Supplemental Digital Content is available in the text.
Abstract
Background:
Keloids are a dermal fibroproliferative scar of unknown etiology. There is no good animal model for the study of keloids, which hinders the development and assessment of treatments for keloids.
Methods:
Human keratinocytes and dermal fibroblasts were isolated from 3 human skin tissues: normal skin, white scars, and keloids. A mixed-cell slurry containing keratinocytes and dermal fibroblasts was poured into a double chamber implanted on the back of NOD/Shi-scid/IL-2Rγnull mice. After 12 weeks, the recipient mice had developed reconstituted human skin tissues on their backs. These were harvested for histological studies.
Results:
Macroscopically, the reconstituted skins derived from both normal skin and white scars were similar to normal skin and white scars in humans, respectively. Keloid-derived reconstituted skins exhibited keloid-like hypertrophic nodules. Histological findings and immunohistochemical staining confirmed that the reconstituted skin tissues were of human origin and the keloid-derived reconstituted skin had the typical features of human keloids such as a hypertrophic dermal nodule, collagen type composition, orientation of collagen fibers, and versican expression.
Conclusion:
The mouse model with humanized keloid tissue presented here should be a useful tool for future keloid research.
INTRODUCTION
Keloids are dermal fibroproliferative scars that result from pathological wound healing, in which there is excessive deposition of extracellular matrix. Although several factors, especially mechanical tension1 and transforming growth factor β signaling,2 are known to be involved in keloid formation, the molecular mechanism of their formation is not fully understood.
Keloids are benign, but their treatment is challenging. Surgical excision or steroid injection can be effective in the short term, but the keloid often recurs. There is less recurrence when adjuvant radiotherapy is conducted after surgical excision of the keloid,3 although the application of radiotherapy to a benign tumor is controversial.
Attempts to assess current therapies for keloids and to develop new ones have been hindered by the lack of appropriate animal models. Animals do not develop keloids after dermal trauma, so it has been necessary to adopt creative approaches in the development of model systems for keloid pathogenesis. Some animal models have been developed by grafting human keloid tissue or keloid-derived cells onto other species, in which the keloid-derived extracellular matrix or the artificial scaffold were also transplanted, respectively.4-8 This is a serious drawback of these experimental models because the extracellular matrix of keloid was not newly generated in vivo.
Here, we report on the development of a novel animal model system based on the chamber technique for skin reconstitution. This technique permits the reconstitution of full-thickness human skin, including the dermal–epidermal junction.9 We established a mouse model with humanized keloids with this technique, and their extracellular matrix was produced in vivo by keloid-derived cells.
MATERIALS AND METHODS
Human Skin Samples
Tissue samples of 3 keloids, 2 white scars, and 2 normal skins were obtained from patients at the time they underwent clinically indicated surgical procedures and after they had granted informed consent (see table, Supplemental Digital Content 1, which displays the patient information, http://links.lww.com/PRSGO/A428). The ethical committee of Jichi Medical University approved this study using clinical samples, and this study was conducted according to the Declaration of Helsinki Principles.
Table 1.
Patient Information

Primary Cultures of Human Keratinocytes and Fibroblasts
Primary culture of human dermal fibroblasts was established using an explant culture technique. Human keratinocytes were isolated as previously described.10 Fibroblasts were cultured in Dulbecco’s modified Eagle’s medium, supplemented with 10% fetal bovine serum, penicillin (100 IU/mL), streptomycin (100 mg/mL), and amphotericin B (0.25 mg/mL) at 37°C in a humidified incubator with 5% CO2. Keratinocytes were cultured in EpiLife (Thermo Fisher Scientific, Yokohama, Japan), supplemented with human keratinocyte growth supplement, penicillin (100 IU/mL), streptomycin (100 mg/mL), and amphotericin B (0.25 mg/mL) at 37°C in a humidified 5% CO2 incubator. Only low-passage cultures (passage 3–4) were utilized in this study.
