Abstract
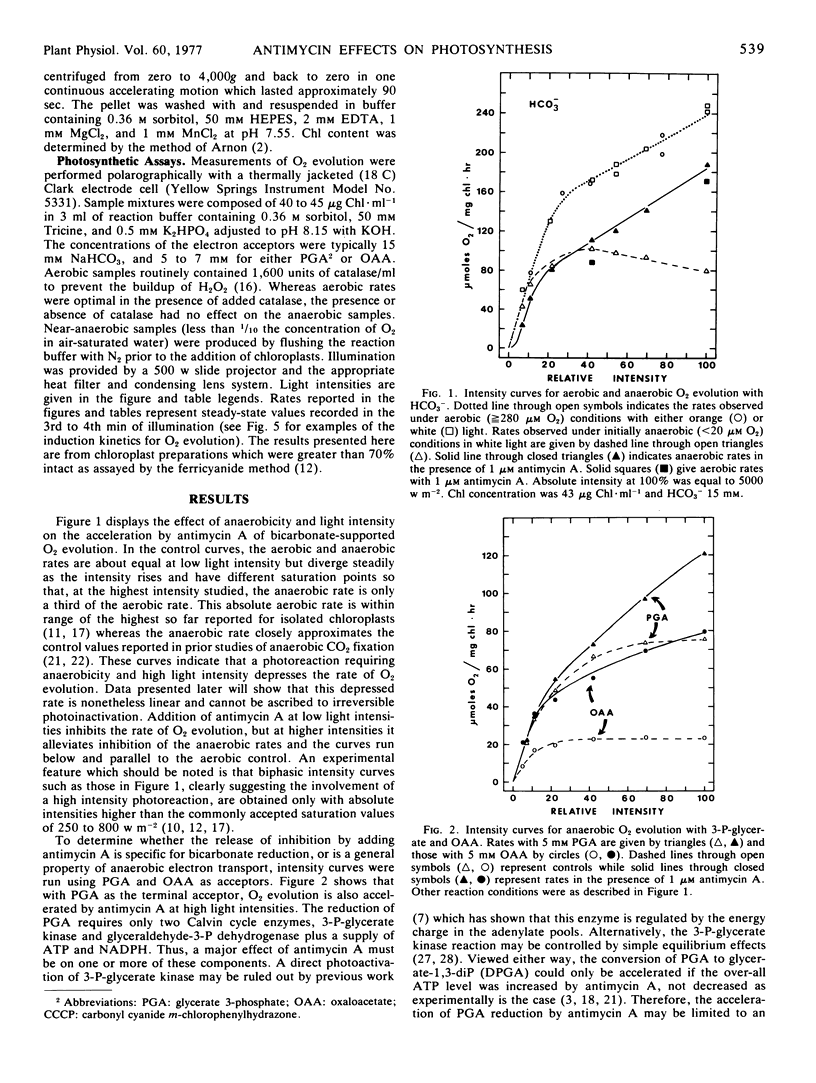
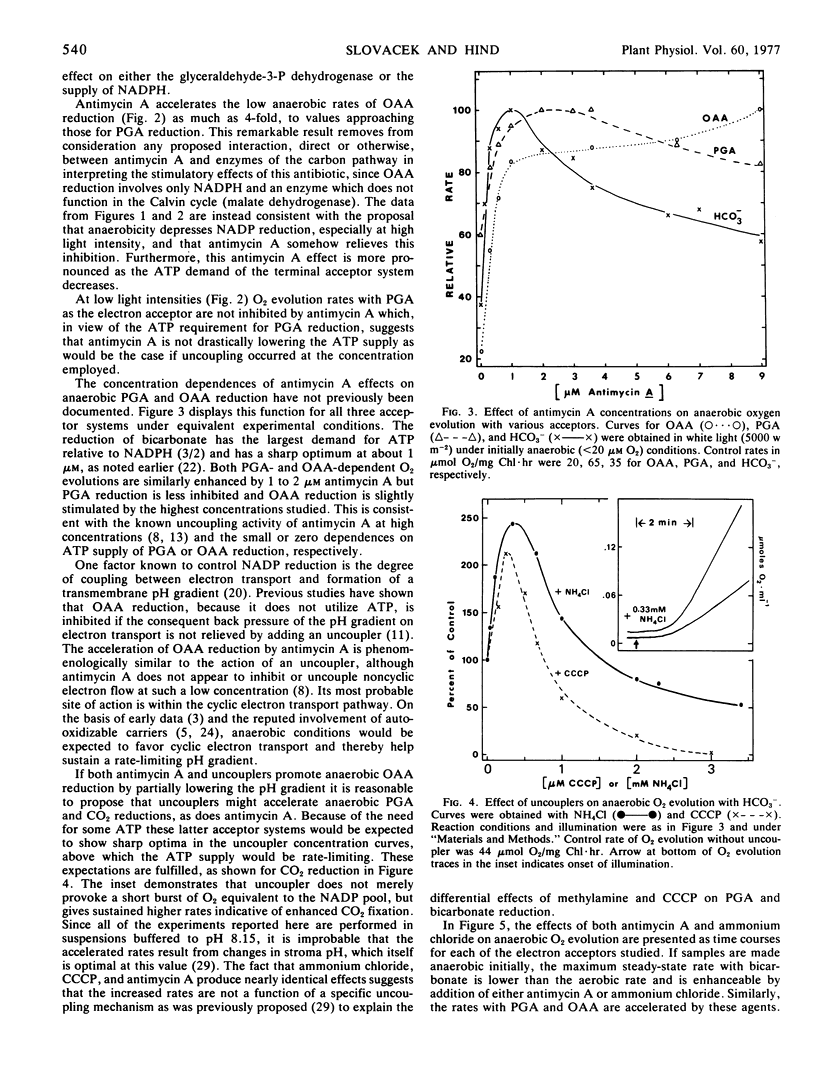
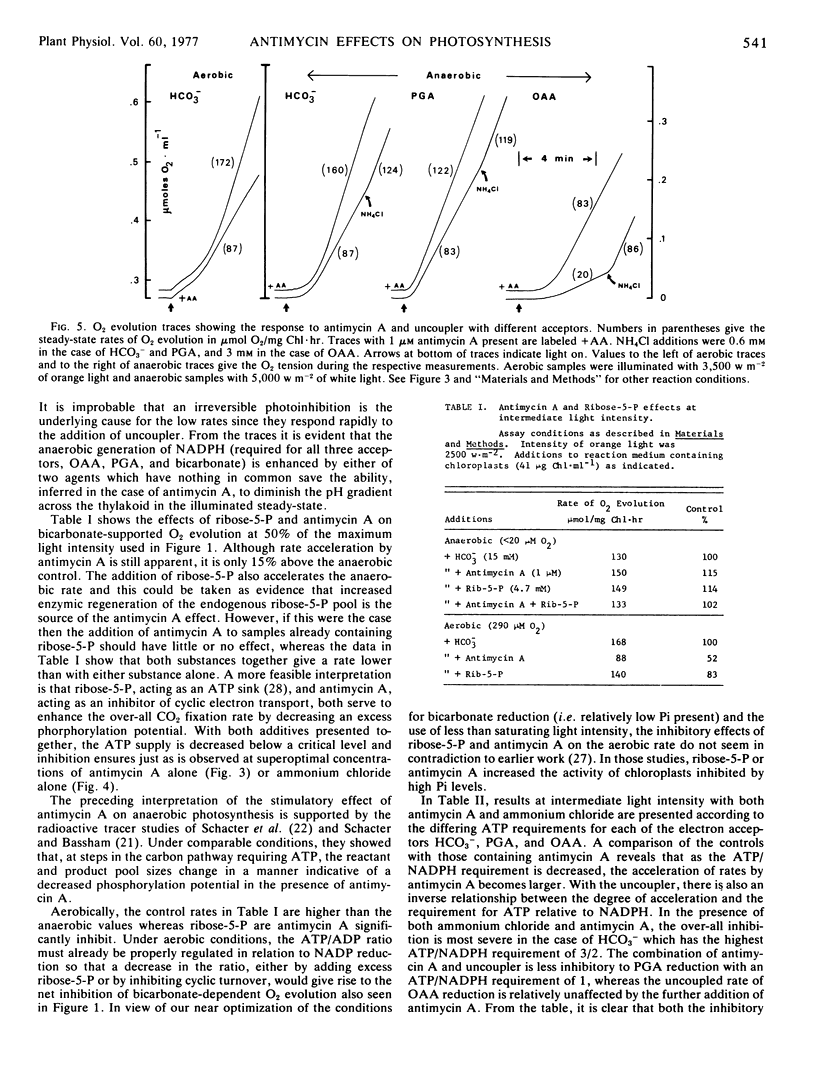
Anaerobiosis depresses the light- and bicarbonate-saturated rates of O2 evolution in intact spinach (Spinacia oleracea) chloroplasts by as much as 3-fold from those observed under aerobic conditions. These lower rates are accelerated 2-fold or more by the addition of 1 μm antimycin A or by low concentrations of the uncouplers 0.3 mm NH4Cl or 0.25 μm carbonyl cyanide m-chlorophenylhydrazone. Oxaloacetate and glycerate 3-phosphate reduction rates are also increased by antimycin A or an uncoupler under anaerobic conditions. At intermediate light intensities, the rate accelerations by either antimycin A or uncoupler are inversely proportional to the adenosine 5′-triphosphate demand of the reduction process for the acceptors HCO3−, glycerate 3-phosphate, and oxaloacetate. The acceleration of bicarbonate-supported O2 evolution may also be produced by adding an adenosine 5′-triphosphate sink (ribose 5-phosphate) to anaerobic chloroplasts. The above results suggest that a proton gradient back pressure resulting from antimycin A-sensitive cyclic electron flow is responsible for the depression of light-saturated photosynthesis under anaerobiosis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. Role of ferredoxin in photosynthesis. Naturwissenschaften. 1969 Jun;56(6):295–305. doi: 10.1007/BF00602160. