Abstract
Background
Mothers whose infants are born with complex congenital heart disease (CCHD) experience stress during their infant’s hospitalization in a pediatric cardiac intensive care unit (PCICU).
Objectives
This study addressed 2 research questions: (1) What are the parental stressors for mothers whose infants with CCHD are in the PCICU? And (2) What are the relationships of trait anxiety and 3 parental stressors to the parental stress response of state anxiety in mothers whose infants with CCHD are in the PCICU?
Methods
This descriptive correlational study included 62 biological mothers of infants admitted to a PCICU within 1 month of birth who had undergone cardiac surgery for CCHD. Maternal and infant demographics and responses to the Parental Stressor Scale: Infant Hospitalization and the State-Trait Anxiety Inventory were collected at 3 major PCICUs across the United States.
Results
Mothers’ scores revealed that infant appearance and behavior was the greatest stressor, followed by parental role alteration, then sights and sounds. The combination of trait anxiety and parental role alteration explained 26% of the variance in maternal state anxiety. Mothers with other children at home had significantly higher state anxiety than did mothers with only the hospitalized infant.
Conclusions
Results from this study revealed factors that contribute to the stress of mothers whose infants are born with CCHD and are hospitalized in a PCICU. Nurses are in a critical position to provide education and influence care to reduce maternal stressors in the PCICU, enhance mothers’ parental role, and mitigate maternal state anxiety.
Congenital heart disease (CHD) is the most common birth defect, with a rate of 8 per 1000 live births.1 When infants are born with complex congenital heart disease (CCHD), they require surgery during the early weeks of life with inpatient care provided in a pediatric cardiac intensive care unit (PCICU). Parents experience stress as a result of their infants’ diagnosis and hospitalization.2–4 The parental stress associated with this very serious and acute period surrounding open heart surgery is often overwhelming.
Parental stress in neonatal intensive care units (NICUs) and pediatric intensive care units (PICUs) has been well described throughout the past 3 decades. Past research on parental stress has been largely focused on parents of premature infants or on older children in the PICU, but little work has been done to explore the stress of parents who have newborns undergoing cardiac surgery soon after birth.5–7 These studies provide a foundation for understanding parental stress in the PCICU, but are insufficient to describe the unique experience for parents of infants undergoing neonatal open heart surgery.
With the improvements in survival after neonatal cardiac surgery, the needs of these medically fragile infants have become more complex. The cardiac diagnoses of many of these infants are made in utero at approximately 20 weeks’ gestation. Therefore, parents begin to experience stress even before the infant is born and admitted to a PCICU.2 In addition, the postoperative recovery is often a nonlinear process, requiring multidisciplinary care to manage comorbid conditions and complications, including prolonged intubation, chylothorax, bleeding, infection, cardiac arrest, seizures, failure to thrive, and feeding difficulties.8,9 Because infants often experience some of these complications, parental stress is further exacerbated. Moreover, parents of children with CHD report higher amounts of stress than parents of healthy children or parents of children with other diseases.10–12
The source of stress appears to emanate from the infant, parent, and environment. Depending on the severity of CCHD, infants may need to be immediately separated from their parents at birth for resuscitation and intervention in the PCICU.13 The physical separation of parent and child can cause significant stress for parents.14–16 In the PCICU, infants may appear blue or in respiratory distress. They often require various technologies, tubes, medications, and wires for care and monitoring.17,18 The appearance and behavior of the infant creates stress for parents in addition to the sights and sounds of the critical care environment. Parents are unable to provide for their infants’ basic needs, such as clothing, feeding, and comforting, altering their sense of parental role with the infant.19 Mothers experience greater stress and anxiety than fathers do when their infant/ child is in the critical care environment.20–22 This stress is often accompanied by a myriad of postpartum issues, such as physical discomfort, fatigue, hormonal changes, and the challenge of initiating lactation through mechanical breast pumping. Therefore, when examining stress, mothers and fathers must be examined separately.