Animal Models
The immune-deficient NOD/Shi-scid,IL-2RγKO mice (NOG mice)11 were purchased from Central Institute of Experimental Animals (Kawasaki, Japan), and 21 NOG mice (3 mice per sample) were used as recipients. A spontaneous in vivo cell-sorting model (chamber technique) was generated as previously described9 with some modifications. Briefly, a mixed-cell slurry containing approximately 6 × 106 keratinocytes and 6 × 106 dermal fibroblasts was trypsinized and mixed in 300 µL Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum. A circle 1 cm in diameter of upper back skin was removed from NOG mice, using surgical scissors. Double chambers (Renner-Gmbh, Güglingen, Germany) were implanted on the back of NOG mice with a purse-string suture of 6-0 nylon. The mixed-cell suspension was poured into the chamber through the 1-mm hole on the top of the chamber, so that it was applied directly onto the mouse muscle fascia. The upper third of the upper chamber was cut off and covered with a metal mesh 1 week after implantation to evaporate the solution inside the chamber gradually. The purse-string suture was removed 4 weeks after implantation, and the double chamber was allowed to fall off spontaneously from the skin approximately 6 weeks after implantation. After 12 weeks, the reconstituted human skins on the back of the recipient mice were harvested for histological studies (Fig. 1).
Fig. 1.
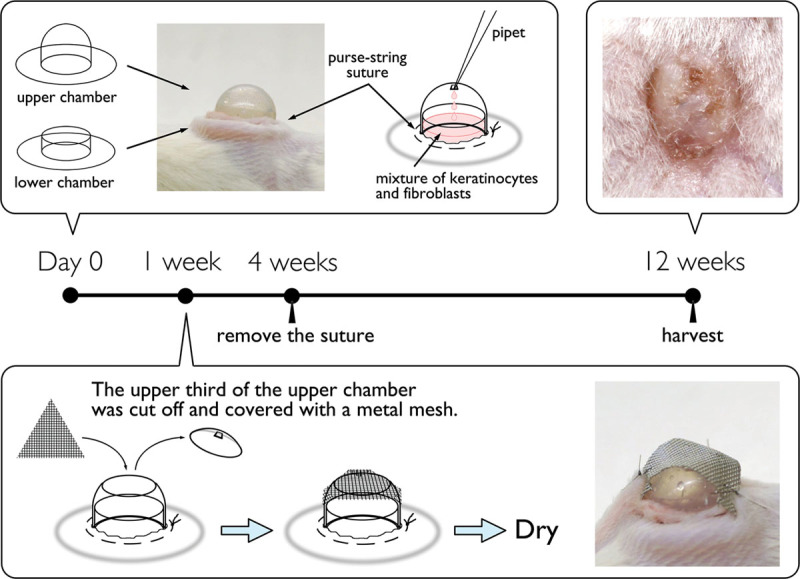
Schematic diagrams of development of the reconstituted human skins. Day 0: a circle 1 cm in diameter of upper back skin was removed from NOG mice, using surgical scissors. Double chambers were implanted on the back of NOG mice with a purse-string suture of 6-0 nylon. The 300 µL of mixed-cell suspension containing approximately 6 × 106 keratinocytes and 6 × 106 dermal fibroblasts was poured into the chamber through the 1-mm hole on the top of the chamber. One week: the upper third of the upper chamber was cut off and covered with a metal mesh to evaporate the solution inside the chamber gradually. Four weeks: the purse-string suture was removed and the double chamber was allowed to fall off spontaneously from the skin. Twelve weeks: the reconstituted human skins were harvested for histological studies.
Histological Analysis and Immunohistology
Samples were fixed in 4% paraformaldehyde, embedded in paraffin, sectioned, and stained. Gomori’s 1-step trichrome staining and picrosirius red staining were performed with Gomori’s Trichrome Stain Kit and Picrosirius Red Stain Kit (Polysciences, Inc., Warrington, Pa.), respectively. For immunohistochemistry, the following antibodies were used: rabbit antiversican, rabbit antiinvolucrin (Sigma-Aldrich, Tokyo, Japan), mouse antivimentin (Dako, Tokyo, Japan), rabbit antihuman collagen I, and rabbit antihuman collagen III (Abcam, Tokyo, Japan). For visualization with diaminobenzidine, EnVision Plus System (Dako, Tokyo, Japan) was used.
