Abstract
We previously reported that CD137 (encoded by Tnfrsf9) deficiency suppressed type 1 diabetes (T1D) progression in nonobese diabetic (NOD) mice. We also demonstrated that soluble CD137 produced by regulatory T cells contributed to their autoimmune suppressive function in this model. These results suggest that CD137 can either promote or suppress T1D development in NOD mice depending on where it is expressed. Here, we show that NOD.Tnfrsf9−/− CD8 T cells had significantly reduced diabetogenic capacity, while absence of CD137 in non-T and non-B cells had a limited impact on T1D progression. In contrast, NOD.Tnfrsf9−/− CD4 T cells highly promoted T1D development. We further demonstrated that CD137 was important for the accumulation of β-cell autoreactive CD8 T cells but was dispensable for their activation in pancreatic lymph nodes. The frequency of islet infiltrating CD8 T cells was reduced in NOD.Tnfrsf9−/− mice in part due to their decreased proliferation. Furthermore, CD137 deficiency did not suppress T1D development in NOD mice expressing the transgenic NY8.3 CD8 T cell receptor. This suggests that increased precursor frequency of β-cell autoreactive CD8 T cells in NY8.3 mice obviated a role for CD137 in diabetogenesis. Finally, blocking CD137-CD137L interaction significantly delayed T1D onset in NOD mice. Collectively, our results indicate that one important diabetogenic function of CD137 is to promote the expansion and accumulation of β-cell autoreactive CD8 T cells, and in the absence of CD137 or its interaction with CD137L, T1D progression is suppressed.
Introduction
CD137 is a costimulatory molecule belonging to the TNF receptor superfamily (1). Expression of CD137 is induced on activated T cells, and it interacts with CD137 ligand (CD137L) expressed on antigen-presenting cells (APCs). Engagement of CD137 recruits TRAF1 and TRAF2, activates ERK, JNK, p38 MAPK, and NF-κB pathways, and leads to enhanced activation and survival of T cells (2–8). In addition to T cells, CD137 is also expressed on myeloid cells and activated NK cells to elicit various immune modulating functions (9, 10). Previous studies have also indicated a role of CD137 in the regulation of several autoimmune diseases including type 1 diabetes (T1D) (11).
Genetic susceptibility contributes to T1D development. In the NOD mouse model, more than 30 insulin-dependent diabetes susceptibility (Idd) loci have been identified, although our understanding of the underlying genes and the mechanisms by which they regulate T1D is incomplete (12). The Idd9.3 locus was mapped to a 1.2 Mb region at the distal end of Chromosome 4 (13). The C57BL/10 (B10)-derived Idd9.3 region confers T1D resistance in NOD mice (14, 15). Compared to the B10 allele, two nonsynonymous single nucleotide polymorphisms and one 3 base-pair insertion have been reported in the coding region of NOD Tnfrsf9, a major candidate gene for the Idd9.3 region (14). CD137 (the protein product of Tnfrsf9) in NOD mice is hypofunctional. NOD T cells stimulated via CD137 proliferated less, and produced significantly lower levels of IL-2, than those isolated from the B10-derived Idd9.3 congenic strain (NOD.Idd9.3) (13).
Many costimulatory molecules have been found on T cells (11, 16). However, it is not completely known if individual costimulatory molecules play distinct roles in promoting the diabetogenic activity of T cells. In the NOD model of T1D, CD137 has been mostly studied for its role in FOXP3+ CD4+ regulatory T cells (Tregs) mediated diabetes suppression. Previous studies have demonstrated that a subset of Tregs constitutively express CD137 (17–21). The hypofunctional NOD Tnfrsf9 allele was associated with significantly decreased numbers of CD137+ Tregs in NOD mice compared to NOD.Idd9.3 (21). CD137+ Tregs were more suppressive than the CD137-negative subset in vitro and were the primary cellular source of soluble CD137 (21). Compared to the NOD.Idd9.3 congenic strain, NOD mice also had significantly lower levels of serum soluble CD137 (21). Importantly soluble CD137 actively suppressed CD4 T cell activation in vitro in an APC-independent but CD137 ligand (CD137L)-dependent fashion, and protected NOD mice from T1D (21, 22). Collectively, these results indicate that CD137 has a diabetes protective role most likely through a Treg dependent mechanism, and its functional impairment contributes to T1D development in NOD mice. On the other hand, NOD mice transgenically expressing an agonistic anti-CD137 single chain variable domain (Fv) in islets developed accelerated T1D, suggesting a diabetes promoting role of CD137 (23).
To further test the role of CD137 in T1D development, we used zinc-finger nucleases to target Tnfrsf9 and generate two different knockout alleles directly in NOD mice (24). Both mutant alleles abolished CD137 protein expression (24). Compared to wild-type NOD mice, T1D development was significantly suppressed in the absence of CD137, indicating a diabetes promoting role of this costimulatory molecule (24). T1D suppression in CD137-deficient NOD mice was found to be in part due to reduced diabetogenic activity of their T cells (24). These results indicate that CD137 plays a non-redundant role among other costimulatory molecules in supporting the pathogenic function of β-cell autoreactive T cells. As CD137 contributes to Treg mediated immunosuppression, our observation that unfractionated CD137-deficient T cells were less diabetogenic than the wild-type counterparts also suggested a critical disease promoting role of this costimulatory molecule in at least a subset of β-cell autoreactive T effector cells. In this report, we further determined the subset of T cells that require CD137 to fully exert their diabetogenic function. We analyzed the role of CD137 in CD4 versus CD8 T cells and further investigated if expression of this costimulatory molecule differentially affects leukocyte populations in the islet inflammatory environment.
Materials and Methods
Mouse strains
NOD/ShiLtDvs (NOD), NOD.Cg-Tg(TcraTcrbNY8.3)1Pesa/DvsJ (NOD.NY8.3), NOD.129S7(B6)-Rag1tm1Mom/J (NOD.Rag1−/−), and NOD.B6-Ptprcb/6908MrkTacJ (NOD.CD45.2) mice were obtained from The Jackson Laboratory and subsequently maintained at the Medical College of Wisconsin by brother-sister mating. NOD.Tnfrsf9−/− mice were generated using zinc-finger nucleases as previously described (24), and are currently maintained at the N4 generation. The generation of NOD.Tnfrsf9−/−.Rag1−/− mice was accomplished by outcrossing NOD.Tnfrsf9−/− mice to the NOD.Rag1−/− strain followed by intercrossing. NOD.Rag1−/−.NY8.3 mice were generated through outcrossing NOD.NY8.3 mice to the NOD.Rag1−/− strain, which was then followed by backcrossing the F1 progeny to NOD.Rag1−/− mice. NOD.Tnfrsf9−/−.NY8.3 mice were similarly generated by outcrossing NOD.NY8.3 mice to the NOD.Tnfrsf9−/− strain. Female mice were used for all experiments except where indicated.
T cell assays and adoptive transfer studies
Splenic CD8 and CD4 T cells were isolated by negative selection using the respective CD4 and CD8 T cell isolation kits (Miltenyi Biotec). CD8 and CD4 T cells were individually isolated from NOD and NOD.Tnfrsf9−/− mice at 6–8 weeks of age and admixed in a 2:1 CD4:CD8 ratio. NOD.Rag1−/− mice (6–8 weeks) were injected intravenously with a total of 6×106 CD4 and CD8 T cells to test their diabetogenic activity. In separate experiments, splenic CD4 T cells (3x106) isolated from 6–8 week old NOD or NOD.Tnfrsf9−/− mice were infused into NOD.Rag1−/−.NY8.3 recipients. To evaluate the function of CD137 in non-T and non-B cells, total splenic T cells (5x106) were isolated from 6–8 week old NOD mice by negative selection using a Pan T cell isolation kit II (Miltenyi Biotec) and transferred into 6 week old NOD.Rag1−/− and NOD.Tnfrsf9−/−.Rag1−/− mice. The purity of the isolated T cell populations was routinely higher than 92% as determined by flow cytometry. For in vivo cell proliferation experiments, CD8 T cells were isolated from 7–10 week old NOD.NY8.3 and NOD.Tnfrsf9−/−.NY8.3 mice and labeled with 5μM eFluor®670 (eBioscience, San Diego, CA) in Hanks’ balanced salt solution (HBSS) at 37° C for 10 minutes. After washing, 5x106 labeled CD8 T cells were then transferred into 7–9 week old NOD recipients. At six days post-transfer, spleens and pancreatic lymph nodes (PLNs) were harvested and analyzed via flow cytometry to determine cell proliferation. For CFSE CD8 T cell proliferation studies, minimacs purified CD8 T cells were labeled with CFSE and proliferation calculated as described (22). Some cells were treated with recombinant soluble CD137 which was produced as described (22).
Generation of Mixed Bone Marrow Chimeras
Bone marrow (BM) cells were collected from femurs and tibias of 7–11 week old NOD, NOD.Tnfrsf9−/−, and NOD.CD45.2 mice. T cells were depleted by using anti-CD3e microbeads (Miletenyi Biotec). (NOD x NOD.CD45.2) F1 mice (6–8 weeks) were lethally irradiated (1100 rads) and infused with T cell depleted BM cells (5x106) isolated from the indicated strains. After 10–12 weeks, spleens from recipients were removed and analyzed by flow cytometry.
Flow Cytometry Analysis
Fluorochrome-labeled antibodies specific for CD8 (53-6.72), CD4 (RM4-5), CD3 (145-2C11), CD44 (IM7.8.1), CD45.1 (A20), CD45.2 (104), and Ki-67 (So1A15) were purchased from BD Biosciences (San Jose, CA), BioLegend (San Diego, CA), or eBioscience (San Diego, CA). MHC I (Kd) tetramers loaded with an islet specific glucose-6-phosphatase catalytic subunit related protein (IGRP) peptide (VYLKTNVFL, amino acid residues 206-214) were obtained from the NIH tetramer core facility. Single cell suspension was prepared from the spleen, PLN, thymus, and bone marrow (BM) of mice at the indicated age. BM was isolated from both femurs of individual mice by injecting HBSS in a prepared opening at the proximal end of the bone, resulting in the efflux of marrow through a prepared hole made in the distal end. Red blood cells were lysed with the ACK lysis buffer. Cells were then resuspended in FACS buffer for MHC class I tetramer or antibody staining. Cells were first blocked with Fc block (BD Biosciences) at room temperature for 10 minutes, and then stained with the indicated antibodies for 30 minutes at 4° C. For analyzing IGRP206-214 reactive CD8 T cells, cells were incubated with MHC class I tetramers and Fc block for 15 minutes at room temperature. Indicated antibodies were then added to stain the cells for an additional 30 minutes at 4° C. Stained cells were washed once with the FACS buffer and acquired using the FACSCalibur or LSRII flow cytometer (BD Biosciences). All flow cytometric data were analyzed with the FlowJo software (Tree Star, Ashland, OR).
