA-B27 expression
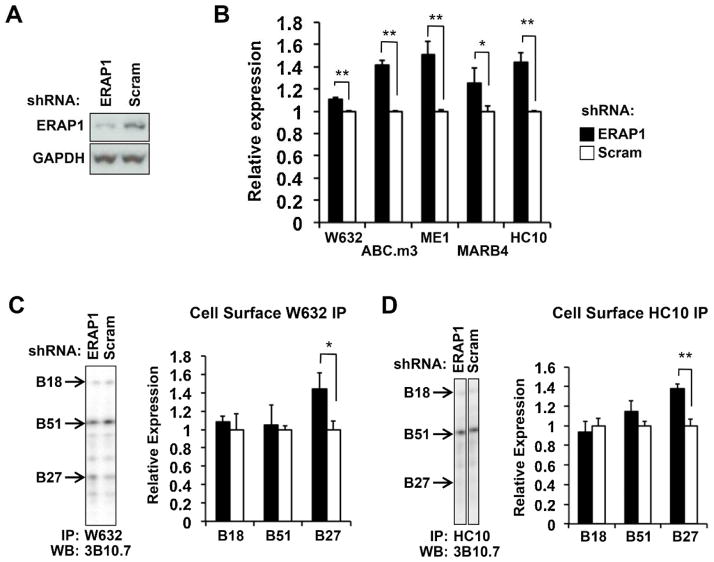
U937.B27 cells transduced with shRNA targeting ERAP1 exhibited approximately 65% reduction of ERAP1 protein expression compared with scrambled shRNA transduced U937.B27 cells (Figure 2A). These cells exhibit increased expression of folded HLA class I heavy chains (W6/32), β2m-free heavy chains (HC10), and forms of HLA-B27 recognized by ME1, HLA.ABC.m3, and MARB4 (Figure 2B). To determine whether loss of ERAP1 expression was affecting B alleles other than HLA-B27, W6/32 and HC10 immunoprecipitates of cell surface proteins were examined by isoelectric focusing. Consistent increases in HLA-B27 were observed with ERAP1 knockdown for both W6/32 and HC10, while the amount of HLA-B18 and HLA-B51 remained unchanged (Figure 2C,D). Examination of whole cell lysates revealed more folded HLA-B27 in the absence of ERAP1, while the total HC10-reactive material was no different for the 3 HLA-B alleles. (Supplemental Figure S1A,B shows whole cell lysates. Supplemental Figure S1C shows longer exposure of Figure 2D enabling visualization of the HLA-B27 band.)
Figure 2. ERAP1 knockdown increases HLA-B27 expression.
(A) Whole cell extracts from U937.B27 cells stably expressing ERAP1 or scrambled (Scram) shRNA were subjected to SDS-PAGE and blotted for ERAP1 and GAPDH as a loading control. (B) ERAP1 shRNA knockdown and control (Scram) U937.B27 cells were stained with the antibodies indicated, and analyzed by flow cytometry. Relative expression is mean fluorescence intensity for ERAP1 knockdown cells compared to cells transfected with scrambled shRNA (set to 1). Data are from 2–4 independent experiments done in triplicate. (C,D) Cell surface HLA class I was IPd with W6/32 (C) or HC10 (D), treated with N-glycanase, and quantitated by IEF and Western blotting. Representative blots (left panels) and quantitative results (right panels) are shown. Longer exposures are required to better visualize the B27 band in (D). Since this results in overexposure of the B51 band, we show only the shorter exposure here. A longer exposure is shown in Supplemental Figure S1C. Relative expression is the mean (+/−SEM) of triplicate samples, with expression in scrambled shRNA cells normalized to 1. * P < 0.05; **P < 0.01.
To establish the specificity of antibodies during cell surface immunoprecipitation, and to ensure that antibodies were not gaining access to the intracellular compartment during this procedure, an isotype control antibody (IgG2a) was carried through the same procedure. After incubation with isotype control antibody, cells were washed and lysed, and lysates then incubated with protein A-sepharose, followed by electrophoresis and immunoblotting with a secondary antibody against the isotype control (anti-mouse IgG). The isotype control does not precipitate any HLA class I from the cell surface, nor does it gain access to the intracellular compartment (Supplemental Figure S1D,E). Intracellular isotype control would be protected from washing, captured by protein A-sepharose, and visualized by secondary antibodies. No isotype control antibody was detected after the cell surface immunoprecipitation protocol unless it was added directly to the cell lysate as a positive control (Figure S1E).
