Abstract
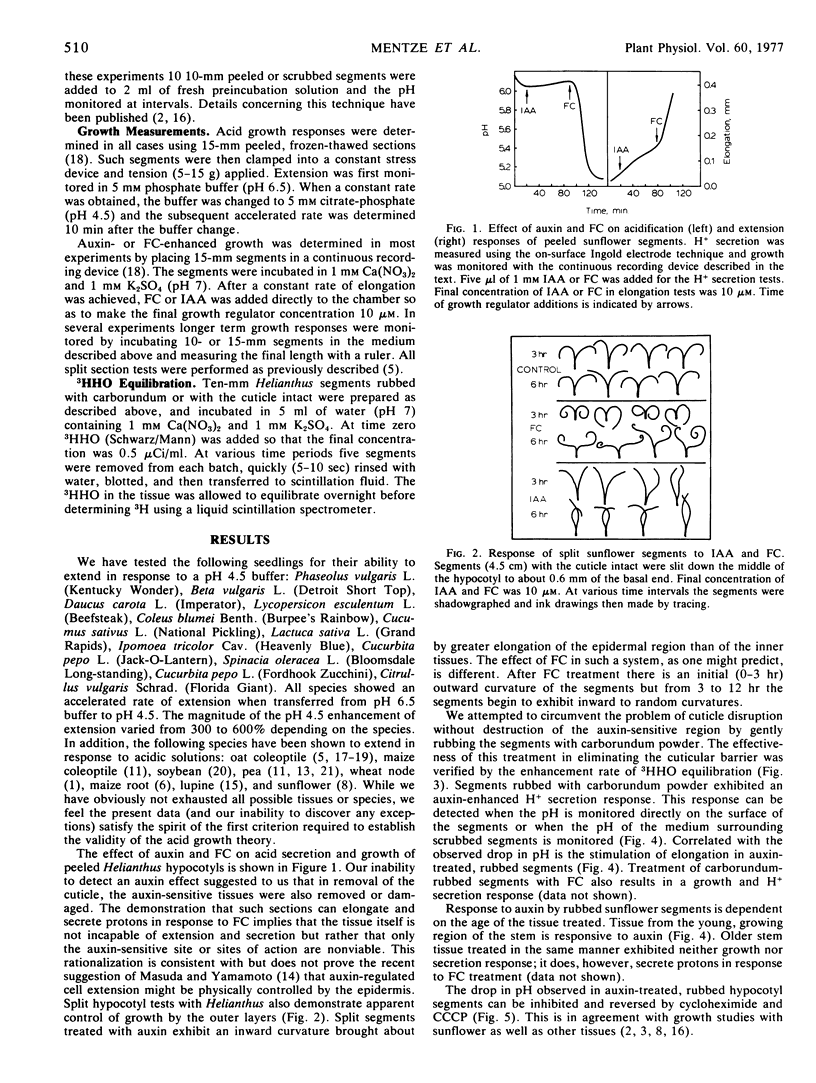
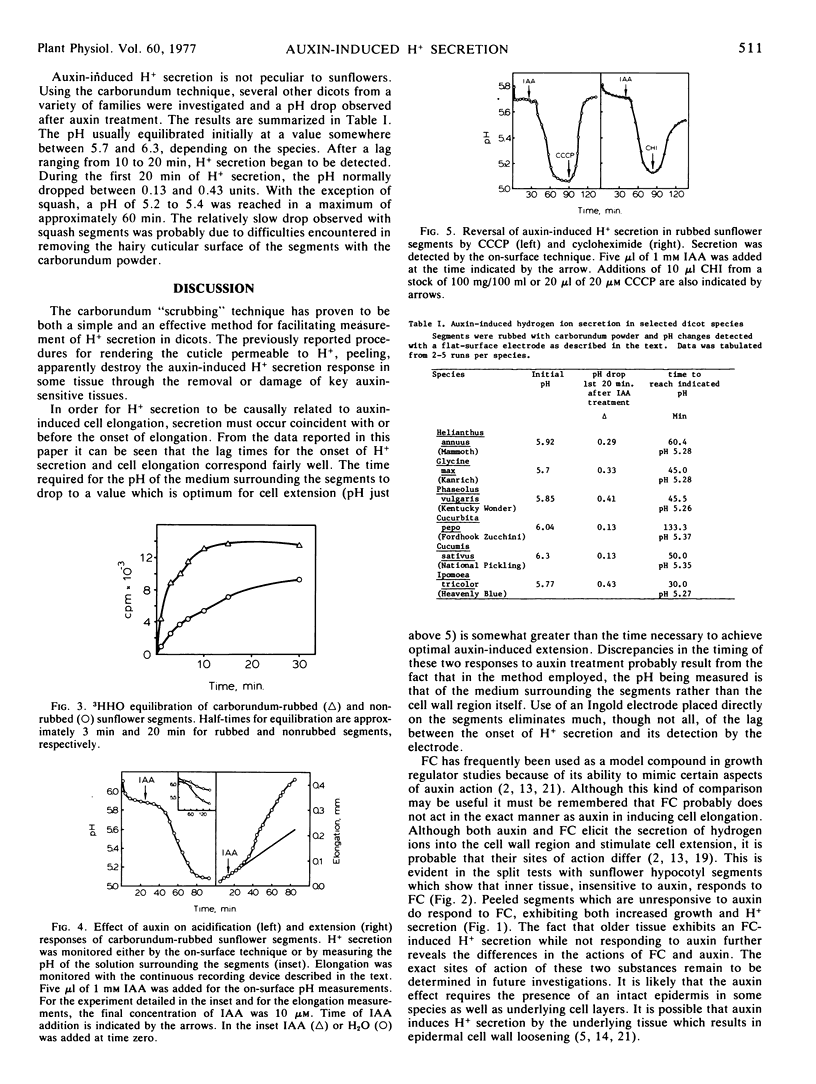
We have examined the ability of Helianthus hypocotyl segments as well as segments from a variety of other species to elongate in response to H+ and to secrete H+ in response to auxin and fusicoccin. In all cases a positive response was obtained when the cuticular barrier was abraded with carborundum. Removal of the cuticular barrier by “peeling” prevented detection of both auxin-induced elongation and H+ secretion. Fusicoccin-induced growth and acid secretion are not prevented by peeling. These results suggest considerable tissue selectivity with respect to auxin action but considerably less specificity with respect to fusicoccin. It seems likely that in many dicots auxin-enhanced proton secretion and elongation are controlled by the epidermis and/or closely associated cell layers. The data presented in this paper provide further support for the acid growth theory of auxin action.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleland R. E. Kinetics of Hormone-induced H Excretion. Plant Physiol. 1976 Aug;58(2):210–213. doi: 10.1104/pp.58.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. Auxin-induced hydrogen ion excretion from Avena coleoptiles. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3092–3093. doi: 10.1073/pnas.70.11.