The number of stressors faced by parents influences their stress response.23 Anxiety is the predominant manifestation of stress at the bedside and is the most referred to in published reports as a stress response.24–28 Two types of anxiety exist: trait anxiety and state anxiety.29 Trait anxiety is an individual’s tendency to perceive a stressful situation as threatening and respond with elevated levels of state anxiety. State anxiety is the amount of anxiety experienced at a specific moment in time. This study focused on state anxiety as a stress response of mothers in the PCICU during the first few weeks after the infant’s surgery for CCHD.
Purposes of the Study
The primary purpose of this study was to explore the stressors and stress response of mothers whose infants with CCHD were being cared for in the PCICU. This study addressed 2 research questions: (1) What are the parental stressors for mothers whose infants with CCHD are in the PCICU? and (2) What are the relationships of trait anxiety and 3 parental stressors to the parental stress response of state anxiety in mothers whose infants with CCHD are in the PCICU?
Theoretical Framework
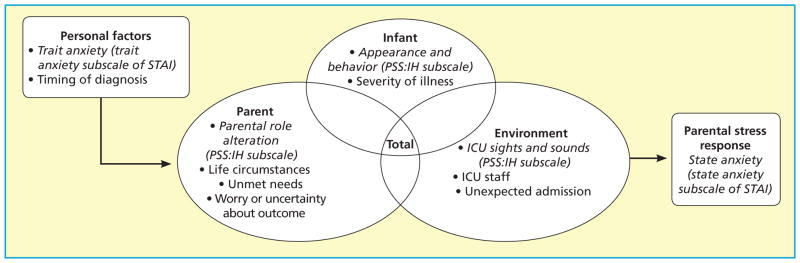
This study used the PCICU Parental Stress Model, which was based on a synthesis of the literature, revisions from prior stress theories developed by Miles and colleagues,30–32 and adjustments to fit research in the PCICU environment (see Figure). The PCICU Parental Stress Model posits that stress is an overarching concept encompassing a stress stimulus, or stressor, that elicits a reaction, or stress response. The model describes parental stress in the critical care setting as emanating from personal factors, such as trait anxiety, and 3 categories of parental stressors: infant, parent, and environment. These sources of stress combine to contribute to parental stress response.
Figure.
The Pediatric Cardiac Intensive Care Unit (PCICU) Parental Stress Model, with application to this study italicized. Abbreviations: ICU, intensive care unit; PSS:IH, Parental Stressor Scale: Infant Hospitalization; STAI, State-Trait Anxiety Inventory.
Methods
This study used a descriptive correlational design to identify the relationships among parental stressors and stress response.
Setting
Data collection occurred at 3 major PCICUs at children’s hospitals across the United States, 2 in the northeast mid-Atlantic region and 1 in the west. Approval was obtained from the institutional review board at each hospital.
Sample
Mothers who were at least 18 years of age, English speaking, with full-term infants who had undergone cardiac surgery for CCHD were approached for participation in this study. Mothers were excluded if their infants had other genetic or congenital abnormalities diagnosed or if the infant was undergoing end-of-life care. Informed consent was obtained from each study participant before data collection.
Research Instruments
Study participants completed the Parental Stressor Scale: Infant Hospitalization (PSS:IH) and the State-Trait Anxiety Inventory (STAI). The PSS:IH is designed to measure parents’ perception of stressors related to the hospitalization of an infant being cared for in any type of hospital unit.33 PSS:IH has 3 subscales: infant appearance and behavior, parental role alteration, and sights and sounds. The PSS:IH has established validity and reliability.33 Mothers rated each item on a scale from 1 to 5. A total mean score was computed for each mother. The internal consistency reliability (Cronbach α) was 0.91. Reliability for each subscale was as follows: 0.83 for infant appearance and behavior, 0.81 for parental role alteration, and 0.81 for sights and sounds.