Assessment of T1D and Insulitis development
T1D and insulitis development were assessed as previously described (25). Briefly, T1D development was monitored weekly using urine glucose strips (Diastix, Bayer) with onset defined by two consecutive readings of > 250 mg/dl. At the end of diabetes incidence study, pancreata of non-diabetic NOD.Rag1−/− T cell recipients were fixed in a 10% formalin solution and sectioned at four non-overlapping levels. Granulated β cells were stained with aldehyde fuchsin and leukocytes with an H&E counterstain. Islets were individually scored as follows: 0, no lesions; 1, peri-insular leukocytic aggregates, 2, <25% islet destruction; 3, >25% islet destruction; and 4, complete islet destruction.
Islet isolation and analysis of infiltrates
Islet infiltration cells were prepared as previously described (26). Pancreata from 9–12 week old NOD and NOD.Tnfrsf9−/− mice were inflated with a collagenase P solution (5 units/mL, Roche Diagnostics) in HBSS via the bile duct using a 30 gauge needle. The inflated pancreata were incubated at 37° C for 30 minutes. After digestion, the pancreata were washed 3 times with HBSS containing 2% FBS. Each individual pancreas was then resuspended in 5 ml complete RPMI and plated on a 60x15 mm glass petri dish. Islets from 2 to 7 mice of the same strain were hand-picked and pooled into a single petri dish under a dissecting microscope. Islets were then transferred to a 1.5 ml microcentrifuge tube and spun down. After the removal of the supernatant, islets were resuspended in 500 μl of enzyme-free Gibco® cell dissociation buffer to obtain single cell suspension. Cells were then washed once with 1 ml of the HBSS, resuspended in FACS buffer, and stained with the indicated fluorochrome-conjugated antibodies. Samples were then analyzed on the LSRII flow cytometer. For intracellular staining with anti-Ki-67, samples were first stained for surface markers and then fixed/permeabilized for 2–4 hours using the FOXP3 staining buffer set from eBioscience. After fixation, the cells were washed twice with permeabilization buffer and then stained with anti-Ki-67 for 30 minutes. After two additional washes, samples were analyzed by flow cytometry.
Immunofluorescence staining
Pancreata from 10-week-old NOD and NOD.Tnfrsf9−/− mice were removed and placed in Tissue-Tek® O.C.T. compound from Sakura® Finetek (Torrance, CA) and flash-frozen in isopentate cooled with liquid nitrogen for 60 seconds. Sections were cut at 5 um and allowed to air-dry for at least 30 minutes. Samples were fixed in 75% acetone/25% EtoH for 5 minutes at room temperature. Slides were then immediately moved into TBST at which point sections were placed on slides and fixed. Serum free protein block from DAKO (Carpinteria, CA) was applied for 5 minutes, after which samples were incubated with primary antibody cocktail diluted with antibody diluent (DAKO) for 90 minutes at room temperature. Primary antibodies used were anti-CD8a (sc-18913 from Santa Cruz, Dallas, TX), anti-CD4 (sc-13573 from Santa Cruz), insulin (A0564 from DAKO), and B220 (14-0542-85 from eBioscience). After a 5 minute wash, slides were incubated with secondary antibody for 45 minutes at room temperature. Secondary antibodies used were donkey anti-rat (A21208 from Invitrogen, Carlsbad, CA) and goat anti-guinea pig (ab175714 from Abcam, Cambridge, United Kingdom). After washing, slides were stained with DAPI for 10 minutes. Coverslips were applied with Prolong Gold (ThermoFisher, Waltham, MA) and allowed to cure for 30 minutes. Whole slide scanning was performed by using the Olympus® VS120 virtual slide microscope (Olympus, Center Valley, PA). Islets with a diameter of 100 μm or larger were analyzed for the presence of B cells as well as CD4 and CD8 T cells. The area of each islet was calculated (μm2) using ImageJ (27). The cell counts per 100μm2 of CD4 T cells, CD8 T cells, and B cells were then determined for each islet.
Anti-CD137L injection
Starting at 9 weeks of age, female NOD mice were injected intraperitoneally with 250 μg of anti-CD137L (TKS-1) or an isotype control antibody (2A3) every other week for a total of 10 weeks (5 injections). Both anti-CD137L and the isotype control antibody were purchased from Bio X Cell (West Lebanon, NH). Mice were observed every week for the development of T1D as described above.
Statistical analysis
Mann-Whitney test, unpaired t test, or Wilcoxon matched-pairs signed rank test was used for comparison between two groups as indicated. Log-rank test was used for the analysis of T1D incidence. All statistical analyses were performed using the GraphPad Prism 6 software (La Jolla, CA).
Results
CD137 expression in CD4 and CD8 T cells respectively suppresses and promotes T1D development
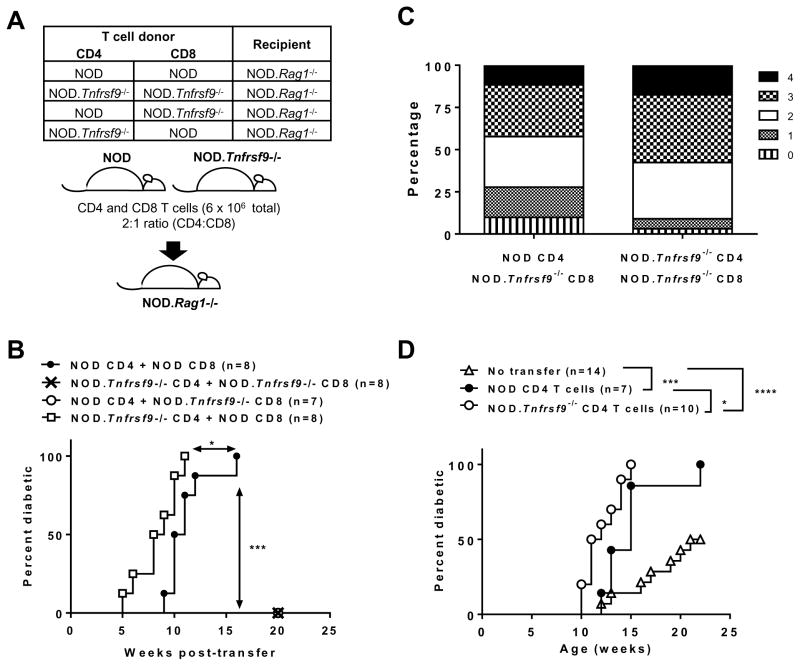
We previously demonstrated that T1D development in NOD.Tnfrsf9−/− mice was suppressed in part due to the reduced diabetogenic activity of their T cells, however we did not identify the role of CD137 in the different subsets (24). A subset of FOXP3+ Tregs constitutively express CD137 on the cells surface and are the main cellular source of soluble CD137 (21). NOD mice have reduced frequencies and numbers of CD137+ Tregs as well as the level of soluble CD137 in the serum compared to the diabetes resistant NOD.Idd9.3 congenic strain (21). Furthermore, recombinant soluble CD137 suppressed CD4 T cells proliferation in vitro and T1D progression in NOD mice (22). Contrary to these results, we showed that CD137 promoted the diabetogenic activity of unfractionated T cells in an adoptive transfer system (24). One possibility that can reconcile these seemingly inconsistent findings is that CD137 plays distinct roles in different T cell subsets. Absence of CD137 signaling has been shown to exert distinct effects in CD4 and CD8 T cells (28). Thus, we tested the function of CD137 in CD4 and CD8 T cells independently. CD4 and CD8 T cells were individually isolated from NOD or NOD.Tnfrsf9−/− mice, mixed in a “criss-cross” design (Figure 1A), and transferred into NOD.Rag1−/− recipients. As expected, CD4 and CD8 T cells from NOD mice induced high incidence of diabetes, but NOD.Rag1−/− recipients of both cell types from NOD.Tnfrsf9−/− donors did not (Figure 1B). Interestingly, T1D development in NOD.Rag1−/− recipients of NOD.Tnfrsf9−/− CD8 and NOD CD4 T cells was also strongly suppressed (Figure 1B). In contrast, diabetes development was significantly accelerated in NOD.Rag1−/− recipients of NOD CD8 and NOD.Tnfrsf9−/− CD4 T cells compared to those infused with both cell populations from the NOD donors (Figure 1B). To rule out the possibility that differences in T1D progression in the four recipient groups were due to differential T cell reconstitution, spleens from a subset of recipients were analyzed for the presence of CD4 and CD8 T cells at diabetes onset or at the end of the incidence study. The results do not support the possibility that differential T cell repopulation was the underlying reason of the observed T1D incidence (Supplementary Figure 1). Histological analysis was performed at the end of diabetes incidence study to determine the level of islet infiltrations in non-diabetic NOD.Rag1−/− recipients. As shown in Figure 1C, in the two transfer groups that did not develop diabetes, NOD.Rag1−/− recipients of NOD.Tnfrsf9−/− CD8 and NOD.Tnfrsf9−/− CD4 T cells had worse insulitis (increased scores 3 and 4) compared to recipients of NOD.Tnfrsf9−/− CD8 and NOD CD4 T cells that had less severe insulitis (increased scores 0 and 1). Thus, even though neither group of NOD.Tnfrsf9−/− CD8 T cell recipients developed diabetes, insulitis was more advanced in the presence of NOD.Tnfrsf9−/− CD4 T cells. The lack of T1D in recipients of knockout CD4 and CD8 cells was surprising given the fact that NOD.Tnfrsf9−/− mice still develop T1D, albeit at a later time point. One explanation for this result is a possible role for CD137 on APCs. CD137 is also expressed in non-T cells (1), so its expression in other cell populations could contribute to diabetes development in NOD mice. Particularly, CD137 has a regulatory role in dendritic cells, an antigen-presenting cell subset important for T1D development in NOD mice (29–31). To initially test this possibility, we transferred T cells isolated from NOD mice into NOD.Rag1−/− or NOD.Tnfrsf9−/−.Rag1−/− recipients. As shown in Supplementary Figure 2, diabetes development did not significantly differ between these two recipient groups, indicating that CD137 expression in non-T and non-B cells did not play an important role in T1D. Therefore, the lack of T1D in mice receiving NOD.Tnfrsf9−/− CD8 T cells likely reflects differences in β-cell antigen stimulated survival/expansion in vivo or accumulation in the islet (see below).
