Abstract
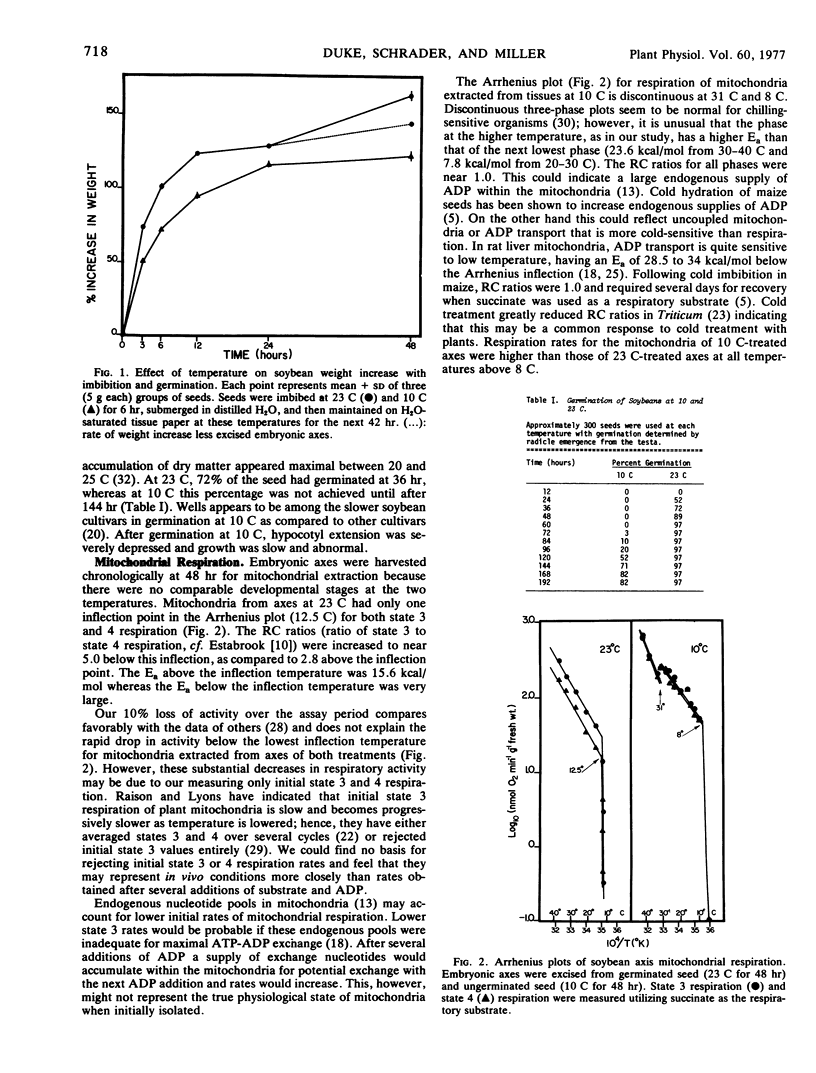
The influence of low temperature on soybean (Glycine max [L.] Merr. cv. Wells) energy transduction via mitochondrial respiration and dehydrogenases was investigated in this study during imbibition and germination. Mitochondria were isolated from embryonic axes of seeds treated at 10 and 23 C (control) by submergence in H2O for 6 hours and maintenance for an additional 42 hours in a moist environment. Arrhenius plots of initial respiration rates revealed that those from cold-treated axes had respiratory control (RC) ratios of near 1.0 above an inflection in the plot at 8 C. Arrhenius plots of control axes mitochondrial respiration showed RC ratios of 2.8 above and 5.0 below an inflection temperature of 12.5 C. Energies of activation for mitochondrial respiration between 20 and 30 C for the cold and control treatments were 7.8 and 15.6 kcal/mole, respectively. These data indicate possible differences in mitochondrial membranes, degree of mitochondrial integrity, and mitochondrial enzyme complement between the two treatments.