The STAI is a well-established research instrument that has been used by researchers for more than 30 years. The STAI has two 20-item subscales: one for state anxiety and one for trait anxiety.29 The state anxiety scale measures how persons feel at this current moment. The trait anxiety scale measures a person’s tendency toward anxiety by asking people how they generally feel. Mothers rated each item on a scale from 1 to 4. A total score was computed for each mother participating in the study. The internal consistency reliability (Cronbach α) was 0.95 for the state anxiety scale and 0.91 for the trait anxiety scale.
Results
Participants included 62 biological mothers of infants admitted to the PCICU within 1 month of birth. The sample size was sufficient for a power of 80% with a moderate effect size. The overall majority of the mothers were white, non-Hispanic (n = 47), married, well-educated, with 1 or 2 children (Table 1). Almost three-quarters of the mothers (n = 45) received a prenatal diagnosis on their infants’ CHD. Infants were nearly evenly distributed between males (n = 29) and females (n = 32) and univentricular (n = 33) and biventricular (n = 28) CHD. Infants had a variety of cardiac defects and surgeries. At birth, infants were full-term with appropriate birth weight for gestational age. At the time of data collection, infants were a mean age of 16.79 days old and had undergone cardiac surgery just more than 1 week earlier (Table 2).
Table 1.
Maternal and infant demographics
| Variable | Category | No. (%)a |
|---|---|---|
| Maternal race | American Indian/Alaskan Native | 1 (2) |
| Black/African American | 6 (10) | |
| White | 48 (77) | |
| Unknown/other | 7 (11) | |
|
| ||
| Marital status | Single | 14 (23) |
| Married | 37 (60) | |
| Divorced | 1 (2) | |
| Partnered | 5 (8) | |
|
| ||
| Level of education | Partial high school | 4 (6) |
| Graduated high school | 5 (8) | |
| Partial college | 15 (24) | |
| College graduate | 23 (37) | |
| Master’s degree | 7 (11) | |
| PhD/doctoral degree | 1 (2) | |
|
| ||
| Diagnosis | Hypoplastic left heart syndrome | 19 (31) |
| Transposition of the great arteries (D-loop) | 12 (19) | |
| Ventricular septal defect | 10 (16) | |
| Pulmonary stenosis (valvular and subvalvular) | 9 (15) | |
| Coarctation of the aorta | 8 (13) | |
| Atrioventricular canal | 6 (10) | |
| Double-outlet right ventricle | 4 (6) | |
| Tetralogy of Fallot | 4 (6) | |
| Other | 40 (65) | |
|
| ||
| Surgery | Norwood procedure | 13 (21) |
| Aortic arch repair | 6 (10) | |
| Arterial switch operation | 6 (10) | |
| Atrial septal defect closure | 5 (8) | |
| Coarctation end to end | 3 (5) | |
| Tetralogy of Fallot repair | 3 (5) | |
| Other | 32 (52) | |
Percentages may not total 100% because of missing data, multiple diagnoses, or multiple surgeries.
Table 2.
Descriptive statistics for selected infant characteristics
| Variable | Mean | SD | Range |
|---|---|---|---|
| Gestational age at birth, weeks | 38.92 | 0.93 | 37–41 |
| Birth weight, g | 3379.28 | 409.12 | 2530–4336 |
| Infant’s age at the time of data collection, days | 16.79 | 9.37 | 4–44 |
| Length of time postoperatively at data collection, days | 9.11 | 9.73 | 1–46 |
The parental stressors for mothers were identified on the PSS:IH. The mean score for the total PSS:IH was 3.52 (SD, 0.72), with scores ranging from 1.70 to 4.81. Infant appearance and behavior had the highest subscale score (mean, 4.03; SD, 0.86). The second highest mean score was for the parental role alteration subscale (mean, 3.77; SD, 0.81). The sights and sounds subscale had the lowest mean score (mean, 2.52; SD, 0.85). Rank order by means for all 22 items on the PSS:IH revealed that the 5 strongest stressors, with mean scores from 4.05 to 4.56, referred to mothers’ not being able to parent or comfort their baby or protect their baby from pain. These items included, in rank order beginning with the highest: “seeing your child in pain,” “not being able to protect your baby from pain and painful procedures,” “when your child looks afraid, upset, or cries a lot,” “not being able to comfort or help your baby,” and “being separated from your baby.” Descriptive statistics for the STAI revealed that mothers’ state anxiety scores (mean, 44.18; SD, 13.30) were significantly higher (t=6.74, df=61, P < .001) than their trait anxiety scores (mean, 33.79; SD, 8.92).