Figure 1. CD137 expression in CD4 and CD8 T cells differentially controls their diabetogenic activity.
(A) Schematic diagram showing the experimental design of the CD8 and CD4 T cell co-transfer into NOD.Rag1−/− recipients. (B) T1D incidence study of NOD.Rag1−/− recipients depicted in Figure 1A. CD4 and CD8 T cells isolated from 6–8 week old female NOD and/or NOD.Tnfrsf9−/− mice were injected into 6–8 week old NOD.Rag1−/− females. Recipients were analyzed for the development of T1D weekly over the course of 20 weeks post-transfer. (C) Histological analysis of NOD.Rag1−/− recipients in Figure 1B that did not progress to T1D at the end of incidence study. Islets were scored 0 to 4 based on the severity of insulitis: 0, no lesions; 1, peri-insular leukocytic aggregates, 2, <25% islet destruction; 3, >25% islet destruction; and 4, complete islet destruction. The result is presented as the relative proportion of each score. More than 200 islets were scored in each recipient group. (D) T1D incidence study of NOD.Rag1−/−.NY8.3 female recipients of 3x106 CD4 T cells from 6–8 week old NOD and NOD.Tnfrsf9−/− female mice. Recipients were infused with purified CD4 T cells at 6–8 weeks old and followed for the development of T1D every week over the course of 22 weeks post transfer. *P < 0.05, ***P < 0.005, ***P < 0.001 by Log-rank test.
To further confirm a diabetes protective role of CD137 when expressed in CD4 T cells, we conducted a different adoptive transfer experiment. In this system, CD4 T cells from either NOD or NOD.Tnfrsf9−/− donors were transferred into NOD.Rag1−/−.NY8.3 recipients. β-cell autoreactive CD8 T cells of the NY8.3 clonotype require help from CD4 T cells to fully exert their diabetogenic activity (32). Thus, the diabetogenic function of CD4 T cells can be tested by transferring them into NOD.Rag1−/−.NY8.3 recipients (33). Consistent with previous studies, T1D development was further enhanced in NOD.Rag1−/−.NY8.3 mice in the presence of adoptively transferred NOD CD4 T cells (Figure 1D). Compared to NOD CD4 T cells, those isolated from the NOD.Tnfrsf9−/− strain further accelerated diabetes development in the NOD.Rag1−/−.NY8.3 recipients (Figure 1D). Collectively, the results from the T cell adoptive transfer experiments indicated that CD137 expression in CD8 T cells was critical for the progression of T1D in NOD mice; conversely CD137 had a diabetes protective role in CD4 T cells.
CD137 deficiency leads to proportionally reduced islet infiltrating CD8 T cells
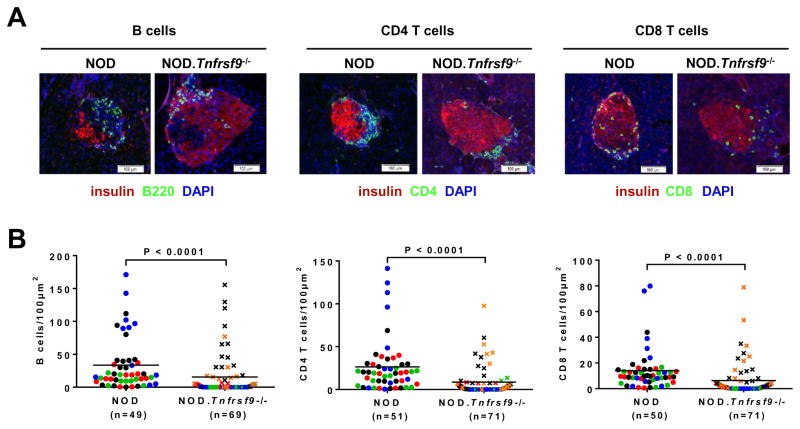
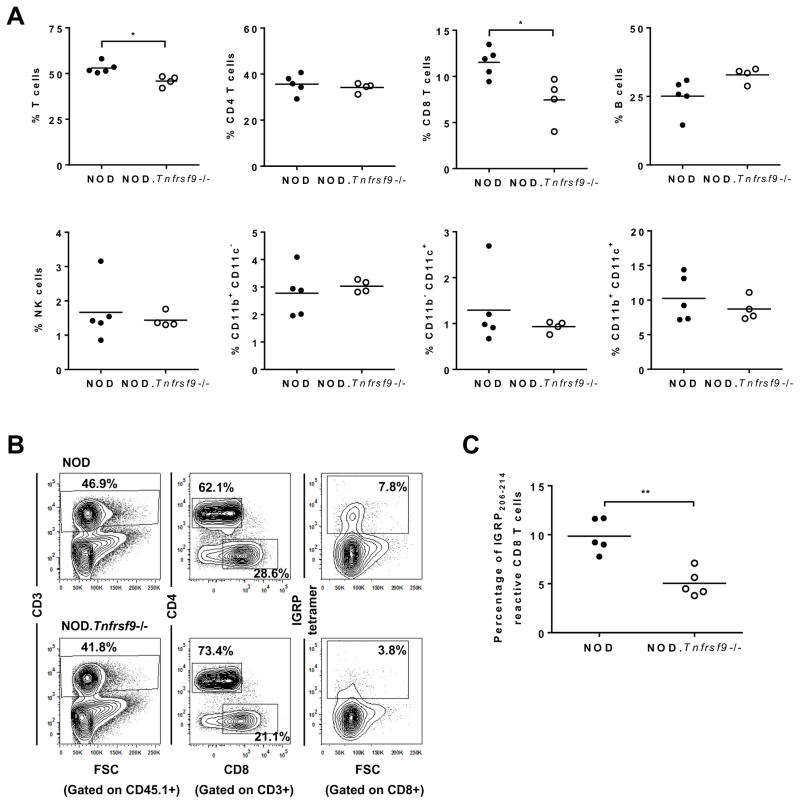
We previously showed that insulitis was reduced in the NOD.Tnfrsf9−/− stock compared to wild-type NOD mice (24). However, it was not known if intra-islet accumulation of all leukocyte populations was similarly affected or certain immune cell subsets were preferentially altered. Immunofluorescence staining was performed on pancreatic sections of NOD and NOD.Tnfrsf9−/− mice to directly determine CD4, CD8, and B cell populations surrounding and infiltrating the islets. Although we did not find a difference in the localization of these lymphocyte populations relative to the insulin producing β-cells, accumulation of all three cell types was reduced in NOD.Tnfrsf9−/− islets compared to those in NOD mice (Figure 2). In general, there was concordance of infiltration among B cells, CD4 T cells, and CD8 T cells. This indicated that the increase of lymphocyte infiltrations found in NOD islets is independent of the cell type. To further determine if intra-islet accumulation of certain cell types was proportionally altered in the absence of CD137, we isolated islet associated leukocytes and analyzed them by flow cytometry. There was a decreased percentage of T cells in NOD.Tnfrsf9−/− islets compared to those in NOD mice, due to a reduced frequency of CD8 but not CD4 T cells (Figure 3A). On the other hand, there were no significant differences in the percentages of B cells, myeloid cells, and NK cells (Figure 3A). NOD CD8 T cells accumulate in pancreatic islets in an antigen specific manner (34). Thus, these results indicate that CD137 is important for the accumulation of β-cell autoreactive CD8 T cells in islets. We also analyzed islet associated IGRP206-214 reactive CD8 T cells in NOD and NOD.Tnfrsf9−/− mice by MHC class I tetramer staining. More of these β-cell autoreactive CD8 T cells accumulated in NOD than in NOD.Tnfrsf9−/− islets (Figures 3B and 3C). CD137 deficiency therefore preferentially affected intra-islet accumulation of β-cell autoreactive CD8 T cells.
Figure 2. Accumulation of B cells, CD4 T cells, and CD8 T cells in islets is reduced in the NOD.Tnfrsf9−/− strain compared to NOD mice.
Pancreata from 10-week-old NOD and NOD.Tnfrsf9−/− females were processed for immunofluorescence staining to determine the numbers of islet infiltrating B cells, CD4 T cells, and CD8 T cells as described in Materials and Methods. (A) Representative immunofluorescence staining of islets from NOD and NOD.Tnfrsf9−/− female mice (B220, CD4, and CD8 = green; insulin = red; DAPI = blue). (B) Numbers of B cells, CD4 T cells, or CD8 T cells per 100μm2 of islet area. Each symbol represents one islet. Islets from the same mouse are color coded. Results are pooled from 4 NOD and 5 NOD.Tnfrsf9−/− mice. Statistical analysis was carried out by Mann-Whitney test.
Figure 3. Intra-islet accumulation of CD8 T cells is proportionally reduced in the NOD.Tnfrsf9−/− strain compared to NOD mice.
(A) Flow cytometric analysis of various islet infiltrating leukocyte populations. Islets were isolated from 9–12 week old NOD and NOD.Tnfrsf9−/− female mice and dissociated into single cell suspension. Leukocytes were identified by CD45.1 expression and the frequencies of different cell populations, including total T cells (CD3+), CD4 T cells (CD3+ CD4+), CD8 T cells (CD3+ CD8+), B cells (CD19+), NK cells (CD3− DX5+), and myeloid cells (CD11b+ CD11c−, CD11b+ CD11c+, and CD11b− CD11c+) were calculated as percentages of total CD45.1+ cells. Each symbol represents cells pooled from 2–7 mice. *P < 0.05 by Mann-Whitney test. (B and C) The frequencies of islet associated IGRP206-214 reactive CD8 T cells in NOD and NOD.Tnfrsf9−/− female mice by MHC class I tetramer staining. Islet cells pooled from 2–5 mice (10–12 weeks old) were stained with antibodies against CD45.1, CD3, CD4, and CD8 as well as the IGRP206-214 MHC I tetramer. (B) One representative experiment is shown. (C) Summarized results from five independent experiments. **P < 0.01 by Mann-Whitney test.
