Abstract
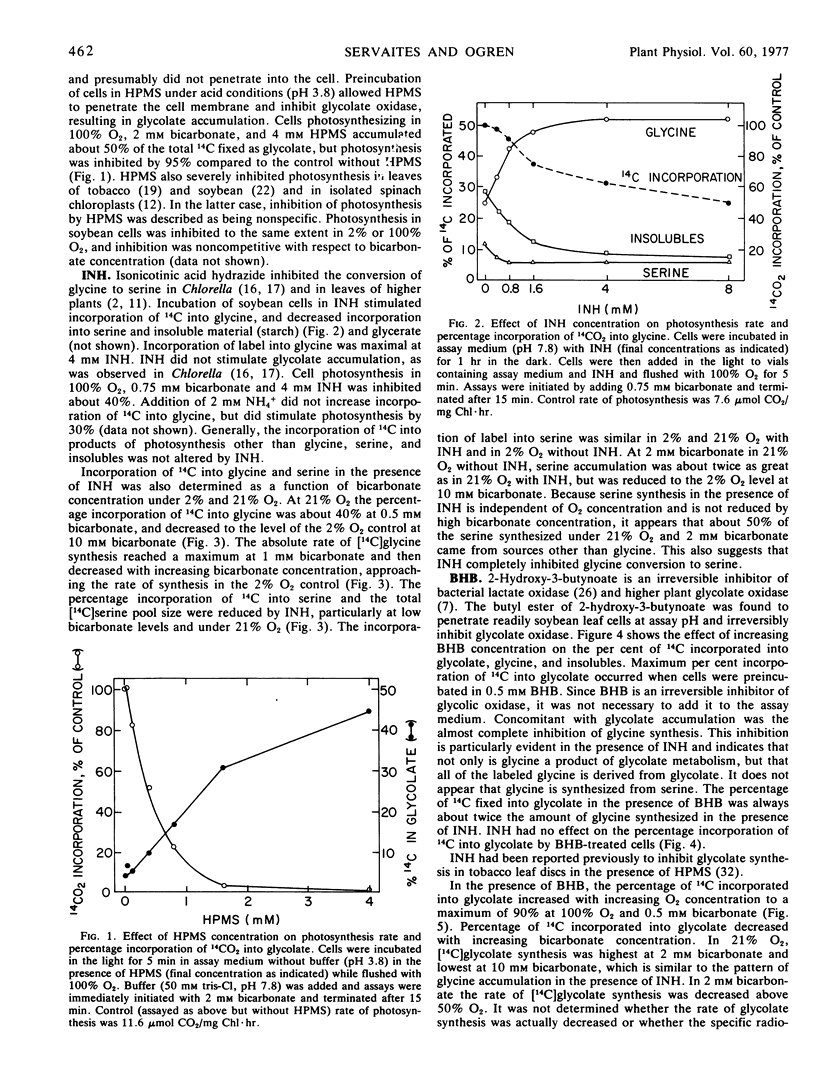
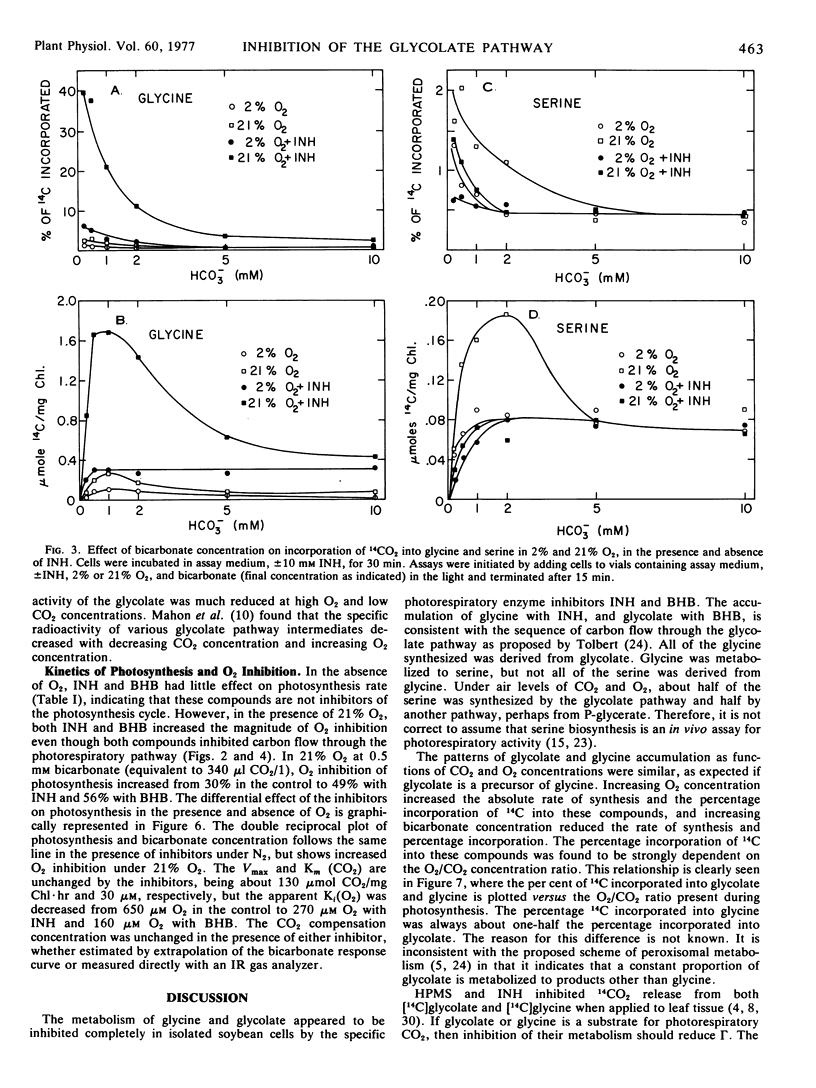
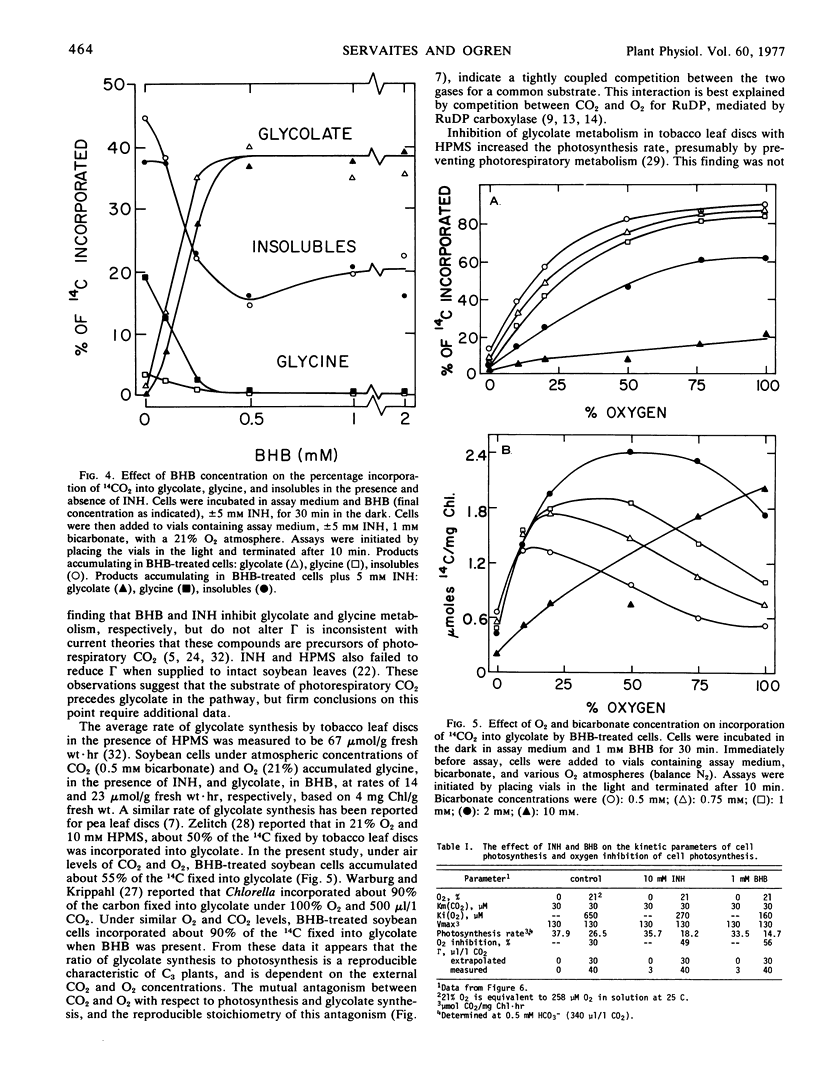
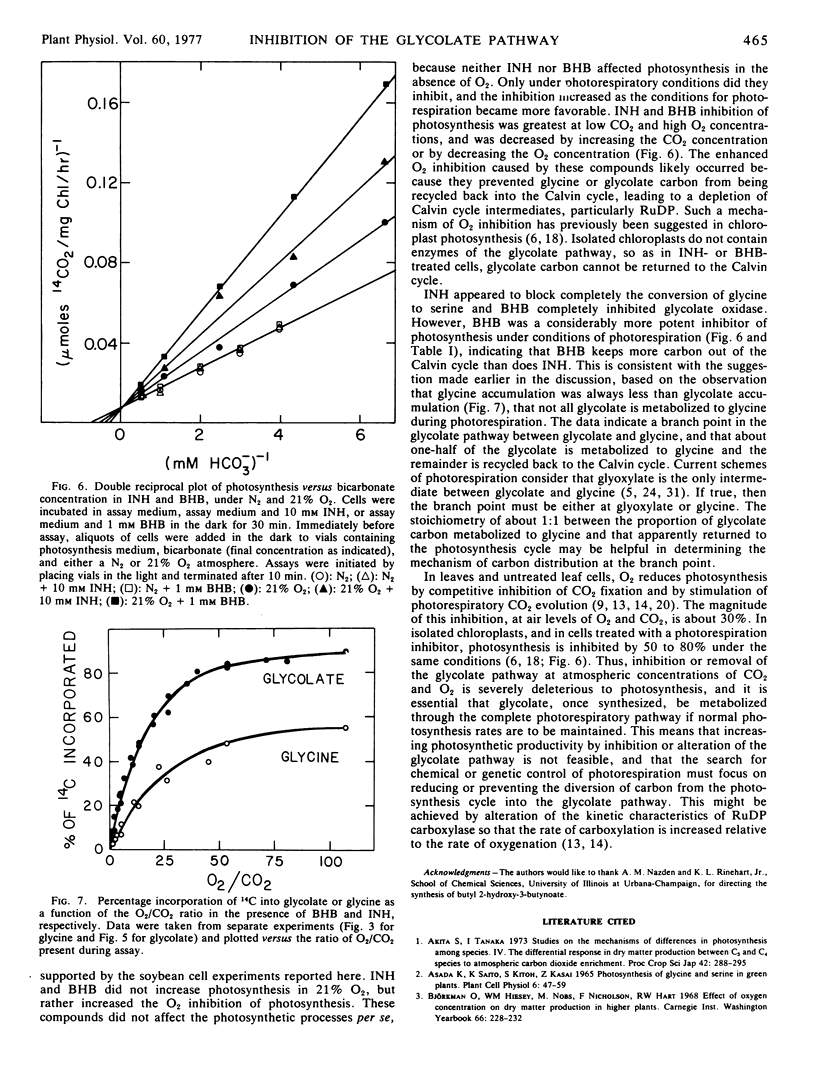
Isolated soybean (Glycine max [L.] Merr.) leaf cells were treated with three inhibitors of the glycolate pathway in order to evaluate the potential of such inhibitors for increasing photosynthetic efficiency. Preincubation of cells under acid conditions in α-hydroxypyridinemethanesulfonic acid increased 14CO2 incorporation into glycolate, but severely inhibited photosynthesis. Isonicotinic acid hydrazide (INH) increased the incorporation of 14CO2 into glycine and reduced label in serine, glycerate, and starch. Butyl 2-hydroxy-3-butynoate (BHB) completely and irreversibly inhibited glycolate oxidase and increased the accumulation of 14C into glycolate. Concomitant with glycolate accumulation was the reduction of label in serine, glycerate, and starch, and the elimination of label in glycine. The inhibitors INH and BHB did not eliminate serine synthesis, suggesting that some serine is synthesized by an alternate pathway. The per cent incorporation of 14CO2 into glycolate by BHB-treated cells or glycine by INH-treated cells was determined by the O2/CO2 ratio present during assay. Photosynthesis rate was not affected