Stepwise multiple regression was computed to determine the relationships among the 4 predictor variables (maternal trait anxiety, parental role alteration, infant appearance and behavior, and sights and sounds) and maternal state anxiety. Although maternal trait anxiety (r=0.46), parental role alteration (r = 0.32), and sights and sounds (r = 0.37) were significantly correlated with state anxiety (P ≤ .006), only maternal trait anxiety and parental role alteration entered into the regression (Table 3). Maternal trait anxiety and parental role alteration were not significantly related (r = 0.20, P = .06). Sights and sounds failed to enter the equation because of multicollinearity.
Table 3.
Stepwise regression predicting maternal state anxiety
| Model | R | R2 | R2Δ | F | df | P |
|---|---|---|---|---|---|---|
| 1. Trait anxiety | 0.46 | 0.21 | 0.21 | 16.03 | 1 | <.001 |
| 2. Parental role alteration | 0.51 | 0.26 | 0.05 | 4.27 | 1 | .04 |
Independent t tests were used to explore differences between groups based on the class of the infant’s CCHD (univentricular vs biventricular physiology), the timing of the diagnosis (prenatal vs postnatal), the number of children (1 child vs more than 1 child), and the time of data collection (< 1 week postoperatively vs ≥ 1 week postoperatively). No significant differences were found between univentricular and biventricular groups or between prenatal and postnatal timing of diagnosis on any of the stressor or anxiety measures. State anxiety scores were significantly higher (t = −2.26, df = 51, P = .03) for the 24 mothers with more than 1 child (mean, 48.07; SD, 13.33) than for the 28 mothers with only 1 child (mean, 40.21; SD, 11.97). In addition, state anxiety scores were significantly higher (t = 2.03, df=40, P = .049) for mothers whose infants were less than 1 week postoperative (mean, 47.95; SD, 13.70) than for mothers whose infants were 1 week or more postoperative (mean, 40.24; SD, 10.77).
Discussion
This study is the first to examine maternal stressors and stress response in a PCICU. The results of this study confirm that mothers experience stress while their infants are hospitalized in a PCICU. Mothers in this study perceived stressors from parental role alteration, infant appearance and behavior, and sights and sounds of the PCICU. The highest rated stressor in this study was infant appearance and behavior. Using a revised version of the PSS:IH, the PSS:CH, Franck and colleagues3 also reported that child appearance and behavior was the highest rated stressor for both mothers and fathers across 5 separate time points throughout the PCICU hospitalization.
Parental role alteration was the second highest source of stress for mothers in this study. Items within this subscale reflect the mother’s ability to safeguard her baby from harm or discomfort as well as her ability to be close, care for, and hold her baby. Mothers perceived stress from not being able to perform basic parenting tasks for their babies. Mothering of an infant usually centers around clothing, feeding, diaper changing, and holding. Most, if not all, of these parenting functions are paused during the critical care admission, especially during the immediate postoperative period. Parental role alteration was also reported to be the highest stressor in other studies using the PSS:NICU.20,24,34 The complexity of care that postoperative infants require is often reflected by their critical appearance in a PCICU, which may have influenced why infant appearance and behavior was the highest rated stressor by mothers in this study.