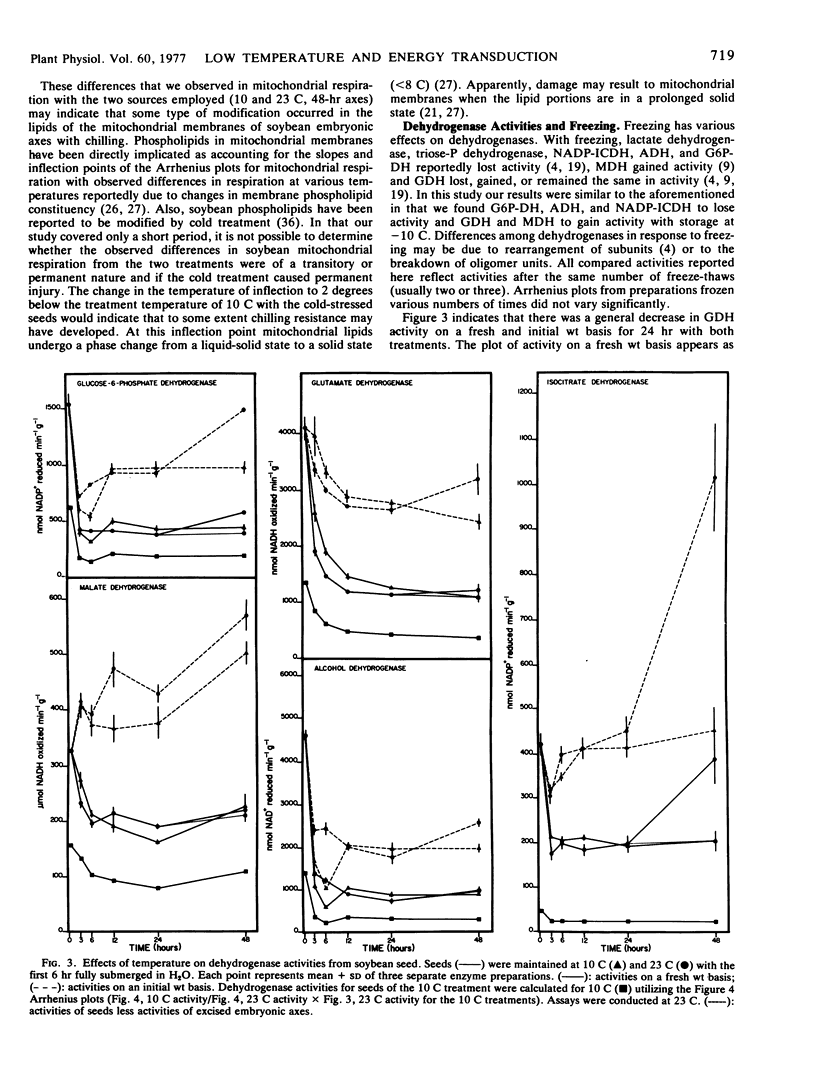
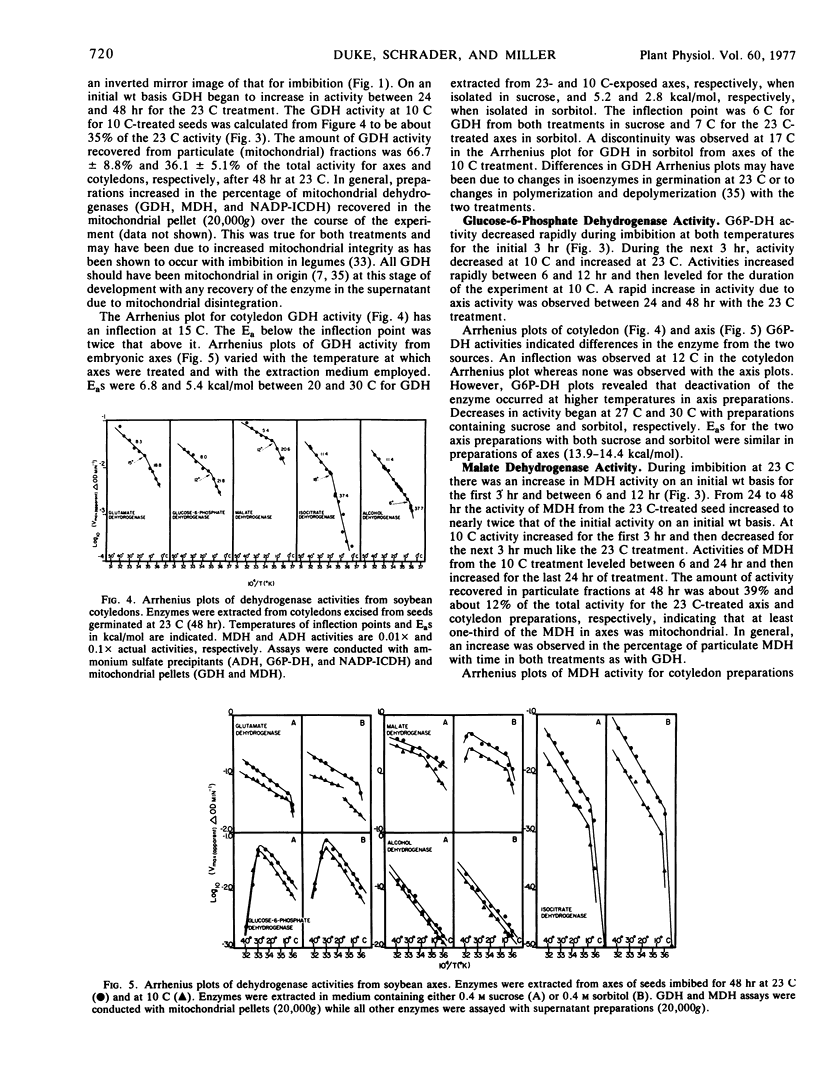
Glutamate dehydrogenase (GDH), malate dehydrogenase (MDH), alcohol dehydrogenase (ADH), glucose-6-phosphate dehydrogenase (G6P-DH), and NADP-isocitrate dehydrogenase (NADP-ICDH) were assayed from whole seeds and axes (after germination) during the 48 hours of temperature treatments. Activity of these dehydrogenases decreased during the first 6 hours with the exception of MDH. After germination at 23 C (48 hours) all five dehydrogenases increased in activity. Arrhenius plots of cotyledon dehydrogenase activities indicated that one inflection temperature between 6 and 18 C was present for each enzyme assayed. Differences were seen in Arrhenius plots of axes dehydrogenase activities with the two temperature treatments in the cases of GDH and MDH from mitochondrial pellets and with differences in enzyme extraction media. These data suggest that the temperature treatments yield differences in mitochondrial enzyme complement. There were no detectable inflection temperatures for the activities of G6P-DH and ADH extracted from axes. Arrhenius plots of NADP-ICDH activity indicated extreme cold sensitivity. The slopes of the plots for axes NADP-ICDH were very similar to those for mitochondrial respiration (23 C treatment) suggesting that this enzyme may limit mitochondrial respiration at low temperature in soybean tissues grown at moderate temperatures.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN E. G. CHANGES IN THE FREE NUCLEOTIDE PATTERN OF PEA SEEDS IN RELATION TO GERMINATION. Biochem J. 1965 May;95:509–514. doi: 10.1042/bj0950509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman E. J., Ikuma H., Stein H. J. Citric Acid cycle activity in mitochondria isolated from mung bean hypocotyls. Plant Physiol. 1976 Sep;58(3):426–432. doi: 10.1104/pp.58.3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry R. A., Ting I. P. Purification, properties, and kinetic observations on the isoenzymes of NADP isocitrate dehydrogenase of maize. Arch Biochem Biophys. 1976 Oct;176(2):501–509. doi: 10.1016/0003-9861(76)90193-4. [DOI] [PubMed] [Google Scholar]