Reduced ERAP1 expression promotes accumulation of disulfide-linked HLA-B27 dimers
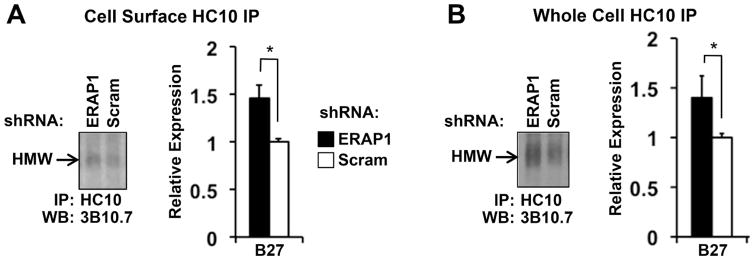
To determine the effect of reduced ERAP1 expression on the accumulation of disulfide-linked HLA-B27 dimers, HC10 immunoprecipates were examined on non-reducing SDS-PAGE. Dimers were consistently increased by about 50% on the cell surface (Figure 3A), with a similar increase detected in whole cell lysates (40%) (Figure 3B). Disulfide-linked dimers were not seen in U937.pSV2 cells, and were eliminated when samples were electrophoresed under reducing conditions, indicating that they are a consequence of HLA-B27 expression and dimerization (data not shown) consistent with previous reports (Dangoria et al., 2002).
Figure 3. ERAP1 knockdown increases expression of disulfide-linked HLA-B27 dimers.
(A) Cell surface complexes were immunoprecipitated from ERAP1 knockdown and control (Scram) U937.B27 cells with HC10 and analyzed by SDS-PAGE under non-reducing conditions. Representative (left) and quantitative (right) results from triplicates are shown. High molecular weight complexes (HMW) migrate in the 85–90 kDa size range and are eliminated when samples are reduced prior to electrophoresis (not shown). (B) Whole cell HC10 immunoprecipitations were performed and analyzed as described in (A). Quantitative results are from three experiments with duplicate gels. * P < 0.05
IFN-γ promotes accumulation of aberrant forms of HLA-B27
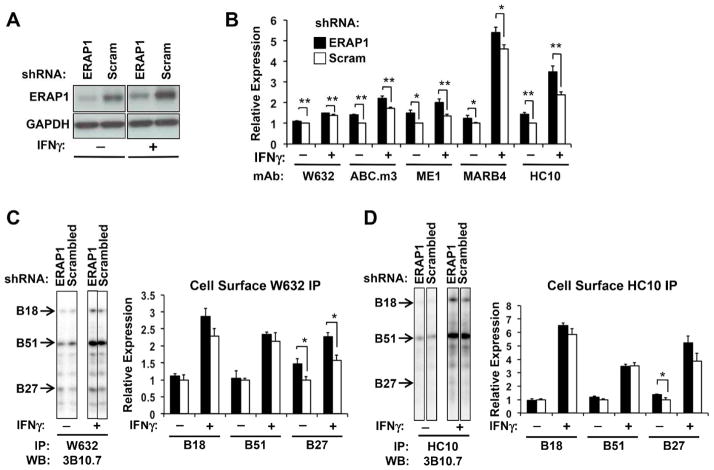
When U937 cells were treated with IFN-γ there was an increase in ERAP1 expression, but cells transduced with ERAP1 shRNA continued to exhibit ERAP1 expression, with levels about 50% of control (scrambled shRNA) cells (Figure 4A). When cell surface complexes were examined by flow cytometry in IFN-γ-treated cells, the increased expression of folded (W6/32) and unfolded (HC10) molecules seen in ERAP1 knockdown cells in the absence of IFN-γ was maintained, as was increased staining with ME1, HLA.ABC.m3, and MARB4 (Figure 4B). Comparing the 3 B alleles using isoelectric focusing of cell surface immunopreciptates, the increase in class I molecules recognized by W6/32 appeared to be largely a consequence of increased HLA-B27 expression, although there was a trend toward increased expression of HLA-B18 as well (Figure 4C). In the cell surface HC10 immunoprecipitates, the difference in total unfolded HLA-B27 also appeared to be increased in ERAP1 knockdown cells, but the difference did not reach statistical significance (P = 0.07) (Figure 4D). Differences in HLA-B alleles immunoprecipitated from whole cell lysates with W6/32 and HC10 before and after IFN-γ treatment are shown in supplemental Figure S2. It is worth noting that the largest IFN-γ-induced increase in cell surface HLA expression detected by flow cytometry was seen with MARB4 and HC10 regardless of whether or not ERAP1 expression was reduced. Both of these antibodies have been shown to recognize HLA-B27 dimers as well as unfolded or aberrant monomers (Dangoria et al., 2002). When cell surface expression of disulfide-linked dimers was specifically assessed using non-reducing SDS-PAGE, dimers of HLA-B27 were substantially increased with IFN-γ treatment (4–5-fold) (Figure 5A), consistent with flow cytometry results (Figure 4B). Increases in disulfide-linked dimers were also observed in whole cell lysates (5–6-fold) (Figure 5B).
Figure 4. ERAP1 effects persist after IFN-γ treatment.
ERAP1 knockdown and scrambled shRNA (Scram) U937.B27 cells were treated with IFN-γ (100 ng/ml) or PBS for 24 hours. (A) Whole cell extracts were subjected to SDS-PAGE and blotted for ERAP1 and GAPDH as a loading control. (B) Cells were stained with the antibodies indicated, and analyzed by flow cytometry. For each antibody, expression in PBS-treated scrambled shRNA cells was normalized to 1. Data are expressed as mean +/−SEM from 2–4 experiments done in duplicate or triplicate. (C,D) Cell surface HLA class I was IPd with W6/32 (C) or HC10 (D) and quantitated by IEF and Western blotting as described in the legend to Figure 2 (C,D). Representative blots (left panels) and quantitative results (right panels) are shown. The representative images for no IFNγ treatment are the same as those shown in Figure 2C,D, and are included here to allow comparison with +IFNγ. Expression is the mean (+/−SEM) of triplicate samples, relative to PBS-treated scrambled shRNA cells. * P < 0.05; **P < 0.01.