The 2 highest rated items in the PSS:IH were on the infant appearance and behavior subscale and the parental role alteration subscale. These items both addressed infant pain from the mother’s perspective: seeing the infant in pain, and being unable to comfort or prevent pain. Parental worry about infant pain contributes to parental stress in the NICU.22,35
The subscale sights and sounds was the lowest rated stressor in this study, consistent with other published reports3,33,36; however, sights and sounds still contributed a moderate amount of perceived stress for mothers. Every patient in the PCICU is connected to a cardiopulmonary monitor that audibly alarms when the patient’s vital signs move outside set parameters. A mother can observe the monitor and often becomes very in tune with her infant’s vital signs. The physical layout of the PCICUs for most participants in this study was open, with bay-style rooms that allowed parents to see and hear the activity at another patient’s bedside. The results of this study suggest that these aspects of the PCICU environment create stress for mothers visiting their infants.
Although infant appearance and behavior was the highest rated stressor, only trait anxiety and parental role alteration entered into the multiple regression to predict state anxiety scores. Research has documented the link between parental role alteration and stress response in other ICU settings.22,34 Shaw and colleagues21 reported that parental role alteration was the most strongly correlated variable to stress response, as measured by acute stress disorder, in 40 parents of infants hospitalized in a NICU. In the classic study by Miles and colleagues,32 trait anxiety and parental role alteration influenced stress response, as measured by state anxiety. The mean trait anxiety scores of mothers in our study fell within the 50th percentile of normative mean published values for working women aged 19 to 49 years, which is an expected finding as this study’s sample was a group of healthy young women.29 In contrast, the mean state anxiety scores of mothers in our study were high, around the 83rd percentile.29 State anxiety scores were significantly higher than trait anxiety scores in our sample. State anxiety in 119 mothers of hospitalized NICU infants was reported with mean scores of 51.53 (SD, 14.96) by Shields-Poe and Pinelli22 and 51.25 (SD, 14.10) by Franck and colleagues.35 Although these means are higher than those of mothers in our study, trait anxiety scores published by Shields-Poe and Pinelli (mean, 39.2; SD, 11.02) and Franck et al (mean, 41.61; SD, 11.02) were also higher than the means reported in our study. State anxiety in pregnant women who had an infant with a prenatal diagnosis of CCHD was also high (mean, 44.14; SD, 14.69). Trait anxiety scores for these pregnant women were also higher than normative mean published values, potentially influencing state anxiety scores.4 Although trait anxiety is a nonmodifiable factor affecting state anxiety, it can be screened for and identified early so that psychosocial supports and interventions can be provided to mediate maternal state anxiety.
The results of this study also showed that mothers with more than 1 child experienced higher stress response than did mothers with only the hospitalized infant. Mothers who have other children may need additional support to cope with the hospitalization as they manage competing responsibilities of caring for children at home and watching over their infant in the PCICU. Finally, maternal state anxiety was significantly higher less than 1 week postoperatively when compared with maternal state anxiety scores obtained 1 week postoperatively or more. Franck and colleagues3 reported that parental stress was reduced in the PCICU when measured on postoperative day 8, in comparison with stress scores measured preoperatively, on postoperative day 3, and on postoperative day 5. This study adds to growing evidence that the first week postoperatively is a period of extreme stress for mothers. Additional psychosocial supports should be offered to help mothers cope during the immediate postoperative period.
Limitations
This study had a relatively homogeneous sample, which decreases generalizability to the greater population. Maternal perception of stressors and stress response was measured at only 1 point in time after the infant had surgery. Mothers’ perception of stressors and stress response may change throughout the infant’s hospitalization. In this study, only one type of stress response, state anxiety, was measured. Mothers may experience a variety of stress responses. The relationships between stressors and multiple stress responses need to be explored. Finally, this study relied on self-reported measures to examine perception of stressors and stress response. Objective data were not gathered for these variables.
Conclusions and Implications
This study was the first to explore the stressors and stress response of mothers whose infants with CCHD were being cared for in a PCICU. The results of this study confirm that these mothers perceive stressors from their infants’ appearance and behavior, parental role alteration, and the sights and sounds of the PCICU. Mothers also experienced a heightened stress response of state anxiety. Trait anxiety and parental role alteration were the best predictors of state anxiety for mothers in the PCICU. Additional research is needed to build on this leading study.