CD137 intrinsically promotes the accumulation of β-cell autoreactive CD8 T cells by enhancing their intra-islet proliferation
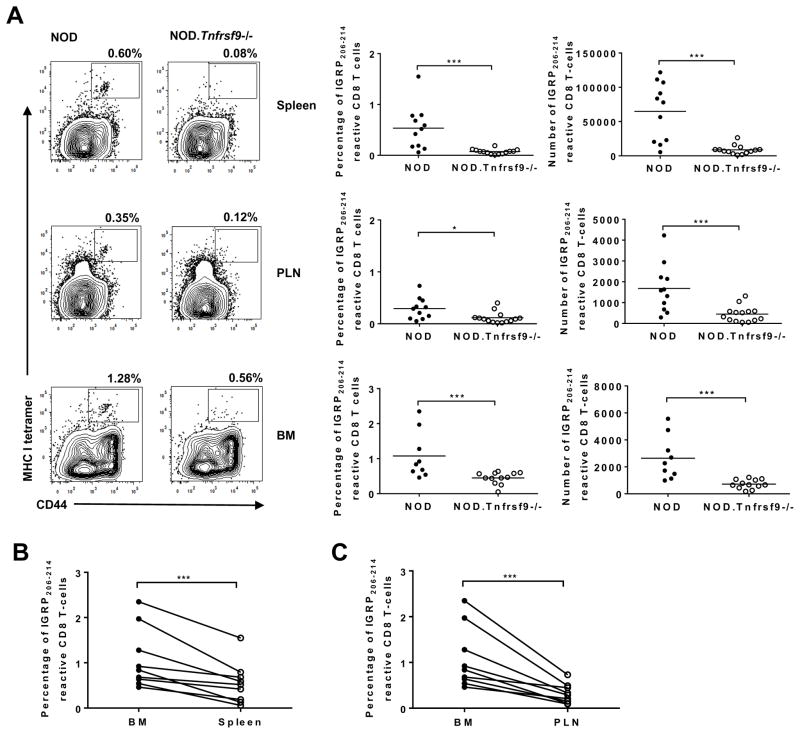
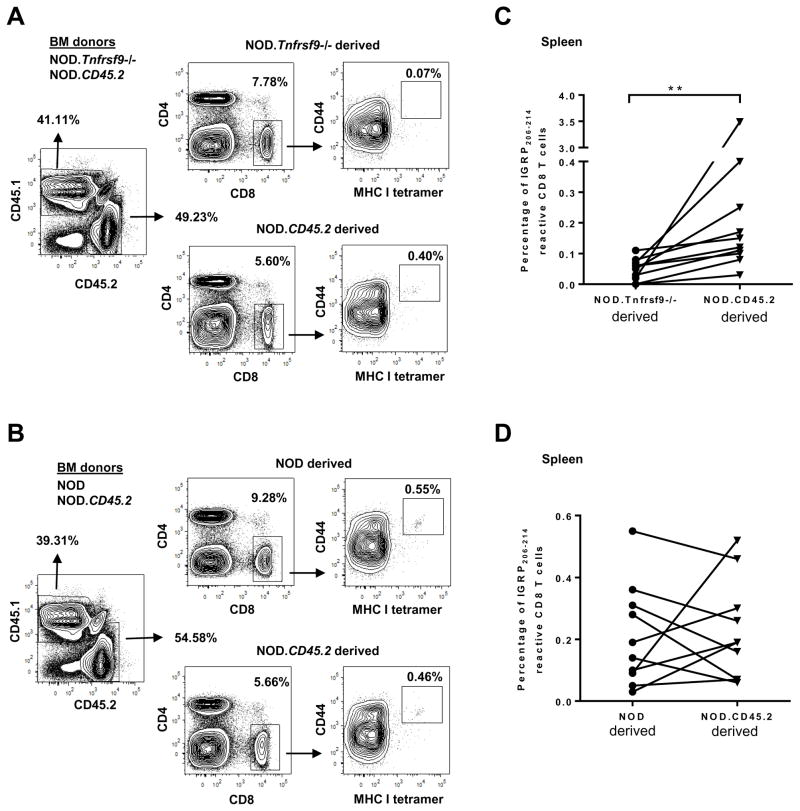
We next determined if β-cell autoreactive CD8 T cells are systemically reduced in NOD.Tnfrsf9−/− mice. We evaluated the presence of IGRP206-214 reactive CD8 T cells in the spleen, PLN, and BM of 10–14 week-old mice by tetramer staining. Both the percentage and number of CD44high effector/memory IGRP206-214 reactive CD8 T cells in the spleen, PLN, and BM were higher in NOD than in NOD.Tnfrsf9−/− mice (Figure 4A). Interestingly, IGRP206-214 reactive CD8 T cells were enriched in the BM relative to the spleen and PLN of NOD mice (Figures 4B and 4C), consistent with the previous observation that BM harbored proportionally more β-cell autoreactive T cells than did the spleen (35). To further determine if CD137 intrinsically promoted the accumulation of IGRP206-214 reactive CD8 T cells, mixed BM chimeras were generated. BM cells from NOD.CD45.2 and NOD.Tnfrsf9−/− (expressing CD45.1) donors were transferred in a 1:1 ratio into (NOD x NOD.CD45.2) F1 recipients. This experimental design allowed us to simultaneously identify the origins of donor cells as well as radiation resistant host cells. At 10 to 12 weeks after BM reconstitution, we analyzed the frequency of CD44high IGRP206-214 reactive CD8 T cells in the spleens. The results showed that NOD.CD45.2 BM gave rise to a significantly higher percentage of CD44high IGRP206-214 reactive CD8 T cells compared to those of the NOD.Tnfrsf9−/− origin (Figures 5A and 5C). To rule out the possibility that different CD45 alleles could have affected the accumulation of IGRP206-214 reactive CD8 T cells, control chimeras were generated using BM cells from NOD (expressing CD45.1) and NOD.CD45.2 donors. The percentages of CD44high IGRP206-214 reactive CD8 T cells from both origins were found to be comparable in the spleens of the recipients (Figures 5B and 5D), further confirming that CD137 intrinsically promoted the accumulation of IGRP206-214 reactive CD8 T cells.
Figure 4. The frequencies and numbers of IGRP206-214 reactive CD8 T cells in the spleen, PLN, and BM are significantly lower in NOD.Tnfrsf9−/− than in NOD mice.
(A) Cells were isolated from the spleen, PLN, and BM, and strained with antibodies against CD8 and CD44 as well as IGRP206-214 loaded MHC class I tetramers. Representative flow cytometry profiles are shown in the left panels. The percentages and numbers of IGRP206-214 MHC class I tetramer staining of the spleen, PLN, and BM from 10–14 week old NOD and NOD.Tnfrsf9−/− female mice from 4 independent experiments are summarized respectively in the middle and right panels. In one experiment, BM from two mice of each strain were not analyzed. *P < 0.05, ***P < 0.005 by Mann-Whitney test. (B and C) The frequency of IGRP206-214 reactive CD8 T cells is higher in the BM than in the spleen (B) and PLN (C). Paired analysis of IGRP206-214 MHC class I tetramer staining for BM versus spleen and BM versus PLN in NOD mice. ***P < 0.005 by Wilcoxon matched-pairs signed rank test.
Figure 5. CD137 intrinsically promotes the accumulation of IGRP206-214 reactive CD8 T cells.
Lethally irradiated (NOD x NOD.CD45.2)F1 mice were infused with equal number of T cell depleted BM cells (2.5x106 per donor strain) from NOD.CD45.2 and NOD.Tnfrsf9−/− (A and C) or NOD.CD45.2 and NOD mice (B and D). The frequencies of CD44high IGRP206-214 reactive CD8 T cells were analyzed in the spleens of the F1 recipients by MHC I tetramer staining at 10–12 weeks post BM reconstitution. The origin of the splenocytes was determined by CD45.1 and CD45.2 expression. The gating strategy is shown in panels A and B. The summarized results of two independent experiments are shown in panels C and D. **P < 0.01 by Wilcoxon matched-pairs signed rank test.
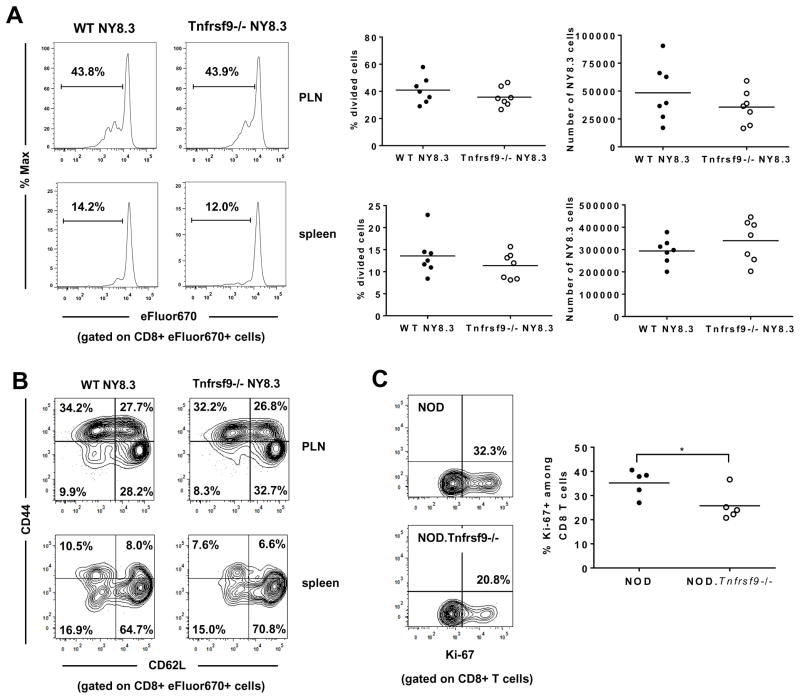
CD137 may promote the accumulation of β-cell autoreactive CD8 T cells by enhancing their activation in PLNs and/or subsequent proliferation in the islets when they re-encounter the cognate antigens. To test the former possibility, we transferred CD137 sufficient or deficient NY8.3 CD8 T cells into NOD mice and analyzed their in vivo activation (proliferation and CD44/CD62L expression) in the spleen and PLN. We did not observe differences in proliferation or the expression levels of CD44 and CD62L between CD137 sufficient and deficient NY8.3 CD8 T cells (Figures 6A and 6B) in either location. We also did not find a difference when the percentages of cells in each division were compared between CD137 sufficient and deficient NY8.3 CD8 T cells (data not shown). We next determined if proliferation of β-cell autoreactive CD8 T cells in pancreatic islets is reduced in the absence of CD137 by Ki-67 staining (36, 37). Ki-67 expression was analyzed in islet associated T cells isolated from NOD and NOD.Tnfrsf9−/− mice. We observed a higher percentage of islet infiltrating Ki-67+ CD8 T cells in NOD than in NOD.Tnfrsf9−/− mice (Figure 6C). On the other hand, no difference was found in CD4 T cells (Supplementary Figure 3). These results indicated that CD137 promoted accumulation of β-cell autoreactive CD8 T cells at least in part through enhancing their proliferation in the islets.