Figure 5. Cell surface disulfide-linked dimers increased by IFN-γ.
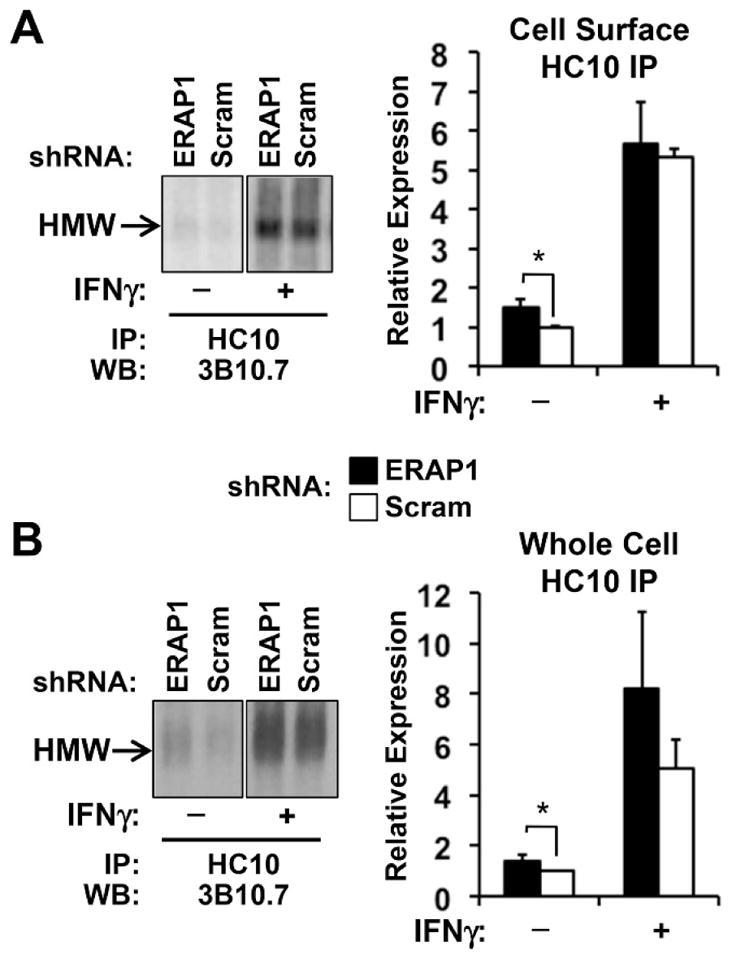
ERAP1 knockdown and scrambled shRNA (Scram) U937.B27 cells were treated with IFN-γ (100 ng/ml) or PBS for 24 hours. (A) Cell surface complexes were immunoprecipitated with HC10 and analyzed by SDS-PAGE under non-reducing conditions. Representative (left) and quantitative (right) results from triplicates are shown. High molecular weight complexes (HMW) migrate in the 85–90 kDa size range and are eliminated when samples are reduced prior to electrophoresis (not shown). (B) Whole cell lysate HC10 immunoprecipitations were performed and analyzed as described in (A). Quantitative results are from three experiments with duplicate gels. * P < 0.05
Discussion
We investigated whether reduced ERAP1 expression would affect the accumulation of different forms of HLA-B27 in monocytic cells, and whether effects of ERAP1 would differ between B alleles. Our results show that ERAP1 knockdown increases the accumulation of HLA-B27 on the cell surface as recognized by monoclonal antibodies HLA.ABC.m3, ME1, and MARB4. Staining with W6/32 and HC10 was also increased by ERAP1 knockdown. Since W6/32 and HC10 are not HLA-B27-specific, we measured the amount of HLA-B27 compared to other B alleles by immunoprecipitation, isoelectric focusing, and immunoblotting. This revealed that the increase in cell surface HLA-B was due to HLA-B27 and not HLA-B51 or HLA-B18. Moreover, we found that disulfide-linked, β2m-free HLA-B27 dimers accumulate on the cell surface when ERAP1 expression is reduced.