Nurses are in a critical position to provide education and influence care to reduce maternal perception of stressors, enhance mothers’ parental role, and mitigate mothers’ state anxiety. Nurses can support mothers through this stressful time. Mothers may also benefit from increased education on interpreting their infants’ behavioral cues and signs of pain as well as how to respond to those cues in the setting of the PCICU. Nurses can facilitate the enhancement of parental role for mothers as their infant recovers from cardiac surgery by allowing holding and participation in feeding, changing, or clothing the infant as appropriate. Finally, nurses can screen mothers for high trait anxiety and identify mothers at risk for heightened stress response. Interventions can be created to assist mothers in coping with their anxiety, such as the use of mindful meditation or cognitive behavioral therapy. The results of this study can guide continuing education on parental stress and prompt cultural change within PCICUs to provide additional psychological support to mothers.
Acknowledgments
The following institutions participated in the study: Widener University School of Nursing, Chester, Pennsylvania; Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania; Phoenix Children’s Hospital, Phoenix, Arizona; Nemours/Alfred I. duPont Hospital for Children, Wilmington, Delaware; and the University of Pennsylvania School of Nursing, Philadelphia, Pennsylvania. The authors express gratitude to the members of the research teams at each participating site: Barbara Fasick, RN-NIC, MS, PPCNP-BC, PCNS-BC, Wilma M. Berends, MSN, Michele Osborn, RN, MSN, CCRN, Tess Wright, RN, BSN, CCRN, Desiree Fleck, RN, PhD, and Darcy Brodecki, BS.
Footnotes
To purchase electronic or print reprints, contact American Association of Critical-Care Nurses, 101 Columbia, Aliso Viejo, CA 92656. Phone, (800) 899-1712 or (949) 362-2050 (ext 532); fax, (949) 362-2049; reprints@aacn.org.
FINANCIAL DISCLOSURES
This research study was supported by the national Institutes of Nursing Research (NINR R01 NR002093), The National Institutes of Health/National Center for Advancing Translational Sciences (UL1TR001878), and a grant from the Eta Beta Chapter of Sigma Theta Tau.
Contributor Information
Amy Jo Lisanti, Postdoctoral fellow at the University of Pennsylvania School of Nursing, and a clinical nurse specialist/nurse researcher at Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania.
Lois Ryan Allen, Professor emeritus of nursing, Widener University School of Nursing, Chester, Pennsylvania.
Lynn Kelly, Professor emeritus of nursing, Widener University School of Nursing, Chester, Pennsylvania.
Barbara Medoff-Cooper, Professor, University of Pennsylvania School of Nursing, and a nurse scientist at Children’s Hospital of Philadelphia.