Image Analysis
The horizontal width of human-derived epidermises in the histological sections of the reconstituted skins, which could be distinguished from mouse epidermis microscopically, and the areas of the reconstituted skins under the human-derived epidermis were quantified using ImageJ software (https://imagej.nih.gov/ij/) in microscopic fields photographed at 2× magnification. The thicknesses of the reconstituted skins were obtained by dividing the area by the horizontal width.
Statistics
Statistically significant differences were determined using the Student’s t test. Differences were considered significant when P < 0.05.
RESULTS
Development of Humanized Skin, White Scar, or Keloid Models
All recipient mice had reconstituted human skin on their backs 12 weeks after they received the mixed-cell suspension graft. The macroscopic appearances of the reconstituted skin derived from normal skin and white scars were similar to the original skin and scars, respectively. The keloid-derived reconstituted skin had a keloid-like hypertrophied nodule (Fig. 2). The human origin of the reconstituted skins (normal skin, white scar, and keloids) was confirmed with immunohistochemistry for the human-specific antibodies involucrin, collagen type 1 (Fig. 3), vimentin, and collagen type 3 (see figure, Supplemental Digital Content 2, which displays reconstituted human skins immunostained with human-specific antibodies, antivimentin, and anticollagen type 3, http://links.lww.com/PRSGO/A429).
Fig. 2.
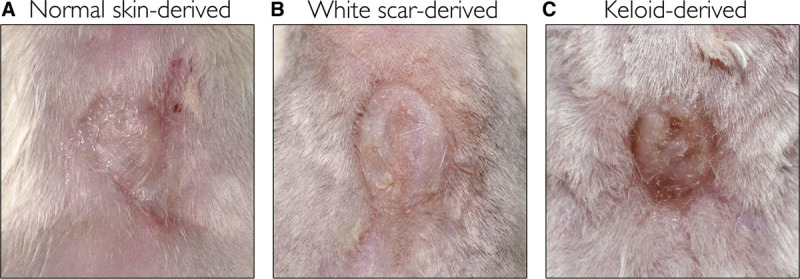
Representative appearance of the reconstituted human skins. A, Derived from normal skins. B, Derived from white scars. C, Derived from keloids.
Fig. 3.
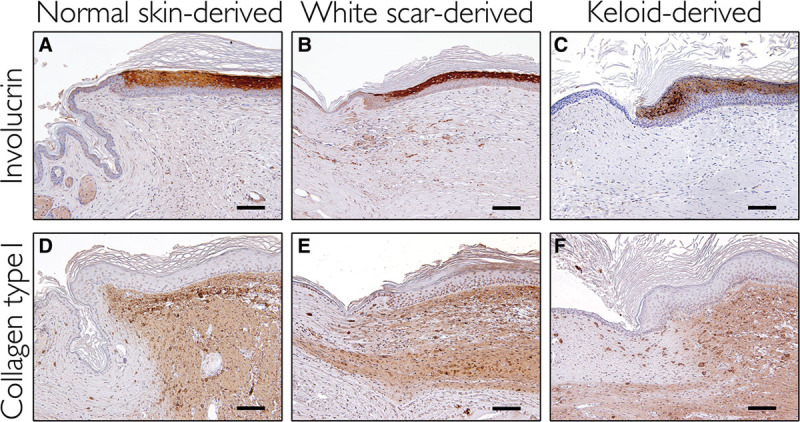
Reconstituted human skins immunostained with human-specific antibodies, antiinvolucrin, and anticollagen type 1. A and D, Derived from normal skins. B and E, Derived from white scars. C and F, Derived from keloids. Scale bar = 100 µm.
Histological Findings
Image analyses of histological sections were performed to quantify the thickness of the reconstituted skins. Consistent with the macroscopic findings, keloid-derived reconstituted skins were significantly thicker than skins derived from normal skin or white scar tissue (Fig. 4).
Fig. 4.