The panel of antibodies used here exhibits distinct but also overlapping specificities that warrant further discussion. HLA class I complexes recognized by W6/32 and HC10 are largely non-overlapping, with W6/32 being specific for a conformational epitope on folded heavy chain-peptide-β2m complexes, and HC10 restricted to a subset of β2m-free HLA-B and C heavy chains (Barnstable et al., 1978; Stam et al., 1986). In previous studies we demonstrated that HC10 recognizes unfolded (β2m-free) and dimerized forms of the HLA-B27 heavy chain (Dangoria et al., 2002; Turner et al., 2007; Turner et al., 2005). It is worth mentioning that cell lysis conditions, particularly the detergents used, could influence the spectrum of HLA class I heavy chains (and therefore HLA-B27 heavy chains) recognized by HC10. For example, under milder conditions than those used here, some heavy chains may be partially folded and β2m associated. We also reported that W6/32 recognizes a small pool of dimers (‘folded’ dimers) that differ from the species immunoprecipitated by HC10 (Dangoria et al., 2002). Thus, the increase in W6/32-reactive forms of HLA-B27 in ERAP1 knockdown cells could include ‘folded’ dimers. Since W6/32-reactive complexes were the least affected by ERAP1 knockdown, we did not further pursue their composition. ME1 recognizes a conformational epitope present primarily on trimolecular complexes. Interestingly, MARB4 has been shown to recognize HLA-B27 displaying peptides longer than the canonical 8–10 amino acids (Urban et al., 1994) as well as β2m-free HLA-B27 heavy chains (Malik et al., 2002). Since ME1 pre-incubation was reported to block staining of HLA-B27 with MARB4, and HLA-B27 complexes immunopurified with ME1 contain MARB4-reactive complexes (Malik et al., 2002), ME1 may recognize some forms of HLA-B27 with long peptides. This is consistent with peptide elution studies of HLA-B27 expressed in ERAP1 knockdown cells and immunopurified with ME1 (Chen et al., 2014). Little is known about the fine specificity of HLA.ABC.m3 and thus we do not know whether there is overlap with long peptide complexes recognized by MARB4. Taken together, our results provide direct evidence that reduced ERAP1 expression increases the accumulation of aberrant disulfide-linked complexes of HLA-B27 displayed on the cell surface. Increased staining with MARB4 suggests greater accumulation of HLA-B27 with longer peptides, consistent with recent peptide elution studies done with ERAP1 knockdown cells (Chen et al., 2014). Thus, increased staining with ME1 could represent recognition of complexes with longer peptides, although we cannot rule greater expression of canonical HLA-B27 complexes when ERAP1 is deficient.
The separation of immunoprecipitated heavy chains by isoelectric focusing enabled quantitative assessment of the expression of 3 different HLA-B alleles immunoprecipitated with W6/32 and HC10, and revealed a differential effect of ERAP1 knockdown on HLA-B27. Whereas both folded (W6/32) and β2m-free and aberrant (HC10-reactive) HLA-B27 heavy chains were increased by ERAP1 knockdown, no increase (or decrease) was seen for either HLA-B51 or B18. This is of interest particularly with regard to HLA-B51, which interacts with ERAP1 in conferring risk for Behçet’s disease (Kirino et al., 2013). The ERAP1 variants associated with Behçet’s disease differ from those associated with ankylosing spondylitis, although functional analyses have not been reported. Nevertheless, the differential effect of ERAP1 knockdown on HLA-B27 and HLA-B51 expression suggests that diminished peptide editing and an altered supply of peptides in the ER has a differential effect on these two alleles. Given the dramatically different phenotypes of ankylosing spondylitis and Behçet’s disease, it would not be surprising for the ERAP1-HLA interaction to have allele-specific consequences. The lack of HLA-B51 or -B18-specific antibody reagents (such as HLA.ABC.m3 and MARB4 for HLA-B27) precludes a more detailed evaluation of these alleles. It may be that HLA-B51 also displays longer peptides when ERAP1 expression is reduced, but that these complexes replace others such total expression is not increased. It will be of interest to explore effects of ERAP1 on HLA-Cw6 as well as HLA-B51, since the ERAP1-C6 gene-gene interaction is important in predisposition to psoriasis.