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Kalberer P. P. Ferredoxin-activated fructose diphosphatase of spinach chloroplasts. Resolution of the system, properties of the alkaline fructose diphosphatase component, and physiological significance of the ferredoxin-linked activation. J Biol Chem. 1971 Oct 10;246(19):5952–5959. [PubMed] [Google Scholar]
- Drechsler Z., Nelson N., Neumann J. Antimycin A as an uncoupler and electron transport inhibitor in photoreactions of chloroplasts. Biochim Biophys Acta. 1969 Sep 16;189(1):65–73. doi: 10.1016/0005-2728(69)90226-6. [DOI] [PubMed] [Google Scholar]
- Egneus H., Heber U., Matthiesen U., Kirk M. Reduction of oxygen by the electron transport chain of chloroplasts during assimilation of carbon dioxide. Biochim Biophys Acta. 1975 Dec 11;408(3):252–268. doi: 10.1016/0005-2728(75)90128-0. [DOI] [PubMed] [Google Scholar]
- Heber U., Kirk M. R. Flexibility of coupling and stoichiometry of ATP formation in intact chloroplasts. Biochim Biophys Acta. 1975 Jan 31;376(1):136–150. doi: 10.1016/0005-2728(75)90212-1. [DOI] [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Heber U. Stoichiometry of reduction and phosphorylation during illumination of intact chloroplasts. Biochim Biophys Acta. 1973 Apr 27;305(1):140–152. doi: 10.1016/0005-2728(73)90239-9. [DOI] [PubMed] [Google Scholar]
- Hind G. Light-induced changes in cytochrome b-559 in spinach chloroplasts. Photochem Photobiol. 1968 Apr;7(4):369–375. doi: 10.1111/j.1751-1097.1968.tb08025.x. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Edwards G. E. Studies on the pathway of cyclic electron flow in mesophyll chloroplasts of a C4 plant. Biochim Biophys Acta. 1976 Dec 6;449(3):420–433. doi: 10.1016/0005-2728(76)90153-5. [DOI] [PubMed] [Google Scholar]