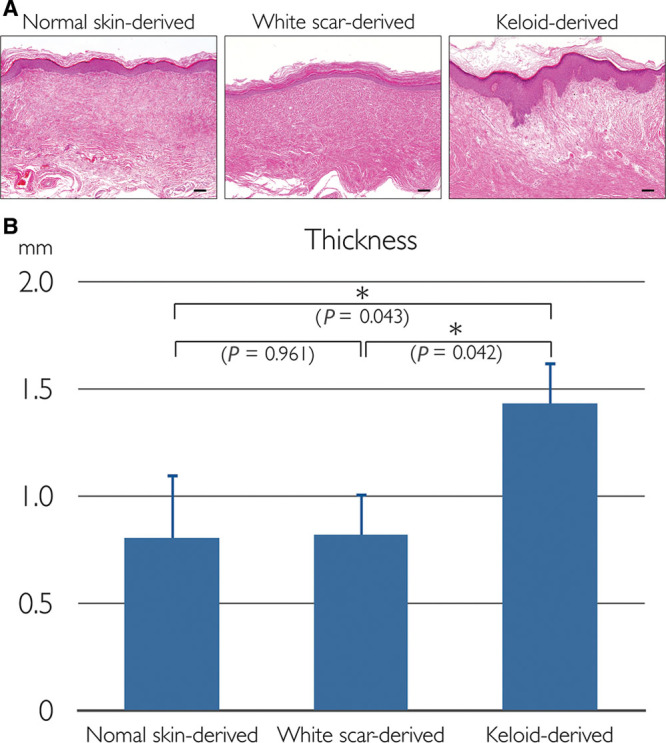
H&E–stained sections and thicknesses of the reconstituted human skins. A, H&E staining. Left, Derived from normal skins. Center, Derived from white scars. Right, Derived from keloids. Scale bars = 100 µm. B, Thickness; data represent means ± standard error of the mean; *P < 0.05. H&E, hematoxylin and eosin.
The accumulation and orientation of collagen fibers in the dermis of the reconstituted skins were assessed by Gomori’s trichrome staining, which stains collagen fibers blue. All the skins assessed had collagen fibers in the dermis. In original normal and white scar skin, collagen fibers were well organized and relatively parallel to the skin surface, whereas the orientation of collagen fibers was more chaotic in original human keloid skin samples. Next, we compared collagen fibers in reconstituted skins. The collagen fibers in keloid-derived reconstituted skins were also chaotic and more perpendicular to the skin surface than those reconstituted from normal or white scar tissue (see figure, Supplemental Digital Content 3, which displays Gomori’s trichrome staining of human and reconstituted skin samples, http://links.lww.com/PRSGO/A430).
Tissues were stained with picrosirius red and then assessed with polarization microscopy. The resulting birefringence patterns are presented in Figure 5 and Supplemental Digital Content 4 (see figure, Supplemental Digital Content 4, which displays histological sections stained with picrosirius red; collagen birefringence pattern was assessed with polarization microscopy, http://links.lww.com/PRSGO/A431). Collagen fibers in normal skin and reconstituted skin derived from normal skin were dark orange, indicating that the dermis of these samples mainly consisted of collagen type 1. On the other hand, collagen fibers in both the original and reconstituted keloids were thin and stained yellow-green, indicating that they contained collagen type 3.
Fig. 5.
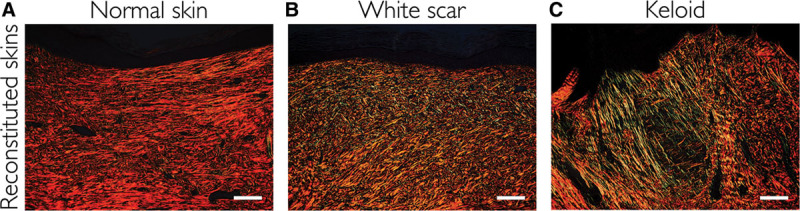
Histological sections stained with picrosirius red. Collagen birefringence pattern was assessed with polarization microscopy. A, Reconstituted skin derived from normal skins. B, Reconstituted skin derived from white scars. C, Reconstituted skin derived from keloids. Scale bars =100 µm.
It was recently demonstrated that versican, an extracellular matrix proteoglycan, is a specific marker for keloids.12 We used immunohistochemical staining for versican to detect its expression. Versican was highly expressed in both the human keloid skin samples and the reconstituted skin from keloid tissue, whereas versican was not detected in the other samples (Fig. 6; see also pdf, Supplemental Digital Content 5, which displays immuno staining for versican, a keloid-specific marker, http://links.lww.com/PRSGO/A432).
Fig. 6.