Several non-synonymous single nucleotide polymorphisms (SNPs) in ERAP1 are associated with ankylosing spondylitis (Burton et al., 2007; Evans et al., 2011; Keidel et al., 2013). For two SNPs, rs30187 (A/G sense strand, resulting in substitution of Arg for Lys at position 528 or p.Lys528Arg) and rs27044 (C/G sense strand results in p.Gln730Glu), the most common allele (listed second) is associated with protection from disease (Burton et al., 2007; Evans et al., 2011; Keidel et al., 2013). For other common variants at positions 349 (p.Met349Val), 575 (p.Asp575Asn), and 725 (p.Arg725Gln), the most common allele (listed first) confers increased risk (Keidel et al., 2013). Several of these variants affect the enzymatic activity of ERAP1. Most notably, p.Lys528Arg was shown to reduce ERAP1 activity (Evans et al., 2011), whereas another study revealed that p.Lys528Arg and p.Gln730Glu could reduce or increase ERAP1 activity depending on the substrate (Evnouchidou et al., 2011; Keidel et al., 2013). Despite some inconsistent results, the majority of evidence suggests that ERAP1 variants reducing aminopeptidase activity are associated with protection from developing ankylosing spondylitis (reviewed in (Tran and Colbert, 2015)). Thus, our results showing that ERAP1 knockdown increases the accumulation of cell surface disulfide-linked HLA-B27 dimers could be interpreted to suggest that its protective role in disease is not mediated through an effect on these complexes. However, Chen et al. recently reported that ERAP1 knockdown reduced the ability of HLA-B27-expressing cells to promote IL-17 production from CD4+ KIR3DL2+ T cells (Chen et al., 2015). In their experiments ERAP1 knockdown reduced the expression of free HLA-B27 heavy chains (HC10-reactive) on the cell surface, leading to the conclusion that the protective effect of ERAP1 loss-of-function might be related to lower expression of aberrant HLA-B27 molecules. Although they did not report the effect of ERAP1 knockdown on disulfide-linked HLA-B27 dimers, the reduction of HC10-reactive heavy chains is the opposite of what we see, and different from what Haroon et al. reported (Haroon et al., 2012). The HLA-B27-specific effects of Chen et al. were observed in transfected HeLa and C1R cells, while we used U937 monocytes. There are several possible reasons for these differences, including cell-specific variation in MHC class I assembly pathway components, the presence of other MHC class I alleles, overexpression of HLA-B27, and the degree of ERAP1 knockdown. It should be emphasized that we used cells where HLA-B27 expression is intermediate between the two other B alleles, and therefore not overexpressed. The relative expression of HLA-B27 and ERAP1 may be a critical determinant in studies of this kind. It is generally thought that peptide supply in the ER is limiting for HLA class I expression, and thus further overexpression of class I in many transfected cells may result in conditions that do not reflect the usual in vivo situation. Additional studies will be needed to resolve these questions before definitive conclusions can be made about protective mechanisms that underlie the ERAP1-HLA-B27 interaction.
It is important to note that while most studies examining the effects of ERAP1 variants have looked at single amino acid changes in isolation, this does not reflect the natural circumstances. ERAP1 genetic variants exist as haplotypes encoding distinct allotypes that differ by several amino acids (Ombrello et al., 2015; Reeves et al., 2014). In addition, co-dominant expression of allotypes increases complexity, making it difficult to interpret results from experiments comparing single amino acid substitutions. Genetic association studies of ERAP1 SNPs have revealed small differences in the frequency of risk vs. protective variants in ankylosing spondylitis. Indeed, given the high allele frequency of protective SNPs (55–70% for rs30187 G and rs27044 G) in the population (Ombrello et al., 2015), the majority of HLA-B27 positive ankylosing spondylitis patients actually carry protective ERAP1 SNPs. When Reeves et al. examined entire haplotypes they found that the pairs of haplotypes in patients were distinct from the pairs found in healthy controls (Reeves et al., 2014). Functional analysis revealed that allotype pairs found in ankylosing spondylitis patients were poor at generating optimal peptides for HLA-B27. Poor generation of MHC class I epitopes was inferred based on the ability of the ERAP1 allotype pairs to increase cell surface expression of HLA-B27, and poor generation of optimal peptides is generally construed as ‘loss-of-function’. However, our data show that reduced ERAP1 expression (loss-of-function) can actually increase HLA-B27 expression in monocytes. Thus, it remains unclear whether poor generation of optimal peptides for HLA-B27 represents ‘loss’ or ‘gain’ of ERAP1 function. The seemingly paradoxical effect of ERAP1 was not seen with other B alleles, and could be due to a tendency of HLA-B27 to escape quality control in the ER when loaded with suboptimal (e.g. longer) peptides. This notion is supported by the observation that HLA-B27 can assemble in the absence of tapasin (Peh et al., 1998), which also results in the accumulation of disulfide-linked HLA-B27 complexes (Dangoria et al., 2002).
Reeves et al. have proposed a complex model where both high and low ERAP1 activity was predicted to be associated with risk (Reeves et al., 2014). Both over- and under-trimming were hypothesized to result in greater accumulation of disulfide-linked HLA-B27 dimers. Here, we provide evidence that loss of ERAP1 expression can result in the accumulation of HLA-B27 heavy chain dimers, providing the first direct demonstration that ERAP1 affects aberrant properties of HLA-B27. Whether over-trimming can result in the same phenomenon is unclear, but could be addressed with overexpression studies. It is also apparent from the accumulated data that there may be important cell- and condition-specific differences in how ERAP1 function affects HLA-B27 that will need to be resolved.
Supplementary Material
Acknowledgments
This work was supported by the NIAMS Intramural Research Program, Z01 AR041184.