Figure 6. Regulation of autoreactive CD8 T cell proliferation by CD137.
(A and B) CD137 is not required for initial activation of β-cell autoreactive CD8 T cells in PLNs. CD8 T cells purified from NOD.NY8.3 and NOD.Tnfrsf9−/−.NY8.3 mice were labelled with eFluor670 cell proliferation dye and transferred into NOD recipients. Six days later, proliferation of adoptively transferred NY8.3 CD8 T cells was analyzed in the spleen and PLN. (A) Representative flow cytometry profiles of cell division are shown on the left. The results of cell proliferation (middle panels) and the recovered numbers (right panels) of adoptively transferred NY8.3 CD8 T cells are summarized from two independent experiments. Each symbol represents one recipient mouse. (B) Representative flow cytometry profiles of CD44 and CD62L expression on the adoptively transferred NY8.3 CD8 T cells. (C) Intra-islet proliferation of CD8 T cells is reduced in the NOD.Tnfrsf9−/− strain compared to the NOD mice. Ki-67 expression was analyzed by flow cytometry in islet infiltrating CD8 T cells from 10–14 week-old NOD and NOD.Tnfrsf9−/− female mice. Representative flow cytometry profiles of the percentages of Ki-67+ CD8 T cells are shown (left). The results are summarized from 5 independent experiments (right). Each symbol represents islet cells pooled from 3–5 mice. *P < 0.05 by Mann-Whitney test.
CD137 deficiency does not impact T1D development in NY8.3 TCR transgenic NOD mice
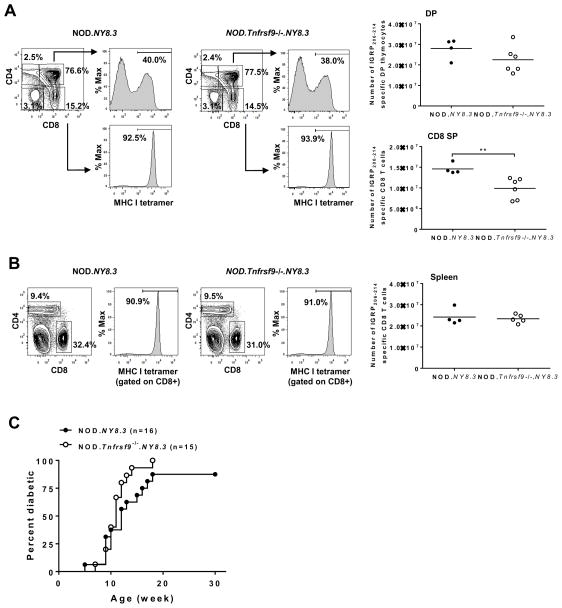
We reasoned that if the T1D promoting role of CD137 is through sustaining the expansion and accumulation of β-cell autoreactive CD8 T cells, its impact on diabetes development would be reduced when the number of these pathogenic effectors is artificially increased. We used NOD mice transgenically expressing the CD8 TCR of the NY8.3 clonotype to test this possibility. The proportions of CD4/CD8 double negative, double positive (DP), and single positive (SP) thymocytes did not significantly differ between 6 to 7 week-old NOD.NY8.3 and NOD.Tnfrsf9−/−.NY8.3 female mice (Figure 7A). We also used MHC class I tetramers to specifically identify DP and CD8 SP thymocytes recognizing Kd restricted IGRP206-214 peptide. NY8.3 CD8 T cells were found to develop normally in the thymus in the absence of CD137, albeit their average number was reduced in the CD8 SP compartment in the NOD.Tnfrsf9−/−.NY8.3 strain compared to NOD.NY8.3 mice (Figure 7A). This was largely due to lower total thymocyte numbers in some of the NOD.Tnfrsf9−/−.NY8.3 than in NOD.NY8.3 females (data not shown). However, the numerical difference of thymic IGRP206-214 reactive CD8 T cells between NOD.NY8.3 and NOD.Tnfrsf9−/−.NY8.3 mice was not observed in a separate analysis where 7 week-old males were compared (data not shown). Analyses of spleens in NOD.NY8.3 and NOD.Tnfrsf9−/−.NY8.3 females did not reveal a numerical difference in IGRP206-214 reactive CD8 T cells (Figure 7B). Importantly, T1D development was comparable between these two NY8.3 TCR transgenic strains (Figure 7C). These results suggested that the function of CD137 in β-cell autoreactive CD8 T cells became less critical when the precursor frequency of these pathogenic T cells was greatly increased in the TCR transgenic mice.
Figure 7. CD137 deficiency does not alter diabetes development in NOD mice transgenically expressing TCRs of the NY8.3 clonal type.
(A) Thymi from 6–7 week-old NOD.NY8.3 and NOD.Tnfrsf9−/−.NY8.3 female mice were analyzed for IGRP206-214 specific double-positive (DP) and CD8 single-positive (SP) thymocytes. Representative flow cytometry profiles for each strain are shown (left and middle panels). The results are summarized in the right panels. Each symbol represents one mouse. **P < 0.01 by Mann-Whitney test. (B) Spleens from 6–7 week-old NOD.NY8.3 and NOD.Tnfrsf9−/−.NY8.3 female mice were analyzed for IGRP206-214 specific CD8 T cells. Representative flow cytometry profiles for each strain are shown (left and middle panels). The results are summarized in the right panels. Each symbol represents one mouse. (C) T1D incidence study of NOD.NY8.3 and NOD.Tnfrsf9−/−.NY8.3 female mice. T1D development was monitored weekly using urine glucose strips with onset defined by two consecutive readings of > 250 mg/dl. Diabetes development is not significantly different between NOD.NY8.3 and NOD.Tnfrsf9−/−.NY8.3 mice.
CD8 T cells are regulated by soluble CD137
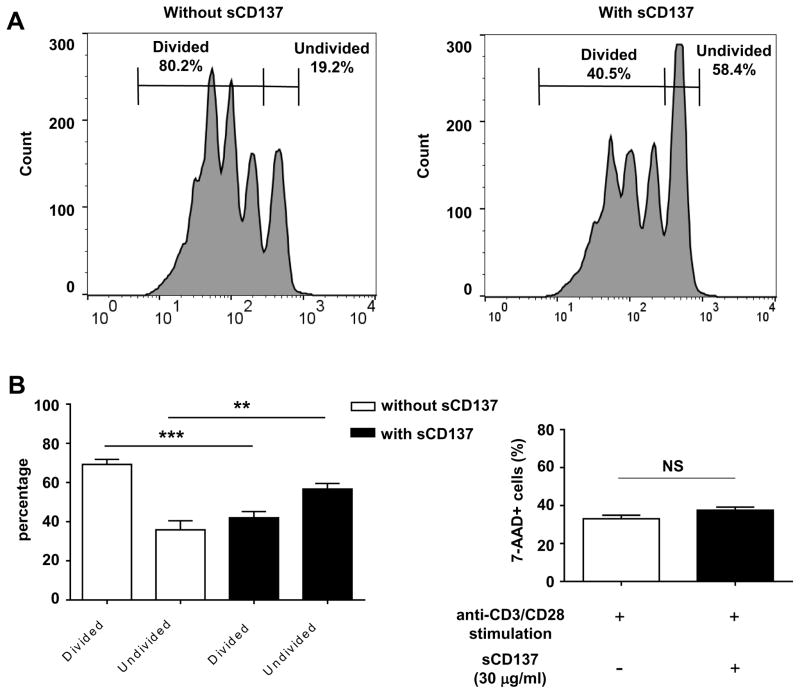
Overall, our results indicated that CD137 promoted the pathogenicity of CD8 T cells but also enhanced the protective capacity of CD4 T cells. We previously showed that CD137+ CD4+ Tregs produced soluble CD137 which could suppress the proliferation of CD4 T cells; however we had not tested the effect of soluble CD137 on CD8 T cells. We purified CD8 T cells and stimulated them with or without recombinant soluble CD137. Soluble CD137 clearly suppressed proliferation of CD8 T cells (Figure 8). We previously published that suppression of CD4 proliferation by soluble CD137 was decreased by co-culture with anti-CD137L antibody (22). We used the same approach and demonstrated that suppression of CD8 cell proliferation by soluble CD137 was blocked by addition of anti-CD137L antibody (Supplementary Figure 4). The result indicates that binding of soluble CD137 to CD137L expressed on CD8 T cells is required for the suppressive activity. This suggests that one mechanism of enhanced pathogenicity in the transfer of CD137-deficient CD4 T cells is due to decreased regulation of CD8 cells via soluble CD137.
Figure 8. Soluble CD137 suppresses CD8 T cell activation.
CD8 T cells purified from 8–10 week-old female NOD mice were labeled by CFSE (final concentration: 0.5 uM), stimulated by 25,000 CD3/CD28 dynabeads and cultured with or without recombinant soluble CD137 (30 ug/ml) on 96-well u-bottom culture plate. After 3 days of culture, CFSE-dilution and 7-AAD positive population was quantified on FACS Calibur. (A) One representative of four experiments for CFSE dilution without soluble CD137 (left) and with soluble CD137 (right) is shown. (B) Soluble CD137 significantly increases the number of undivided CD8 T cells and decreases the number of divided CD8 T cells. Mean percentage of divided and undivided cells is shown (n = 4 experiments). (C) 7-AAD staining shows no difference in CD8 cell death after 3 days of culture with or without soluble CD137 (mean of n = 4 experiments). **P < 0.01; ***P < 0.005 by unpaired t test. sCD137: soluble CD137. NS: not significant.