References
- 1.The impact of congenital heart defects. American Heart Association website; 2015. [Accessed December 19, 2016.]. http://www.heart.org/HEARTORG/Conditions/CongenitalHeartDefects/TheImpactofCongenitalHeartDefects/The-Impact-of-Congenital-Heart-Defects_UCM_001218_Article.jsp. [Google Scholar]
- 2.Brosig CL, Whitstone BN, Frommelt MA, Frisbee SJ, Leuthner SR. Psychological distress in parents of children with severe congenital heart disease: The impact of prenatal versus postnatal diagnosis. J Perinatol. 2007;27:687–692. doi: 10.1038/sj.jp.7211807. [DOI] [PubMed] [Google Scholar]
- 3.Franck LS, Mcquillan A, Wray J, Grocott MPW, Goldman A. Parent stress levels during children’s hospital recovery after congenital heart surgery. Pediatr Cardiol. 2010;31:961–968. doi: 10.1007/s00246-010-9726-5. [DOI] [PubMed] [Google Scholar]
- 4.Rychik J, Donaghue D, Levy S, et al. Maternal psychological stress after prenatal diagnosis of congenital heart disease. J Pediatr. 2013;162:302–307. doi: 10.1016/j.jpeds.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 5.Johnson PA, Nelson GL, Brunnquell DJ. Parent and nurse perceptions of parent stressors in the pediatric intensive care unit. Child Health Care. 1988;17:98–105. doi: 10.1207/s15326888chc1702_7. [DOI] [PubMed] [Google Scholar]
- 6.Miles MS, Funk SG, Kasper MA. The neonatal intensive care unit environment: sources of stress for parents. AACN Clin Issues. 1991;2:346–354. doi: 10.4037/15597768-1991-2022. [DOI] [PubMed] [Google Scholar]
- 7.Pinelli J, Saigal S, Wu YB, et al. Patterns of change in family functioning, resources, coping, and parental depression in mothers and fathers of sick newborns over the first year of life. J Neonat Nurs. 2008;14:156–165. [Google Scholar]
- 8.Padley JR, Cole AD, Pye VE, et al. Five-year analysis of operative mortality and neonatal outcomes in congenital heart disease. Heart Lung Circ. 2011;20(7):460–467. doi: 10.1016/j.hlc.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Schwalbe-Terilli CR, Hartman DH, Nagle ML. Enteral feeding and caloric intake in neonates after cardiac surgery. Am J Crit Care. 2009;18:52–57. doi: 10.4037/ajcc2009405. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg S, Morris P, Simmon RJ, Fowler RS, Levison H. Chronic illness in infancy and parenting stress: a comparison of three groups of parents. J Pediatr Psychol. 1990;15:347–358. doi: 10.1093/jpepsy/15.3.347. [DOI] [PubMed] [Google Scholar]
- 11.Mörelius E, Lundh U, Nelson N. Parental stress in relation to the severity of congenital heart disease in the offspring. Pediatr Nurs. 2002;28(1):28–34. [Google Scholar]
- 12.Uzark K, Jones K. Parenting stress and children with congenital heart disease. J Pediatr Health Care. 2003;17:163–168. doi: 10.1067/mph.2003.22. [DOI] [PubMed] [Google Scholar]
- 13.Hoehn KS, Wernovsky G, Rychik J, et al. Parental decision-making in congenital heart disease. CardiolYoung. 2004;14:309–314. doi: 10.1017/S1047951104003099. [DOI] [PubMed] [Google Scholar]
- 14.Boyd S. Within these walls: moderating parental stress in the NICU. J Neonatal Nurs. 2004;10(3):80–84. [Google Scholar]
- 15.Foster J, Bidewell J, Buckmaster A, Lees S, Henderson-Smart D. Parental stress and satisfaction in the non-tertiary special care nursery. J Adv Nurs. 2008;61:522–530. doi: 10.1111/j.1365-2648.2007.04547.x. [DOI] [PubMed] [Google Scholar]
- 16.Miles MS, Holditch-Davis D. Parenting the prematurely born child: pathways of influence. Semin Perinatol. 1997;21:254–266. doi: 10.1016/s0146-0005(97)80067-5. [DOI] [PubMed] [Google Scholar]
- 17.Board R. Father stress during a child’s critical care hospitalization. J Pediatr Healthcare. 2004;18:244–249. doi: 10.1016/j.pedhc.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Board R, Ryan-Wenger N. Stressors and stress symptoms of mothers with children in the PICU. J Pediatr Nurs. 2003;18:195–202. doi: 10.1053/jpdn.2003.38. [DOI] [PubMed] [Google Scholar]