Non-standard abbreviations used in this paper
- β2m
β2-microglobulin
- HLA
human leukocyte antigen
- MHC
major histocompatibility complex
- ER
endoplasmic reticulum
- ERAP1
ER aminopeptidase 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akram A, Lin A, Gracey E, Streutker CJ, Inman RD. HLA-B27, but not HLA-B7, immunodominance to influenza is ERAP dependent. Journal of Immunology. 2014;192:5520–5528. doi: 10.4049/jimmunol.1400343. [DOI] [PubMed] [Google Scholar]
- Barnstable CJ, Bodmer WJ, Brown G, Galfre G, Milstein C, Williams AF, Zeigler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens - new tools for genetic analysis. Cell. 1978;14:9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Blanchard N, Kanaseki T, Escobar H, Delebecque F, Nagarajan NA, Reyes-Vargas E, Crockett DK, Raulet DH, Delgado JC, Shastri N. Endoplasmic reticulum aminopeptidase associated with antigen processing defines the composition and structure of MHC class I peptide repertoire in normal and virus-infected cells. Journal of Immunology. 2010;184:3033–3042. doi: 10.4049/jimmunol.0903712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowness P. HLA-B27. Annual Review of Immunology. 2015;33:29–48. doi: 10.1146/annurev-immunol-032414-112110. [DOI] [PubMed] [Google Scholar]
- Bowness P, Ridley A, Shaw J, Chan AT, Wong-Baeza I, Fleming M, Cummings F, McMichael A, Kollnberger S. Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. Journal of Immunology. 2011;186:2672–2680. doi: 10.4049/jimmunol.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton PR, Clayton DG, Cardon LR, Craddock N, Deloukas P, Duncanson A, Kwiatkowski DP, McCarthy MI, Ouwehand WH, Samani NJ, et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nature Genetics. 2007;39:1329–1337. doi: 10.1038/ng.2007.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Fischer R, Peng Y, Reeves E, McHugh K, Ternette N, Hanke T, Dong T, Elliott T, Shastri N, et al. Critical role of endoplasmic reticulum aminopeptidase 1 in determining the length and sequence of peptides bound and presented by HLA-B27. Arthritis & Rheumatology. 2014;66:284–294. doi: 10.1002/art.38249. [DOI] [PubMed] [Google Scholar]
- Chen L, Ridley A, Hammitzsch A, Al-Mossawi MH, Bunting H, Georgiadis D, Chan A, Kollnberger S, Bowness P. Silencing or inhibition of endoplasmic reticulum aminopeptidase 1 (ERAP1) suppresses free heavy chain expression and Th17 responses in ankylosing spondylitis. Annals of Rheumatic Disease. 2015 doi: 10.1136/annrheumdis-2014-206996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert RA, Tran TM, Layh-Schmitt G. HLA-B27 misfolding and ankylosing spondylitis. Molecular Immunology. 2014;57:44–51. doi: 10.1016/j.molimm.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Hawari F, Alsaaty S, Lawrence M, Combs CA, Geng W, Rouhani FN, Miskinis D, Levine SJ. Identification of ARTS-1 as a novel TNFR1-binding protein that promotes TNFR1 ectodomain shedding. J Clin Invest. 2002;110:515–526. doi: 10.1172/JCI13847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangoria NS, DeLay ML, Kingsbury DJ, Mear JP, Uchanska-Ziegler B, Ziegler A, Colbert RA. HLA-B27 misfolding is associated with aberrant intermolecular disulfide bond formation (dimerization) in the endoplasmic reticulum. Journal of Biological Chemistry. 2002;277:23459–23468. doi: 10.1074/jbc.M110336200. [DOI] [PubMed] [Google Scholar]
- DeLay ML, Turner MJ, Klenk EI, Smith JA, Sowders DP, Colbert RA. HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis & Rheumatism. 2009;60:2633–2643. doi: 10.1002/art.24763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis SA, Taylor C, McMichael AJ. Recognition of HLA-B27 and related antigens by a monoclonal antibody. Human Immunology. 1982;5:49–59. doi: 10.1016/0198-8859(82)90030-1. [DOI] [PubMed] [Google Scholar]
- Evans DM, Spencer CC, Pointon JJ, Su Z, Harvey D, Kochan G, Oppermann U, Dilthey A, Pirinen M, Stone MA, et al. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nature Genetics. 2011;43:761–767. doi: 10.1038/ng.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evnouchidou I, Kamal RP, Seregin SS, Goto Y, Tsujimoto M, Hattori A, Voulgari PV, Drosos AA, Amalfitano A, York IA, et al. Cutting Edge: Coding single nucleotide polymorphisms of endoplasmic reticulum aminopeptidase 1 can affect antigenic peptide generation in vitro by influencing basic enzymatic properties of the enzyme. Journal of Immunology. 2011;186:1909–1913. doi: 10.4049/jimmunol.1003337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebreselassie D, Spiegel H, Vukmanovic S. Sampling of major histocompatibility complex class I-associated peptidome suggests relatively looser global association of HLA-B*5101 with peptides. Human Immunology. 2006;67:894–906. doi: 10.1016/j.humimm.2006.08.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genetic Analysis of Psoriasis C the Wellcome Trust Case Control C. Strange A, Capon F, Spencer CC, Knight J, Weale ME, Allen MH, Barton A, Band G, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nature Genetics. 2010;42:985–990. doi: 10.1038/ng.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatigny S, Fert I, Blaton MA, Lories RJ, Araujo LM, Chiocchia G, Breban M. Proinflammatory Th17 cells are expanded and induced by dendritic cells in spondylarthritis-prone HLA-B27-transgenic rats. Arthritis & Rheumatism. 2012;64:110–120. doi: 10.1002/art.33321. [DOI] [PubMed] [Google Scholar]