- Kaiser W. The effect of hydrogen peroxide on CO2 fixation of isolated intact chloroplasts. Biochim Biophys Acta. 1976 Sep 13;440(3):476–482. doi: 10.1016/0005-2728(76)90035-9. [DOI] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. Carbon dioxide assimilation by leaves, isolated chloroplasts, and ribulose bisphosphate carboxylase from spinach. Plant Physiol. 1975 Jun;55(6):1087–1092. doi: 10.1104/pp.55.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miginiac-Maslow M., Champigny M. L. Relationship between the Level of Adenine Nucleotides and the Carboxylation Activity of Illuminated Isolated Spinach Chloroplasts: A Study with Antimycin A. Plant Physiol. 1974 Jun;53(6):856–862. doi: 10.1104/pp.53.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIMURA M. Studies on the electron-transfer systems in photosynthetic bacteria. II. The effect of heptylhydroxyquinoline-N-oxide and antimycin A on the photosynthetic and respiratory electron-transfer systems. Biochim Biophys Acta. 1963 Jan 15;66:17–21. doi: 10.1016/0006-3002(63)91163-6. [DOI] [PubMed] [Google Scholar]
- Portis A. R., Jr, McCarty R. E. Quantitative relationships between phosphorylation, electron flow, and internal hydrogen ion concentrations in spinach chloroplasts. J Biol Chem. 1976 Mar 25;251(6):1610–1617. [PubMed] [Google Scholar]
