Abstract
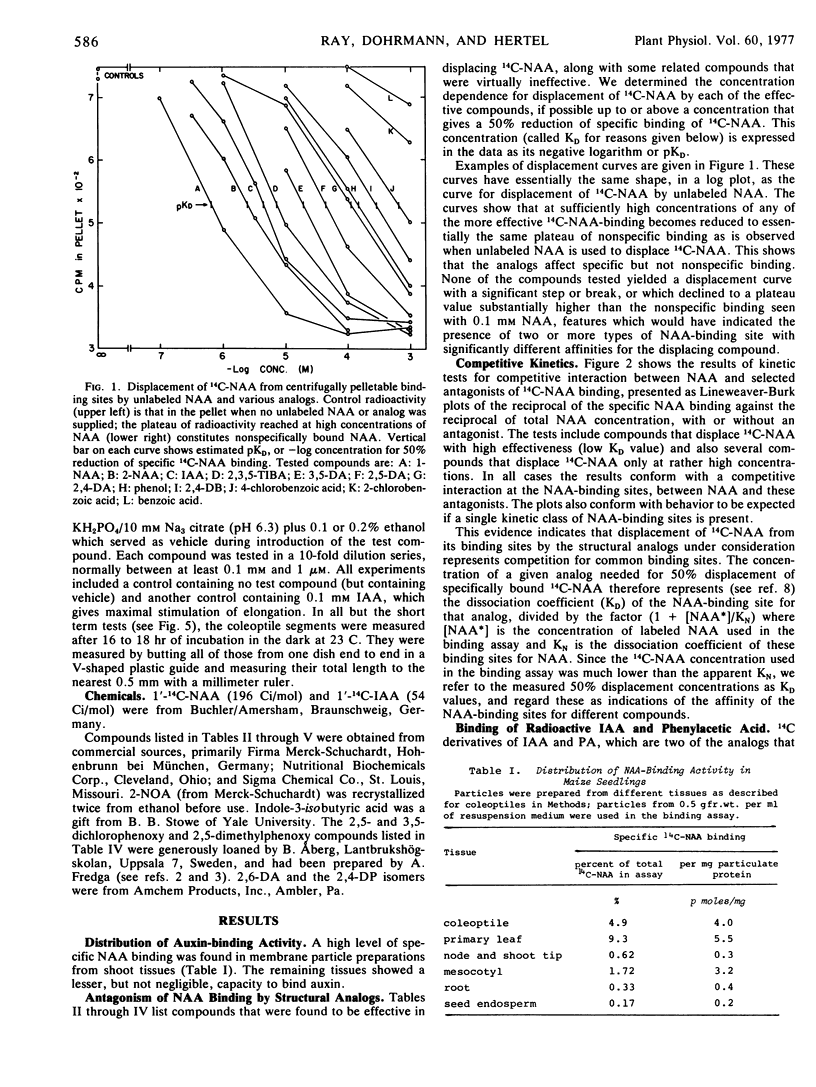
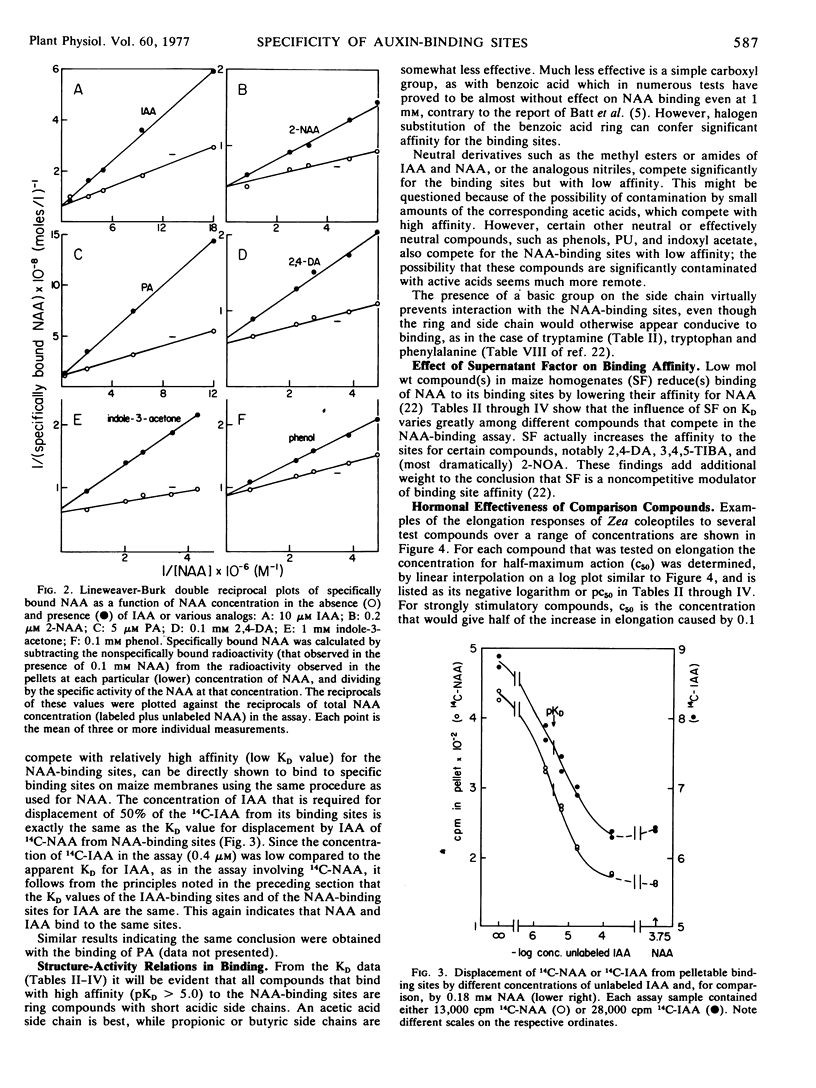
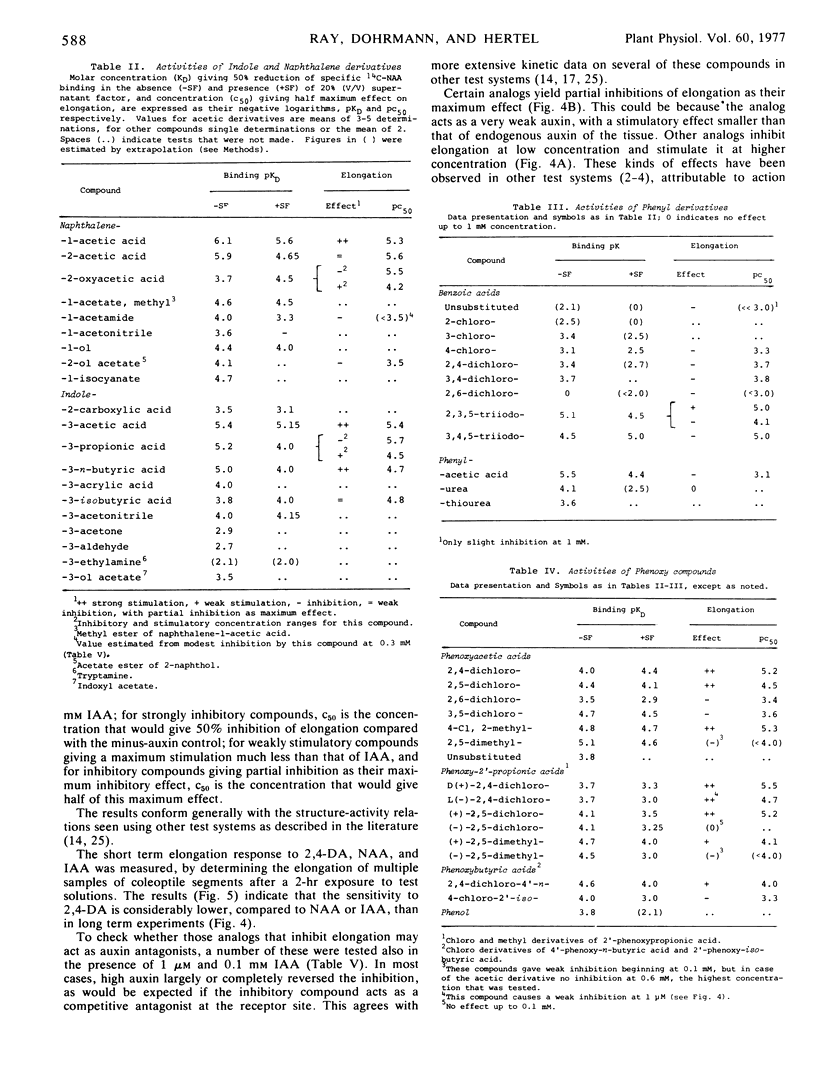
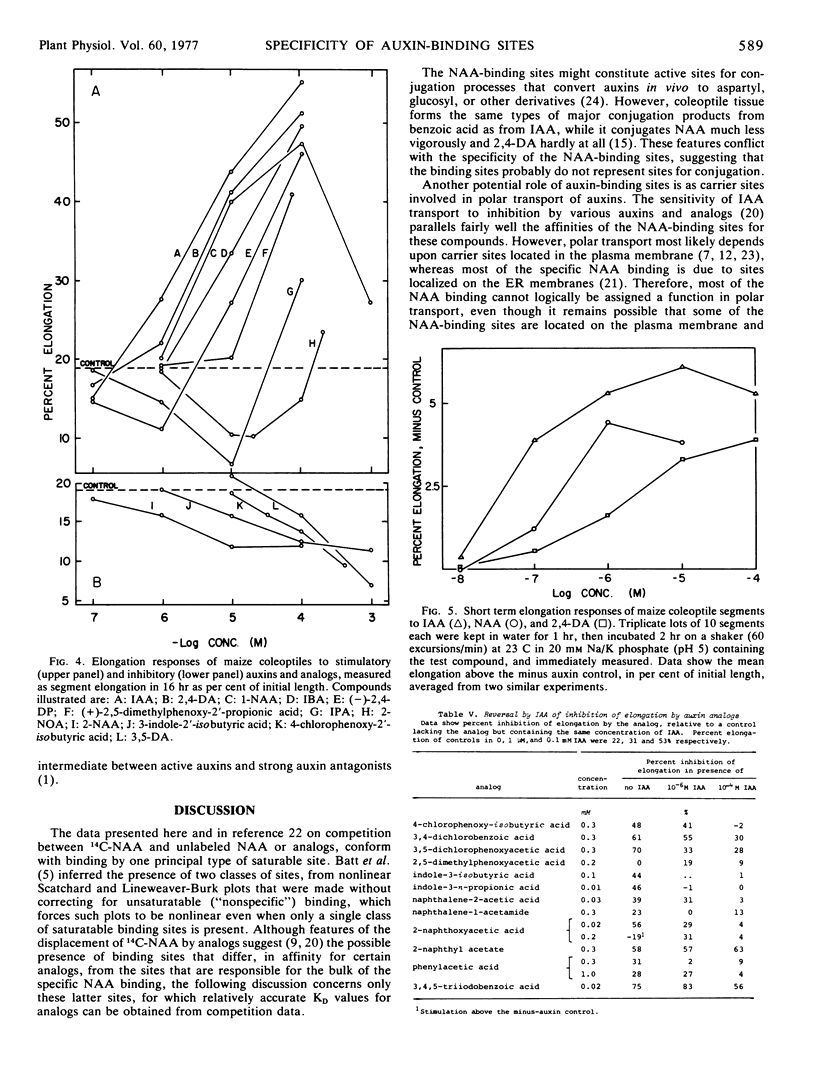
Dissociation coefficients of auxin-binding sites on maize (Zea mays L.) coleoptile membranes were measured, for 48 auxins and related ring compounds, by competitive displacement of 14C-naphthaleneacetic acid from the binding sites. The sites bind with high affinity several ring compounds with acidic side chains 2 to 4 carbons long, and much more weakly bind neutral ring compounds and phenols related to these active acids, most phenoxyalkylcarboxylic acids, and arylcarboxylic acids except benzoic acid, which scarcely binds, and triiodobenzoic acids, which bind strongly. Specificity of the binding is narrowed in the presence of a low molecular weight “supernatant factor” that occurs in maize and other tissues. Activity of many of the analogs as auxin agonists or antagonists in the cell elongation response was determined with maize coleoptiles. These activities on the whole roughly parallel the affinities of the binding sites for