- Goto Y, Ogawa K, Hattori A, Tsujimoto M. Secretion of endoplasmic reticulum aminopeptidase 1 is involved in the activation of macrophages induced by lipopolysaccharide and interferon-gamma. Journal of Biological Chemistry. 2011;286:21906–21914. doi: 10.1074/jbc.M111.239111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Ogawa K, Nakamura TJ, Hattori A, Tsujimoto M. TLR-mediated secretion of endoplasmic reticulum aminopeptidase 1 from macrophages. Journal of Immunology. 2014;192:4443–4452. doi: 10.4049/jimmunol.1300935. [DOI] [PubMed] [Google Scholar]
- Hammer GE, Gonzalez F, Champsaur M, Cado D, Shastri N. The aminopeptidase ERAAP shapes the peptide repertoire displayed by major histocompatibility complex class I molecules. Nature Immunology. 2006;7:103–112. doi: 10.1038/ni1286. [DOI] [PubMed] [Google Scholar]
- Haroon N, Tsui FW, Uchanska-Ziegler B, Ziegler A, Inman RD. Endoplasmic reticulum aminopeptidase 1 (ERAP1) exhibits functionally significant interaction with HLA-B27 and relates to subtype specificity in ankylosing spondylitis. Annals of Rheumatic Disease. 2012;71:589–595. doi: 10.1136/annrheumdis-2011-200347. [DOI] [PubMed] [Google Scholar]
- Keidel S, Chen L, Pointon J, Wordsworth P. ERAP1 and ankylosing spondylitis. Current Opinion in Immunology. 2013;25:97–102. doi: 10.1016/j.coi.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Kirino Y, Bertsias G, Ishigatsubo Y, Mizuki N, Tugal-Tutkun I, Seyahi E, Ozyazgan Y, Sacli FS, Erer B, Inoko H, et al. Genome-wide association analysis identifies new susceptibility loci for Behcet’s disease and epistasis between HLA-B*51 and ERAP1. Nature Genetics. 2013;45:202–207. doi: 10.1038/ng.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz PM, Cresswell P. An epitope common to HLA class I and class II antigens, Ig light chains, and β2-microglobulin. Immunogenetics. 1987;25:228–233. doi: 10.1007/BF00404692. [DOI] [PubMed] [Google Scholar]
- Malik P, Klimovitsky P, Deng LW, Boyson JE, Strominger JL. Uniquely conformed peptide-containing beta 2-microglobulin-free heavy chains of HLA-B2705 on the cell surface. Journal of Immunology. 2002;169:4379–4387. doi: 10.4049/jimmunol.169.8.4379. [DOI] [PubMed] [Google Scholar]
- Neefjes JJ, Ploegh HL. Allele and locus-specific differences in cell surface expression and the association of HLA class I heavy chains with β2-microglobulin: differential effects of inhibition of glycosylation on class I subunit association. European Journal of Immunology. 1988;18:801–810. doi: 10.1002/eji.1830180522. [DOI] [PubMed] [Google Scholar]
- Ombrello MJ, Kastner DL, Remmers EF. Endoplasmic reticulum-associated amino-peptidase 1 and rheumatic disease: genetics. Current Opinion in Rheumatology. 2015;27:349–356. doi: 10.1097/BOR.0000000000000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peh CA, Burrows SR, Barnden M, Khanna R, Cresswell P, Moss DJ, McClusky J. HLA-B27-restricted antigen presentation in the absence of tapasin reveals polymorphism in mechanisms of HLA class I peptide loading. Immunity. 1998;8:531–542. doi: 10.1016/s1074-7613(00)80558-0. [DOI] [PubMed] [Google Scholar]
- Penttinen MA, Heiskanen KM, Mohapatra R, DeLay ML, Colbert RA, Sistonen L, Granfors K. Enhanced intracellular replication of Salmonella enteritidis in HLA-B27-expressing human monocytic cells: dependency on glutamic acid at position 45 in the B pocket of HLA-B27. Arthritis & Rheumatism. 2004;50:2255–2263. doi: 10.1002/art.20336. [DOI] [PubMed] [Google Scholar]
- Ploegh HL. One-dimensional isoelectric focusing of proteins in slab gels. In: Coligan JE, Dunn BM, Ploegh HL, Speicher DW, Wingfield PT, editors. Current Protocols in Protein Science. New York: John Wiley & Sons; 1995. pp. 10.12.11–10.12.18. [DOI] [PubMed] [Google Scholar]
- Reeves E, Colebatch-Bourn A, Elliott T, Edwards CJ, James E. Functionally distinct ERAP1 allotype combinations distinguish individuals with Ankylosing Spondylitis. Proceedings of the National Academy of Sciences USA. 2014;111:17594–17599. doi: 10.1073/pnas.1408882111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saric T, Chang SC, Hattori A, York IA, Markant S, Rock KL, Tsujimoto M, Goldberg AL. An IFN-gamma-induced aminopeptidase in the ER, ERAP1, trims precursors to MHC class I-presented peptides. Nature Immunology. 2002;3:1169–1176. doi: 10.1038/ni859. [DOI] [PubMed] [Google Scholar]