by INH or BHB in the absence of O2, but these compounds increased the O2 inhibition of photosynthesis. This finding suggests that the function of the photorespiratory pathway is to recycle glycolate carbon back into the Calvin cycle, so if glycolate metabolism is inhibited, Calvin cycle intermediates become depleted and photosynthesis is decreased. Thus, chemicals which inhibit glycolate metabolism do not reduce photorespiration and increase photosynthetic efficiency, but rather exacerbate the problem of photorespiration.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chollet R. 14CO2 fixation and glycolate metabolism in the dark in isolated maize (Zea mays L.) bundle sheath strands. Arch Biochem Biophys. 1974 Aug;163(2):521–529. doi: 10.1016/0003-9861(74)90510-4. [DOI] [PubMed] [Google Scholar]
- Ellyard P. W., Gibbs M. Inhibition of photosynthesis by oxygen in isolated spinach chloroplasts. Plant Physiol. 1969 Aug;44(8):1115–1121. doi: 10.1104/pp.44.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewess P. J., Kerr M. W., Whitaker D. P. Inhibition of glycollate oxidase from pea leaves. FEBS Lett. 1975 May 15;53(3):292–296. doi: 10.1016/0014-5793(75)80039-1. [DOI] [PubMed] [Google Scholar]
- Laing W. A. Regulation of Soybean Net Photosynthetic CO(2) Fixation by the Interaction of CO(2), O(2), and Ribulose 1,5-Diphosphate Carboxylase. Plant Physiol. 1974 Nov;54(5):678–685. doi: 10.1104/pp.54.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin B. J., Marker A. F., Whittingham C. P. The metabolism of glycine and glycollate by pea leaves in relation to photosynthesis. Biochim Biophys Acta. 1966 Jun 8;120(2):266–273. doi: 10.1016/0926-6585(66)90346-3. [DOI] [PubMed] [Google Scholar]
- Robinson J. M., Gibbs M. Photosynthetic intermediates, the warburg effect, and glycolate synthesis in isolated spinach chloroplasts. Plant Physiol. 1974 Jun;53(6):790–797. doi: 10.1104/pp.53.6.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C. Rapid isolation of mesophyll cells from leaves of soybean for photosynthetic studies. Plant Physiol. 1977 Apr;59(4):587–590. doi: 10.1104/pp.59.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. W., Tolbert N. E., Ku H. S. Variables Affecting the CO(2) Compensation Point. Plant Physiol. 1976 Aug;58(2):143–146. doi: 10.1104/pp.58.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder F. W. Effect of CO(2) Concentration on Glycine and Serine Formation during Photorespiration. Plant Physiol. 1974 Mar;53(3):514–515. doi: 10.1104/pp.53.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARBURG O., KRIPPAHL G. [Glycolic acid formation in Chlorella]. Z Naturforsch B. 1960 Mar;15B:197–199. [PubMed] [Google Scholar]