Blocking CD137-CD137L interaction dominantly inhibits T1D progression
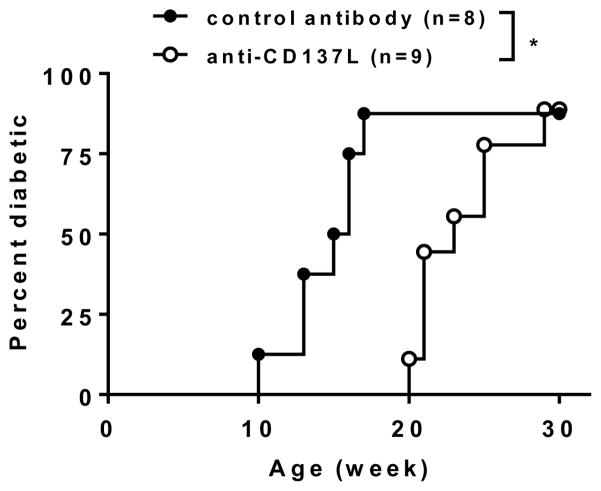
T1D is suppressed in NOD.Tnfrsf9−/− mice albeit immune regulation conferred by Treg-derived soluble CD137 is also lacking. We thus asked if CD137L blockade using a blocking antibody (clone TKS-1) can inhibit T1D development in NOD mice. The same antibody blocked the suppressive function of soluble CD137 on CD4 and CD8 T cell proliferation in vitro (Fig 8 above and ref (22)). Therefore, blocking CD137L in vivo would prevent the suppressive action of soluble CD137 and could enhance T1D development. On the other hand, it would also prevent stimulation of CD137 on CD8 T cells by CD137L, which could suppress T1D. Injections of anti-CD137L significantly delayed the onset of diabetes in NOD mice (Figure 9). However, diabetes suppression was transient and T1D development in the anti-CD137L treated NOD mice reached the same level as in the control group once antibody administration was ceased. Therefore, CD137L blockade dominantly suppressed T1D development in NOD mice but did not provide long-term protection from the disease.
Figure 9. CD137L blockage delays T1D onset in NOD mice.
Starting at 9 weeks of age, NOD female mice were injected every other week for a total of 5 injections with 250μg of anti-CD137L (TKS-1) each treatment or an isotype control antibody. T1D development was monitored weekly using urine glucose strips with onset defined by two consecutive readings of > 250 mg/dl. *P < 0.05 by Log-rank test.
Discussion
An array of costimulatory and coinhibitory molecules modulate the function of T cells, but their individual roles in T1D have not been completely dissected (11). Soluble CD137 produced by Tregs is immunosuppressive, but CD137 deficient NOD mice are more resistant to T1D than the wild-type control (21, 24). To reconcile these seemingly inconsistent results, we asked in which cell types CD137 exerts its diabetogenic function. Using a T cell adoptive transfer approach, we demonstrate here that the diabetogenic activity of CD8 T cells was significantly reduced in the absence of CD137, providing an explanation for T1D suppression observed in NOD.Tnfrsf9−/− mice. Consistent with a role of CD137 in Treg suppressive function, we also showed that CD4 T cells lacking this molecule were more diabetogenic than the wild-type counterparts. Our results also indicate that a balance exists between the diabetes protective and stimulatory roles of CD137 in NOD mice, and the combined effects of its expression (or its absence) in CD4 and CD8 T cells dictate the outcome of diabetes progression.
When pancreatic sections were stained for the presence of B cells, CD4 T cells, and CD8 T cells, lower numbers of all three lymphocyte populations were found in NOD.Tnfrsf9−/− islets compared to those from NOD mice (Figure 2). However, as a proportion of total islet infiltrating leukocytes (CD45+), only CD8 T cells were reduced in NOD.Tnfrsf9−/− mice (Figure 3). These results indicate that accumulation of CD8 T cells is preferentially affected in NOD.Tnfrsf9−/− mice. Previous studies indicate that accumulation of CD8 T cells in the islet is an antigen dependent process (34). Thus, our results indicate that CD137 deficiency reduces the number of islet infiltrating β-cell autoreactive CD8 T cells, an interpretation also supported by the IGRP208-214 MHC class I tetramer analysis. In addition to islets, β-cell autoreactive CD8 T cells were also found to be reduced in the spleen, PLN, and BM as indicated by those reactive to IGRP208-214, the prevalent pathogenic T effectors in NOD mice (38–40). The results from the BM chimera experiments (Figure 5) further demonstrate that the reduction of IGRP206-214 reactive CD8 T cells in NOD.Tnfrsf9−/− mice is due to the absence of CD137 expression on these cells rather than a consequence of delayed T1D development in this strain.
β-cell autoreactive CD8 T cells are activated in PLNs and can be further stimulated to undergo proliferation and differentiation when they re-encounter the cognate antigen in islets (41). To further determine the underlying mechanism of reduced β-cell autoreactive CD8 T cells in NOD.Tnfrsf9−/− mice, we asked if their activation in PLNs or proliferation in islets was affected. Adoptive transfer of CD137 sufficient or deficient NY8.3 CD8 T cells into NOD mice failed to reveal any difference in initial expansion and activation phenotypes (CD44 upregulation and CD62L downregulation) in PLNs. This was not due to lack of CD137 expression on wild-type NY8.3 cells in PLNs upon antigen stimulation. Indeed, we observed CD137 upregulation on adoptively transferred wild-type NY8.3 cells that have been stimulated but have not undergone cell division in PLNs (data not shown). Expression of CD137 was transient since it was not seen on transferred NY8.3 CD8 T cells that have divided at least once (data not shown). Our observation is consistent with a previous study where CD137 was shown to be transiently upregulated on OT-I CD8 T cells after in vivo immunization with OVA (42). We subsequently found that proliferation of islet infiltrating CD8 T cells was significantly lower in NOD.Tnfrsf9−/− than in wild-type NOD mice, indicating that CD137 is important to promote secondary expansion of β-cell autoreactive CD8 T cells in the target tissue. Our observation is reminiscent of the role of CD137 signaling in the secondary CD8 T cell response to influenza infection (43, 44). It remains to be determined if transient expression of CD137 during the initial activation of β-cell autoreactive CD8 T cells is essential and sufficient to sustain their subsequent proliferation in the islets.
The function of CD137-CD137L interaction in CD8 T cells has been studied in several viral infection models as reviewed in (45). In one study, CD8 T cells in CD137L-deficient mice have reduced cytotoxic function in response to murine gammaherpesvirus-68 infection (46). Therefore, CD137 signaling may also enhance the cytotoxic activity of β-cell autoreactive CD8 T cells. Nevertheless, our results suggest that CD137 plays a relatively less critical role in the cytotoxic function of β-cell autoreactive CD8 T cells in NOD mice. This interpretation is based on our observation that T1D development was comparable between NOD.NY8.3 and NOD.NY8.3.Tnfrsf9−/− mice where NY8.3 β-cell autoreactive CD8 T cells represent the main pathogenic effectors. If CD137 is important for the cytotoxic function of NY8.3 CD8 T cells, a delay in T1D development in NOD.NY8.3.Tnfrsf9−/− mice would have been observed. On the other hand, the significantly increased precursor frequency of β-cell autoreactive CD8 T cells in NOD.NY8.3 and NOD.NY8.3.Tnfrsf9−/− likely obviates the need for molecules important for peripheral expansion and accumulation of these pathogenic effectors. Thus, in the condition where the number of NY8.3 CD8 T cells is artificially inflated, CD137 plays a less discernible role in the development of T1D. Previous studies suggest that islet infiltrating autoreactive T cells can exit the site of inflammation to re-circulate and later re-enter the pancreas for subsequent β-cell destruction (47). These observations and our findings support a model in which CD137 expression on β-cell autoreactive CD8 T cells contributes to their increased proliferation and accumulation upon successive rounds of exposure to β cell antigen in PLNs and islets.
Our previous and current findings that CD137 plays roles in both Tregs and β-cell autoreactive T cells are not unique to this costimulatory pathway. Previous studies have shown dual roles of CD28-B7 and ICOS-ICOSL pathways in T1D progression in the NOD mouse model. While CD28 signaling is considered important for T cell activation, NOD mice deficient in this molecule develop rapid onset of diabetes due to largely reduced Tregs (48). ICOS deficient NOD mice are resistant to diabetes development, but blocking ICOS-ICOSL interaction in the BDC2.5 TCR transgenic model causes accelerated T1D onset as a result of impaired Treg function (49–53). Costimulation signals can regulate T1D in NOD mice in an age-dependent manner. Blocking OX40-OX40L at 12 weeks, but not at 6, 9, or 15 weeks, of age protected NOD mice from T1D (54). Conversely, soluble OX40L treatment rapidly induced diabetes development in NOD mice when injected at 12 weeks, but not at 6 weeks, of age (55). In our earlier studies, we showed that treating NOD mice at 6 weeks of age with agonistic anti-CD137 antibody suppressed T1D (20). Similar anti-CD137 treatment accelerated T1D development in NOD-scid recipients of CD4 and CD8 T cells isolated from diabetic NOD donors (20). The age-dependent effects of OX40 and CD137 stimulations likely resulted from differentially targeting of Tregs versus pathogenic T cells whose balance changes during diabetes progression.
Unlike the result of our previous study that a short-term treatment of 6–8 week old NOD females with recombinant soluble CD137 was sufficient to confer long-term protection from T1D (22), we show here that anti-CD137L only temporarily suppressed diabetes development. These results indicate that the in vivo T1D suppressive activity of soluble CD137 is not solely by interfering with the costimulatory interaction between APCs and T cells. The T1D inhibitory effect of soluble CD137 most likely involves its ability to actively suppress T cells by eliciting CD137L reverse signaling (22, 56) and potentially in APCs to promote tolerance, a possibility remains to be tested.