- Saveanu L, Carroll O, Lindo V, Del Val M, Lopez D, Lepelletier Y, Greer F, Schomburg L, Fruci D, Niedermann G, et al. Concerted peptide trimming by human ERAP1 and ERAP2 aminopeptidase complexes in the endoplasmic reticulum. Nature Immunology. 2005;6:689–697. doi: 10.1038/ni1208. [DOI] [PubMed] [Google Scholar]
- Seregin SS, Rastall DP, Evnouchidou I, Aylsworth CF, Quiroga D, Kamal RP, Godbehere-Roosa S, Blum CF, York IA, Stratikos E, et al. Endoplasmic reticulum aminopeptidase-1 alleles associated with increased risk of ankylosing spondylitis reduce HLA-B27 mediated presentation of multiple antigens. Autoimmunity. 2013;46:497–508. doi: 10.3109/08916934.2013.819855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J, Hatano H, Kollnberger S. The biochemistry and immunology of non-canonical forms of HLA-B27. Molecular Immunology. 2014;57:52–58. doi: 10.1016/j.molimm.2013.05.243. [DOI] [PubMed] [Google Scholar]
- Smith JA, Colbert RA. Review: The interleukin-23/interleukin-17 axis in spondyloarthritis pathogenesis: Th17 and beyond. Arthritis & Rheumatology. 2014;66:231–241. doi: 10.1002/art.38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino R, Bockmann RA, Fiorillo MT. HLA-B27 and antigen presentation: at the crossroads between immune defense and autoimmunity. Molecular Immunology. 2014;57:22–27. doi: 10.1016/j.molimm.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Stam NJ, Spits H, Ploegh HL. Monoclonal antibodies raised against denatured HLA-B locus H-chains permit biochemical characterization of certain HLA-C locus products. Journal of Immunology. 1986;137:2299–2306. [PubMed] [Google Scholar]
- Taurog JD, Dorris ML, Satumtira N, Tran TM, Sharma R, Dressel R, van den Brandt J, Reichardt HM. Spondylarthritis in HLA-B27/human beta2-microglobulin-transgenic rats is not prevented by lack of CD8. Arthritis & Rheumatism. 2009;60:1977–1984. doi: 10.1002/art.24599. [DOI] [PubMed] [Google Scholar]
- Tran TM, Colbert RA. Endoplasmic reticulum aminopeptidase 1 and rheumatic disease: functional variation. Current Opinion in Rheumatology. 2015;27:357–363. doi: 10.1097/BOR.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MJ, Delay ML, Bai S, Klenk E, Colbert RA. HLA-B27 up-regulation causes accumulation of misfolded heavy chains and correlates with the magnitude of the unfolded protein response in transgenic rats: Implications for the pathogenesis of spondylarthritis-like disease. Arthritis & Rheumatism. 2007;56:215–223. doi: 10.1002/art.22295. [DOI] [PubMed] [Google Scholar]
- Turner MJ, Sowders DP, DeLay ML, Mohapatra R, Bai S, Smith JA, Brandewie JR, Taurog JD, Colbert RA. HLA-B27 misfolding in transgenic rats is associated with activation of the unfolded protein response. Journal of Immunology. 2005;175:2438–2448. doi: 10.4049/jimmunol.175.4.2438. [DOI] [PubMed] [Google Scholar]
- Urban RG, Chicz RM, Lane WS, Strominger JL, Rehm A, Kenter MJH, Uytde-Haag FGCM, Ploegh H, Uchanska-Ziegler B, Ziegler A. A subset of HLA-B27 molecules contains peptides much longer than nonamers. Proceedings of the National Academy of Sciences USA. 1994;91:1534–1538. doi: 10.1073/pnas.91.4.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utriainen L, Firmin D, Wright P, Cerovic V, Breban M, McInnes I, Milling S. Expression of HLA-B27 causes loss of migratory dendritic cells in a rat model of spondylarthritis. Arthritis & Rheumatism. 2012;64:3199–3209. doi: 10.1002/art.34561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York IA, Brehm MA, Zendzian S, Towne CF, Rock KL. Endoplasmic reticulum aminopeptidase 1 (ERAP1) trims MHC class I-presented peptides in vivo and plays an important role in immunodominance. Proceedings of the National Academy of Sciences USA. 2006;103:9202–9207. doi: 10.1073/pnas.0603095103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York IA, Chang SC, Saric T, Keys JA, Favreau JM, Goldberg AL, Rock KL. The ER aminopeptidase ERAP1 enhances or limits antigen presentation by trimming epitopes to 8–9 residues. Nature Immunology. 2002;3:1177–1184. doi: 10.1038/ni860. [DOI] [PubMed] [Google Scholar]
- Zervoudi E, Saridakis E, Birtley JR, Seregin SS, Reeves E, Kokkala P, Aldhamen YA, Amalfitano A, Mavridis IM, James E, et al. Rationally designed inhibitor targeting antigen-trimming aminopeptidases enhances antigen presentation and cytotoxic T-cell responses. Proceedings of the National Academy of Sciences USA. 2013;110:19890–19895. doi: 10.1073/pnas.1309781110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.