- 19.Seideman RY, Watson MA, Corff KE, Odle P, Haase J, Bowerman JL. Parent stress and coping in NICU and PICU. J Pediatr Nurs. 1997;12:169–177. doi: 10.1016/s0882-5963(97)80074-7. [DOI] [PubMed] [Google Scholar]
- 20.Dudek-Shriber L. Parent stress in the neonatal intensive care unit and the influence of parent and infant characteristics. Am J Occup Ther. 2004;58:509–520. doi: 10.5014/ajot.58.5.509. [DOI] [PubMed] [Google Scholar]
- 21.Shaw RJ, Deblois T, Ikuta L, Ginzburg K, Fleisher B, Koopman C. Acute stress disorder among parents of infants in the neonatal intensive care nursery. Psychosomatics. 2006;47:206–212. doi: 10.1176/appi.psy.47.3.206. [DOI] [PubMed] [Google Scholar]
- 22.Shields-Poe D, Pinelli J. Variables associated with parental stress in neonatal intensive care units. Neonatal Netw. 1997;16(1):29–37. [PubMed] [Google Scholar]
- 23.Lazarus RS. Stress and Emotion: A New Synthesis. New York, NY: Springer; 1999. [Google Scholar]
- 24.Franck LS, Cox S, Allen A, Winter I. Measuring neonatal intensive care unit-related parental stress. J Adv Nurs. 2005;49:608–615. doi: 10.1111/j.1365-2648.2004.03336.x. [DOI] [PubMed] [Google Scholar]
- 25.Huckabay LMD, Tilem-Kessler D. Patterns of parental stress in PICU emergency admission. Dimens Crit Care Nurs. 1999;18(2):36–42. doi: 10.1097/00003465-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Melnyk BM, Alpert-Gillis L, Feinstein NF, et al. Creating opportunities for parent empowerment: program effects on the mental health/coping outcomes of critically ill young children and their mothers. Pediatrics. 2004;113:e597–e607. doi: 10.1542/peds.113.6.e597. [DOI] [PubMed] [Google Scholar]
- 27.Preyde M, Ardal F. Effectiveness of a parent “buddy” program for mothers of very preterm infants in a neonatal intensive care unit. CMAJ. 2003;168:969–973. [PMC free article] [PubMed] [Google Scholar]
- 28.Turan T, Basbakkal Z, Özbek S. Effect of nursing interventions on stressors of parents of premature infants in neonatal intensive care unit. J Clin Nurs. 2008;17:2856–2866. doi: 10.1111/j.1365-2702.2008.02307.x. [DOI] [PubMed] [Google Scholar]
- 29.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. State-Trait Anxiety Inventory for Adults: Manual, Instrument, and Scoring Guide. Palo Alto, CA: Consulting Psychologists Press; 1983. Retrieved from http://www.mindgarden.com. [Google Scholar]
- 30.Holditch-Davis D, Miles MS. Mothers’ stories about their experiences in the neonatal intensive care unit. Neonatal Netw. 2000;19(3):13–21. doi: 10.1891/0730-0832.19.3.13. [DOI] [PubMed] [Google Scholar]
- 31.Miles MS, Carter MC. Assessing parental stress in intensive care units. Am J Matern Child Nurs. 1983;8:354–359. [Google Scholar]
- 32.Miles MS, Carter MC, Hennessey J, Eberly TW, Riddle I. Testing a theoretical model: correlates of parental stress responses in the pediatric intensive care unit. Matern Child Nurs J. 1989;18:207–219. [PubMed] [Google Scholar]
- 33.Miles MS, Brunssen SH. Psychometric properties of the parental stressor scale: infant hospitalization. Adv Neonatal Care. 2003;3:189–196. doi: 10.1016/s1536-0903(03)00138-3. [DOI] [PubMed] [Google Scholar]
- 34.Busse M, Stromgren K, Thorngate L, Thomas K. Parents’ responses to stress in the neonatal intensive care unit. Crit Care Nurse. 2013;33(4):52–60. doi: 10.4037/ccn2013715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franck LS, Cox S, Allen A, Winter I. Parental concern and distress about infant pain. Arch Dis Child Fetal Neonatal Ed. 2004;89:F71–F75. doi: 10.1136/fn.89.1.F71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wereszczak J, Miles MS, Holditch-Davis D. Maternal recall of the neonatal intensive care unit. Neonatal Netw. 1997;16(4):33–40. [PubMed] [Google Scholar]