- Schacter B. Z., Gibbs M., Champigny M. L. Effect of antimycin a on photosynthesis of intact spinach chloroplasts. Plant Physiol. 1971 Oct;48(4):443–446. doi: 10.1104/pp.48.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter B., Bassham J. A. Antimycin A Stimulation of Rate-limiting Steps of Photosynthesis in Isolated Spinach Chloroplasts. Plant Physiol. 1972 Mar;49(3):411–416. doi: 10.1104/pp.49.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater E. C. The mechanism of action of the respiratory inhibitor, antimycin. Biochim Biophys Acta. 1973 Dec 7;301(2):129–154. doi: 10.1016/0304-4173(73)90002-5. [DOI] [PubMed] [Google Scholar]
- TAGAWA K., ARNON D. I. Ferredoxins as electron carriers in photosynthesis and in the biological production and consumption of hydrogen gas. Nature. 1962 Aug 11;195:537–543. doi: 10.1038/195537a0. [DOI] [PubMed] [Google Scholar]
- Teichler-Zallen D., Hoch G. Cyclic electron transport in algae. Arch Biochem Biophys. 1967 Apr;120(1):227–230. doi: 10.1016/0003-9861(67)90620-0. [DOI] [PubMed] [Google Scholar]
- Walker D. A., Slabas A. R., Fitzgerald M. P. Photosynthesis in a reconstituted chloroplast system from spinach. Some factors affecting CO2-dependent oxygen evolution with fructose-1,6-bisphosphate as substrate. Biochim Biophys Acta. 1976 Jul 9;440(1):147–162. doi: 10.1016/0005-2728(76)90120-1. [DOI] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W., Milovancev M. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochim Biophys Acta. 1975 Aug 11;396(2):276–292. doi: 10.1016/0005-2728(75)90041-9. [DOI] [PubMed] [Google Scholar]