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowler M. J., Rayle D. L. Auxin Does Not Alter the Permeability of Pea Segments to Tritium-labeled Water. Plant Physiol. 1974 Feb;53(2):229–232. doi: 10.1104/pp.53.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. B., Cummins W. R. Growth rate and turgor pressure: auxin effect studies with an automated apparatus for single coleoptiles. Plant Physiol. 1974 Dec;54(6):863–869. doi: 10.1104/pp.54.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M., Ray P. M. Promotion of Xyloglucan Metabolism by Acid pH. Plant Physiol. 1975 Sep;56(3):373–376. doi: 10.1104/pp.56.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M., Ray P. M. Rapid Auxin-induced Decrease in Free Space pH and Its Relationship to Auxin-induced Growth in Maize and Pea. Plant Physiol. 1976 Aug;58(2):203–209. doi: 10.1104/pp.58.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Daniels D., Dowler M. J., Rayle D. L. Activation of Avena coleoptile cell wall glycosidases by hydrogen ions and auxin. Plant Physiol. 1974 Feb;53(2):224–228. doi: 10.1104/pp.53.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoef L. N., Stahl C. A. Separation of two responses to auxin by means of cytokinin inhibition. Proc Natl Acad Sci U S A. 1975 May;72(5):1822–1825. doi: 10.1073/pnas.72.5.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]