T1D development in NOD mice can be suppressed not only by CD137 deficiency, but also by introducing the B10-derived Idd9.3 congenic region. The apparent contradiction arises due to the fact that the B10-derived Idd9.3 region contains a more functional Tnfrsf9 allele (13). This may be explained by the dual role of CD137 in Tregs and CD8 T cells to respectively suppress and promote T1D in NOD mice (Figure 10). The collective results described here and published previously suggest that the Tnfrsf9 allele (null, NOD, or B10) influences the balance of Tregs and β-cell autoreactive T cells, which in turn modulates T1D progression. Complete CD137 deficiency inhibits accumulation of β-cell autoreactive CD8 T cells and tips the balance to favor immune regulation and T1D suppression. A more functional B10 CD137 molecule promotes the accumulation of CD137+ Tregs and the level of soluble CD137, which also leads to T1D suppression. On the other hand, a hypofunctional NOD CD137 molecule does not affects the accumulation of β-cell autoreactive CD8 T cells but reduces CD137+ Tregs and the level of soluble CD137, which in turn promotes T1D development. In conclusion, our studies provide a better understanding of how CD137 regulates T1D progression in NOD mice, and may facilitate the development of clinical therapeutics aimed at either blocking the stimulatory function of CD137 or enhancing its suppressive capacity.
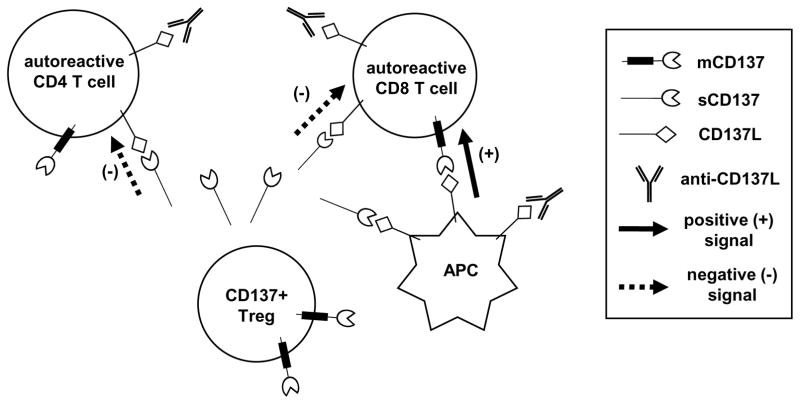
Figure 10. Schematic diagram illustrating the functions of CD137-CD137L interactions in the regulation of β-cell autoreactive T cells.
Treg-derived soluble CD137 (sCD137) binds to CD137L expressed on T cells and suppresses their activation. CD137L expressed by APC binds to membrane CD137 (mCD137) on β-cell autoreactive CD8 T cells to promote their expansion and accumulation. For therapeutic purposes, recombinant sCD137 can suppress activation of β-cell autoreactive T cells through direct binding to CD137L expressed on their surface as well as by blocking the interaction between mCD137 on CD8 T cells and CD137L on APC. CD137L blocking antibody inhibits interaction between mCD137 on CD8 T cells and CD137L on APC. CD137L blocking antibody also binds to CD137L on T cells without inducing an inhibitory effect but it prevents the suppressive function of sCD137.
Supplementary Material
Acknowledgments
This work was supported by NIH grants DK107541 (to Y.-G.C. and W.M.R.), DK097605 (to Y.-G.C.), AI110963 (to Y.-G.C.), and AI125879 (to Y.-G.C.), and an American Diabetes Association grant (to Y.-G.C. and W.M.R.).
We thank Shamim Khaja and Kevin Mueller for maintaining NOD and related mouse strains and Christine Duris at the Children’s Research Institute histology core for excellent technical assistance. We thank the NIH tetramer core facility for providing MHC class I tetramers.
References
- 1.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 2.Arch RH, Thompson CB. 4-1BB and Ox40 are members of a tumor necrosis factor (TNF)-nerve growth factor receptor subfamily that bind TNF receptor-associated factors and activate nuclear factor kappaB. Mol Cell Biol. 1998;18:558–565. doi: 10.1128/mcb.18.1.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jang IK, Lee ZH, Kim YJ, Kim SH, Kwon BS. Human 4-1BB (CD137) signals are mediated by TRAF2 and activate nuclear factor-kappa B. Biochemical and biophysical research communications. 1998;242:613–620. doi: 10.1006/bbrc.1997.8016. [DOI] [PubMed] [Google Scholar]
- 4.Cannons JL, Hoeflich KP, Woodgett JR, Watts TH. Role of the stress kinase pathway in signaling via the T cell costimulatory receptor 4-1BB. Journal of immunology. 1999;163:2990–2998. [PubMed] [Google Scholar]
- 5.Cannons JL, Choi Y, Watts TH. Role of TNF receptor-associated factor 2 and p38 mitogen-activated protein kinase activation during 4-1BB-dependent immune response. Journal of immunology. 2000;165:6193–6204. doi: 10.4049/jimmunol.165.11.6193. [DOI] [PubMed] [Google Scholar]
- 6.Wang C, Wen T, Routy JP, Bernard NF, Sekaly RP, Watts TH. 4-1BBL induces TNF receptor-associated factor 1-dependent Bim modulation in human T cells and is a critical component in the costimulation-dependent rescue of functionally impaired HIV-specific CD8 T cells. Journal of immunology. 2007;179:8252–8263. doi: 10.4049/jimmunol.179.12.8252. [DOI] [PubMed] [Google Scholar]
- 7.Sabbagh L, Pulle G, Liu Y, Tsitsikov EN, Watts TH. ERK-dependent Bim modulation downstream of the 4-1BB-TRAF1 signaling axis is a critical mediator of CD8 T cell survival in vivo. J Immunol. 2008;180:8093–8101. doi: 10.4049/jimmunol.180.12.8093. [DOI] [PubMed] [Google Scholar]
- 8.Sabbagh L, Andreeva D, Laramee GD, Oussa NA, Lew D, Bisson N, Soumounou Y, Pawson T, Watts TH. Leukocyte-specific protein 1 links TNF receptor-associated factor 1 to survival signaling downstream of 4-1BB in T cells. Journal of leukocyte biology. 2013;93:713–721. doi: 10.1189/jlb.1112579. [DOI] [PubMed] [Google Scholar]
- 9.Rajasekaran K, Kumar P, Schuldt KM, Peterson EJ, Vanhaesebroeck B, Dixit V, Thakar MS, Malarkannan S. Signaling by Fyn-ADAP via the Carma1-Bcl-10-MAP3K7 signalosome exclusively regulates inflammatory cytokine production in NK cells. Nature immunology. 2013;14:1127–1136. doi: 10.1038/ni.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SW, Park Y, So T, Kwon BS, Cheroutre H, Mittler RS, Croft M. Identification of regulatory functions for 4-1BB and 4-1BBL in myelopoiesis and the development of dendritic cells. Nature immunology. 2008;9:917–926. doi: 10.1038/ni.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Q, Vignali DA. Co-stimulatory and Co-inhibitory Pathways in Autoimmunity. Immunity. 2016;44:1034–1051. doi: 10.1016/j.immuni.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Driver JP, Serreze DV, Chen YG. Mouse models for the study of autoimmune type 1 diabetes: a NOD to similarities and differences to human disease. Semin Immunopathol. 2011;33:67–87. doi: 10.1007/s00281-010-0204-1. [DOI] [PubMed] [Google Scholar]
- 13.Cannons JL, Chamberlain G, Howson J, Smink LJ, Todd JA, Peterson LB, Wicker LS, Watts TH. Genetic and functional association of the immune signaling molecule 4-1BB (CD137/TNFRSF9) with type 1 diabetes. J Autoimmun. 2005;25:13–20. doi: 10.1016/j.jaut.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Lyons PA, Hancock WW, Denny P, Lord CJ, Hill NJ, Armitage N, Siegmund T, Todd JA, Phillips MS, Hess JF, Chen SL, Fischer PA, Peterson LB, Wicker LS. The NOD Idd9 genetic interval influences the pathogenicity of insulitis and contains molecular variants of Cd30, Tnfr2, and Cd137. Immunity. 2000;13:107–115. doi: 10.1016/s1074-7613(00)00012-1. [DOI] [PubMed] [Google Scholar]
- 15.Yamanouchi J, Puertas MC, Verdaguer J, Lyons PA, Rainbow DB, Chamberlain G, Hunter KM, Peterson LB, Wicker LS, Santamaria P. Idd9.1 locus controls the suppressive activity of FoxP3+CD4+CD25+ regulatory T-cells. Diabetes. 2010;59:272–281. doi: 10.2337/db09-0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward-Kavanagh LK, Lin WW, Sedy JR, Ware CF. The TNF Receptor Superfamily in Co-stimulating and Co-inhibitory Responses. Immunity. 2016;44:1005–1019. doi: 10.1016/j.immuni.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 18.Choi BK, Bae JS, Choi EM, Kang WJ, Sakaguchi S, Vinay DS, Kwon BS. 4-1BB-dependent inhibition of immunosuppression by activated CD4+CD25+ T cells. Journal of leukocyte biology. 2004;75:785–791. doi: 10.1189/jlb.1003491. [DOI] [PubMed] [Google Scholar]
- 19.Zheng G, Wang B, Chen A. The 4-1BB costimulation augments the proliferation of CD4+CD25+ regulatory T cells. Journal of immunology. 2004;173:2428–2434. doi: 10.4049/jimmunol.173.4.2428. [DOI] [PubMed] [Google Scholar]
- 20.Irie J, Wu Y, Kachapati K, Mittler RS, Ridgway WM. Modulating protective and pathogenic CD4+ subsets via CD137 in type 1 diabetes. Diabetes. 2007;56:186–196. doi: 10.2337/db06-0793. [DOI] [PubMed] [Google Scholar]
- 21.Kachapati K, Adams DE, Wu Y, Steward CA, Rainbow DB, Wicker LS, Mittler RS, Ridgway WM. The B10 Idd9.3 Locus Mediates Accumulation of Functionally Superior CD137+ Regulatory T Cells in the Nonobese Diabetic Type 1 Diabetes Model. J Immunol. 2012;189:5001–5015. doi: 10.4049/jimmunol.1101013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kachapati K, Bednar KJ, Adams DE, Wu Y, Mittler RS, Jordan MB, Hinerman JM, Herr AB, Ridgway WM. Recombinant soluble CD137 prevents type one diabetes in nonobese diabetic mice. J Autoimmun. 2013;47:94–103. doi: 10.1016/j.jaut.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Sytwu HK, Lin WD, Roffler SR, Hung JT, Sung HS, Wang CH, Cheng TL, Tsou SC, Hsi SC, Shen KL. Anti-4-1BB-based immunotherapy for autoimmune diabetes: lessons from a transgenic non-obese diabetic (NOD) model. J Autoimmun. 2003;21:247–254. doi: 10.1016/s0896-8411(03)00112-4. [DOI] [PubMed] [Google Scholar]