- Walsh C. T., Schonbrunn A., Lockridge O., Massey V., Abeles R. H. Inactivation of a flavoprotein, lactate oxidase, by an acetylenic substrate. J Biol Chem. 1972 Sep 25;247(18):6004–6006. [PubMed] [Google Scholar]
- ZELITCH I. The relationship of glycolic acid to respiration and photosynthesis in tobacco leaves. J Biol Chem. 1959 Dec;234:3077–3081. [PubMed] [Google Scholar]
- Zelitch I. Alternate pathways of glycolate synthesis in tobacco and maize leaves in relation to rates of photorespiration. Plant Physiol. 1973 Feb;51(2):299–305. doi: 10.1104/pp.51.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I. Comparison of the effectiveness of glycolic Acid and glycine as substrates for photorespiration. Plant Physiol. 1972 Jul;50(1):109–113. doi: 10.1104/pp.50.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I. Improving the efficiency of photosynthesis. Science. 1975 May 9;188(4188):626–633. doi: 10.1126/science.188.4188.626. [DOI] [PubMed] [Google Scholar]
- Zelitch I. Increased rate of net photosynthetic carbon dioxide uptake caused by the inhibition of glycolate oxidase. Plant Physiol. 1966 Dec;41(10):1623–1631. doi: 10.1104/pp.41.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]