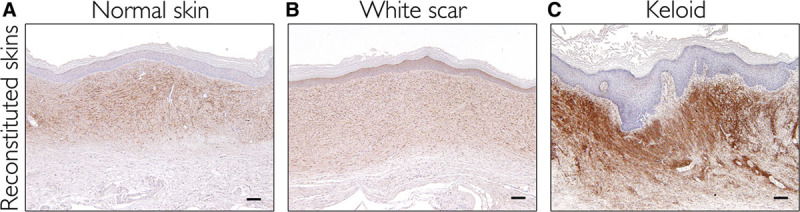
Immunostaining for versican, a keloid-specific marker. A, Reconstituted skin derived from normal skins. B, Reconstituted skin derived from white scars. C, Reconstituted skin derived from keloids. Scale bars = 100 µm.
DISCUSSION
Here, we report on a novel mouse model of human keloids, which has humanized keloid tissue on the back. We developed this model by grafting both keloid-derived keratinocytes and fibroblasts to NOG mice with a spontaneous cell-sorting technique (chamber technique).
The genes governing the pathogenesis associated with keloid formation have not been identified, precluding the development of transgenic systems for the study of keloids. Thus, transplantation of human keloid tissue to immunodeficient mice is a reasonable strategy for generating an in vivo model of keloid formation.13 However, xenografts contain not only cellular components but also donor-derived extracellular matrix, which makes it difficult to be certain that it is the keloid-derived cells that are responsible for any observed keloid phenotype. A related approach was used by Supp et al.6, who grafted engineered human skin substitutes with keloid-derived fibroblasts and keratinocytes to athymic mice, and the resulting grafts were thicker and wider than grafts made with normal skin-derived cells. However, their organotypic skin included an artificial collagen–glycosaminoglycan scaffold and did not have macroscopic or pathological features of keloids. In contrast, our humanized keloid model had keloid-like nodules and chaotic/perpendicular orientation of collagen fibers (predominantly collagen type 3), macroscopic, and pathological features of keloids. Moreover, the extracellular matrix in the reconstituted skin had been newly produced by the grafted keloid fibroblasts, which strongly suggested that the transplanted keloid-derived fibroblasts maintained its original functions under interaction with keloid-derived keratinocytes.
In this study, we did not observe invasiveness of the reconstituted keloid tissue, which is one of the hallmark features of keloids. It may be due to the lack of human cells in the surrounding tissue or bone marrow, which may be the origin of invasive cells.14 Another possibility is that some human-specific microenvironment, such as mechanical force, is essential for keloid invasion.15 Mechanical tension loading to the reconstituted skin in our model may not have been sufficient enough to maintain the invasive phenotype of keloid. Although the invasive phenotype was not reproduced, we observed that transplantation of keratinocytes and fibroblasts produced a keloid-like fibroproliferative lesion. It was indicated that these keloid-derived cells maintained the keloid phenotype in vivo, for at least 12 weeks, and in the absence of a human-specific microenvironment.
In this study, the number of grafted cells and the ratio of fibroblasts to keratinocytes were determined based on the previous results of the double-chamber technique.
Recently, Russel et al.16 demonstrated that treatment of keloid fibroblasts with trichostatin A, an inhibitor of histone deacetylation, resulted in increased expression of secreted frizzled-related protein 1. They proposed the intriguing hypothesis that epigenetic modification is involved in keloid pathogenesis. Although their study does not provide direct evidence for this interpretation, epigenetic modification could explain the long-term maintenance of the phenotype in keloid-derived cells. The hypothesis warrants further study.
The greatest disadvantage to our model and other keloid–xenograft models is that the supply of keloid-derived cells is limited. Because of this limitation, long-term evolution of the reconstituted skins over 12 weeks could not be assessed in this study. Long-term follow-up in the future studies will reveal whether the keloid phenotype of the reconstituted scar tissue is sustainable or not. Generation of immortalized human keloid-derived cell lines may circumvent this limitation and would be a valuable resolution in future studies.