- 24.Chen YG, Forsberg MH, Khaja S, Ciecko AE, Hessner MJ, Geurts AM. Gene targeting in NOD mouse embryos using zinc-finger nucleases. Diabetes. 2014;63:68–74. doi: 10.2337/db13-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serreze DV, Osborne MA, Chen YG, Chapman HD, Pearson T, Brehm MA, Greiner DL. Partial versus full allogeneic hemopoietic chimerization is a preferential means to inhibit type 1 diabetes as the latter induces generalized immunosuppression. J Immunol. 2006;177:6675–6684. doi: 10.4049/jimmunol.177.10.6675. [DOI] [PubMed] [Google Scholar]
- 26.Serreze DV, Wasserfall C, Ottendorfer EW, Stalvey M, Pierce MA, Gauntt C, O’Donnell B, Flanagan JB, Campbell-Thompson M, Ellis TM, Atkinson MA. Diabetes acceleration or prevention by a coxsackievirus B4 infection: critical requirements for both interleukin-4 and gamma interferon. Journal of virology. 2005;79:1045–1052. doi: 10.1128/JVI.79.2.1045-1052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C, Lin GH, McPherson AJ, Watts TH. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol Rev. 2009;229:192–215. doi: 10.1111/j.1600-065X.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 29.Saxena V, Ondr JK, Magnusen AF, Munn DH, Katz JD. The countervailing actions of myeloid and plasmacytoid dendritic cells control autoimmune diabetes in the nonobese diabetic mouse. J Immunol. 2007;179:5041–5053. doi: 10.4049/jimmunol.179.8.5041. [DOI] [PubMed] [Google Scholar]
- 30.Choi BK, Kim YH, Kwon PM, Lee SC, Kang SW, Kim MS, Lee MJ, Kwon BS. 4-1BB functions as a survival factor in dendritic cells. Journal of immunology. 2009;182:4107–4115. doi: 10.4049/jimmunol.0800459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SW, Park Y, Eun SY, Madireddi S, Cheroutre H, Croft M. Cutting edge: 4-1BB controls regulatory activity in dendritic cells through promoting optimal expression of retinal dehydrogenase. J Immunol. 2012;189:2697–2701. doi: 10.4049/jimmunol.1201248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verdaguer J, Schmidt D, Amrani A, Anderson B, Averill N, Santamaria P. Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J Exp Med. 1997;186:1663–1676. doi: 10.1084/jem.186.10.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serra P, Amrani A, Yamanouchi J, Han B, Thiessen S, Utsugi T, Verdaguer J, Santamaria P. CD40 ligation releases immature dendritic cells from the control of regulatory CD4+CD25+ T cells. Immunity. 2003;19:877–889. doi: 10.1016/s1074-7613(03)00327-3. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Tsai S, Shameli A, Yamanouchi J, Alkemade G, Santamaria P. In situ recognition of autoantigen as an essential gatekeeper in autoimmune CD8+ T cell inflammation. Proc Natl Acad Sci U S A. 2010;107:9317–9322. doi: 10.1073/pnas.0913835107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li R, Perez N, Karumuthil-Melethil S, Vasu C. Bone marrow is a preferential homing site for autoreactive T-cells in type 1 diabetes. Diabetes. 2007;56:2251–2259. doi: 10.2337/db07-0502. [DOI] [PubMed] [Google Scholar]
- 36.Tritt M, Sgouroudis E, d’Hennezel E, Albanese A, Piccirillo CA. Functional waning of naturally occurring CD4+ regulatory T-cells contributes to the onset of autoimmune diabetes. Diabetes. 2008;57:113–123. doi: 10.2337/db06-1700. [DOI] [PubMed] [Google Scholar]
- 37.Bresson D, Fousteri G, Manenkova Y, Croft M, von Herrath M. Antigen-specific prevention of type 1 diabetes in NOD mice is ameliorated by OX40 agonist treatment. Journal of autoimmunity. 2011;37:342–351. doi: 10.1016/j.jaut.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lieberman SM, Evans AM, Han B, Takaki T, Vinnitskaya Y, Caldwell JA, Serreze DV, Shabanowitz J, Hunt DF, Nathenson SG, Santamaria P, DiLorenzo TP. Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc Natl Acad Sci U S A. 2003;100:8384–8388. doi: 10.1073/pnas.0932778100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trudeau JD, Kelly-Smith C, Verchere CB, Elliott JF, Dutz JP, Finegood DT, Santamaria P, Tan R. Prediction of spontaneous autoimmune diabetes in NOD mice by quantification of autoreactive T cells in peripheral blood. J Clin Invest. 2003;111:217–223. doi: 10.1172/JCI16409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lieberman SM, Takaki T, Han B, Santamaria P, Serreze DV, DiLorenzo TP. Individual nonobese diabetic mice exhibit unique patterns of CD8+ T cell reactivity to three islet antigens, including the newly identified widely expressed dystrophia myotonica kinase. J Immunol. 2004;173:6727–6734. doi: 10.4049/jimmunol.173.11.6727. [DOI] [PubMed] [Google Scholar]
- 41.Graham KL, Krishnamurthy B, Fynch S, Ayala-Perez R, Slattery RM, Santamaria P, Thomas HE, Kay TW. Intra-islet proliferation of cytotoxic T lymphocytes contributes to insulitis progression. Eur J Immunol. 2012;42:1717–1722. doi: 10.1002/eji.201242435. [DOI] [PubMed] [Google Scholar]
- 42.Dawicki W, Watts TH. Expression and function of 4-1BB during CD4 versus CD8 T cell responses in vivo. European journal of immunology. 2004;34:743–751. doi: 10.1002/eji.200324278. [DOI] [PubMed] [Google Scholar]
- 43.Bertram EM, Lau P, Watts TH. Temporal segregation of 4-1BB versus CD28-mediated costimulation: 4-1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following influenza infection. J Immunol. 2002;168:3777–3785. doi: 10.4049/jimmunol.168.8.3777. [DOI] [PubMed] [Google Scholar]
- 44.Bertram EM, Dawicki W, Sedgmen B, Bramson JL, Lynch DH, Watts TH. A switch in costimulation from CD28 to 4-1BB during primary versus secondary CD8 T cell response to influenza in vivo. Journal of immunology. 2004;172:981–988. doi: 10.4049/jimmunol.172.2.981. [DOI] [PubMed] [Google Scholar]
- 45.Wortzman ME, Clouthier DL, McPherson AJ, Lin GH, Watts TH. The contextual role of TNFR family members in CD8(+) T-cell control of viral infections. Immunological reviews. 2013;255:125–148. doi: 10.1111/imr.12086. [DOI] [PubMed] [Google Scholar]
- 46.Fuse S, Bellfy S, Yagita H, Usherwood EJ. CD8+ T cell dysfunction and increase in murine gammaherpesvirus latent viral burden in the absence of 4-1BB ligand. Journal of immunology. 2007;178:5227–5236. doi: 10.4049/jimmunol.178.8.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chee J, Ko HJ, Skowera A, Jhala G, Catterall T, Graham KL, Sutherland RM, Thomas HE, Lew AM, Peakman M, Kay TW, Krishnamurthy B. Effector-memory T cells develop in islets and report islet pathology in type 1 diabetes. J Immunol. 2014;192:572–580. doi: 10.4049/jimmunol.1302100. [DOI] [PubMed] [Google Scholar]
- 48.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 49.Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199:1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ansari MJ, Fiorina P, Dada S, Guleria I, Ueno T, Yuan X, Trikudanathan S, Smith RN, Freeman G, Sayegh MH. Role of ICOS pathway in autoimmune and alloimmune responses in NOD mice. Clinical immunology. 2008;126:140–147. doi: 10.1016/j.clim.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 51.Hawiger D, Tran E, Du W, Booth CJ, Wen L, Dong C, Flavell RA. ICOS mediates the development of insulin-dependent diabetes mellitus in nonobese diabetic mice. Journal of immunology. 2008;180:3140–3147. doi: 10.4049/jimmunol.180.5.3140. [DOI] [PubMed] [Google Scholar]
- 52.Kornete M, Sgouroudis E, Piccirillo CA. ICOS-dependent homeostasis and function of Foxp3+ regulatory T cells in islets of nonobese diabetic mice. J Immunol. 2012;188:1064–1074. doi: 10.4049/jimmunol.1101303. [DOI] [PubMed] [Google Scholar]
- 53.Prevot N, Briet C, Lassmann H, Tardivel I, Roy E, Morin J, Mak TW, Tafuri A, Boitard C. Abrogation of ICOS/ICOS ligand costimulation in NOD mice results in autoimmune deviation toward the neuromuscular system. European journal of immunology. 2010;40:2267–2276. doi: 10.1002/eji.201040416. [DOI] [PubMed] [Google Scholar]
- 54.Pakala SV, Bansal-Pakala P, Halteman BS, Croft M. Prevention of diabetes in NOD mice at a late stage by targeting OX40/OX40 ligand interactions. European journal of immunology. 2004;34:3039–3046. doi: 10.1002/eji.200425141. [DOI] [PubMed] [Google Scholar]
- 55.Haddad CS, Bhattacharya P, Alharshawi K, Marinelarena A, Kumar P, El-Sayed O, Elshabrawy HA, Epstein AL, Prabhakar BS. Age-dependent divergent effects of OX40L treatment on the development of diabetes in NOD mice. Autoimmunity. 2016:1–14. doi: 10.1080/08916934.2016.1183657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eun SY, Lee SW, Xu Y, Croft M. 4-1BB Ligand Signaling to T Cells Limits T Cell Activation. J Immunol. 2015;194:134–141. doi: 10.4049/jimmunol.1401383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.