the same compounds, especially affinities measured in the presence of supernatant factor, but there are some quantitative discrepancies, especially among phenoxyalkylcarboxylic acids. In view of several factors that can cause receptor affinity and biological activity values to diverge quantitatively among analogs, the findings appear to support the presumption that the auxin-binding sites may be receptors for auxin action.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunton L. L., Wiklund R. A., Van Arsdale P. M., Gilman A. G. Binding of (3H)prostaglandin E1 to putative receptors linked to adenylate cyclase of cultured cell clones. J Biol Chem. 1976 May 25;251(10):3037–3044. [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Koerner D., Schwartz H. L., Surks M. I., Oppenheimer J. H. Binding of selected iodothyronine analogues to receptor sites of isolated rat hepatic nuclei. High correlation between structural requirements for nuclear binding and biological activity. J Biol Chem. 1975 Aug 25;250(16):6417–6423. [PubMed] [Google Scholar]
- Muir R. M., Hansch C. THE RELATIONSHIP OF STRUCTURE AND PLANT-GROWTH ACTIVITY OF SUBSTITUTED BENZOIC AND PHENOXYACETIC ACIDS. Plant Physiol. 1951 Apr;26(2):369–374. doi: 10.1104/pp.26.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee C., Caron M. G., Coverstone M., Lefkowitz R. J. Identification of adenylate cyclase-coupled beta-adrenergic receptors in frog erythrocytes with (minus)-[3-H] alprenolol. J Biol Chem. 1975 Jul 10;250(13):4869–4876. [PubMed] [Google Scholar]
- Ray P. M. Auxin-binding Sites of Maize Coleoptiles Are Localized on Membranes of the Endoplasmic Reticulum. Plant Physiol. 1977 Apr;59(4):594–599. doi: 10.1104/pp.59.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Dohrmann U. Characterization of naphthaleneacetic Acid binding to receptor sites on cellular membranes of maize coleoptile tissue. Plant Physiol. 1977 Mar;59(3):357–364. doi: 10.1104/pp.59.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. T., Mullikin D., Lefkowitz R. J. Identification of alpha-adrenergic receptors in uterine smooth muscle membranes by [3H]dihydroergocryptine binding. J Biol Chem. 1976 Nov 25;251(22):6915–6923. [PubMed] [Google Scholar]