In summary, we developed a mouse model with humanized keloid by grafting the keloid-derived keratinocytes and fibroblasts in a double chamber on the back of NOG mice. The reconstituted keloid tissue exhibited typical macroscopic and histopathological features of human keloid lesions such as a hypertrophic dermal nodule, collagen type composition, orientation of collagen fibers, and versican expression. The reconstituted tissues did not, however, invade adjacent skin, at least within 12 weeks after transplantation. We hope this model proves to be a useful tool for future keloid research.
Supplementary Material
Footnotes
Disclosure: Supported by the Japanese Ministry of Education, Culture, Sports, Science, and Technology (contact grant 23659835 and 15K15655). The Article Processing Charge was paid for by the authors.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Ogawa R, Okai K, Tokumura F, et al. The relationship between skin stretching/contraction and pathologic scarring: the important role of mechanical forces in keloid generation. Wound Repair Regen. 2012;20:149–157.. [DOI] [PubMed] [Google Scholar]
- 2.Washio H, Fukuda N, Matsuda H, et al. Transcriptional inhibition of hypertrophic scars by a gene silencer, pyrrole-imidazole polyamide, targeting the TGF-β1 promoter. J Invest Dermatol. 2011;131:1987–1995.. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa R, Mitsuhashi K, Hyakusoku H, et al. Postoperative electron-beam irradiation therapy for keloids and hypertrophic scars: retrospective study of 147 cases followed for more than 18 months. Plast Reconstr Surg. 2003;111:547–53.; discussion 554. [DOI] [PubMed] [Google Scholar]
- 4.Polo M, Kim YJ, Kucukcelebi A, et al. An in vivo model of human proliferative scar. J Surg Res. 1998;74:187–195.. [DOI] [PubMed] [Google Scholar]
- 5.Shetlar MR, Shetlar CL, Kischer CW, et al. Implants of keloid and hypertrophic scars into the athymic nude mouse: changes in the glycosaminoglycans of the implants. Connect Tissue Res. 1991;26;23–26.. [DOI] [PubMed] [Google Scholar]
- 6.Supp DM, Hahn JM, Glaser K, et al. Deep and superficial keloid fibroblasts contribute differentially to tissue phenotype in a novel in vivo model of keloid scar. Plast Reconstr Surg. 2012;129:1259–1271.. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Luo S. Establishment of an animal model for human keloid scars using tissue engineering method. J Burn Care Res. 2013;34:439–446.. [DOI] [PubMed] [Google Scholar]
- 8.Ishiko T, Naitoh M, Kubota H, et al. Chondroitinase injection improves keloid pathology by reorganizing the extracellular matrix with regenerated elastic fibers. J Dermatol. 2013;40:380–383.. [DOI] [PubMed] [Google Scholar]
- 9.Wang CK, Nelson CF, Brinkman AM, et al. Spontaneous cell sorting of fibroblasts and keratinocytes creates an organotypic human skin equivalent. J Invest Dermatol. 2000;114:674–680.. [DOI] [PubMed] [Google Scholar]
- 10.Aasen T, Izpisúa Belmonte JC. Isolation and cultivation of human keratinocytes from skin or plucked hair for the generation of induced pluripotent stem cells. Nat Protoc. 2010;5:371–382.. [DOI] [PubMed] [Google Scholar]
- 11.Ito M, Hiramatsu H, Kobayashi K, et al. NOD/SCID/gamma©(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182.. [DOI] [PubMed] [Google Scholar]
- 12.Yagi Y, Muroga E, Naitoh M, et al. An ex vivo model employing keloid-derived cell-seeded collagen sponges for therapy development. J Invest Dermatol. 2013;133:386–393.. [DOI] [PubMed] [Google Scholar]
- 13.Hillmer MP, MacLeod SM. Experimental keloid scar models: a review of methodological issues. J Cutan Med Surg. 2002;6:354–359.. [DOI] [PubMed] [Google Scholar]
- 14.LeBleu VS, Taduri G, O’Connell J, et al. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19:1047–1053.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aarabi S, Bhatt KA, Shi Y, et al. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007;21:3250–3261.. [DOI] [PubMed] [Google Scholar]
- 16.Russell SB, Russell JD, Trupin KM, et al. Epigenetically altered wound healing in keloid fibroblasts. J Invest Dermatol. 2010;130:2489–2496.. [DOI] [PMC free article] [PubMed] [Google Scholar]