- Gusta L. V., Weiser C. J. Nucleic Acid and protein changes in relation to cold acclimation and freezing injury of korean boxwood leaves. Plant Physiol. 1972 Jan;49(1):91–96. doi: 10.1104/pp.49.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingebretsen O. C., Sanner T. Activation of NADP-specific isocitrate dehydrogenase by chelating agents. Arch Biochem Biophys. 1976 Oct;176(2):442–448. doi: 10.1016/0003-9861(76)90186-7. [DOI] [PubMed] [Google Scholar]
- Kimelberg H. K., Papahadjopoulos D. Effects of phospholipid acyl chain fluidity, phase transitions, and cholesterol on (Na+ + K+)-stimulated adenosine triphosphatase. J Biol Chem. 1974 Feb 25;249(4):1071–1080. [PubMed] [Google Scholar]
- Krasnuk M., Jung G. A., Witham F. H. Electrophoretic studies of several dehydrogenases in relation to the cold tolerance of alfalfa. Cryobiology. 1976 Jun;13(3):375–393. doi: 10.1016/0011-2240(76)90121-8. [DOI] [PubMed] [Google Scholar]
- Lyons J. M., Raison J. K. Oxidative activity of mitochondria isolated from plant tissues sensitive and resistant to chilling injury. Plant Physiol. 1970 Apr;45(4):386–389. doi: 10.1104/pp.45.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. W., de la Roche I., Pomeroy M. K. Structural and functional responses of wheat mitochondrial membranes to growth at low temperatures. Plant Physiol. 1974 Mar;53(3):426–433. doi: 10.1104/pp.53.3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawa Y., Asahi T. Rapid Development of Mitochondria in Pea Cotyledons during the Early Stage of Germination. Plant Physiol. 1971 Dec;48(6):671–674. doi: 10.1104/pp.48.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff E., Heldt H. W., Klingenberg M. Adenine nucleotide translocation of mitochondria. Kinetics of the adenine nucleotide exchange. Eur J Biochem. 1969 Oct;10(3):484–493. doi: 10.1111/j.1432-1033.1969.tb00715.x. [DOI] [PubMed] [Google Scholar]
- Raison J. K., Lyons J. M. The influence of mitochondrial concentration and storage on the respiratory control of isolated plant mitochondria. Plant Physiol. 1970 Apr;45(4):382–385. doi: 10.1104/pp.45.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison J. K., Lyons J. M., Thomson W. W. The influence of membranes on the temperature-induced changes in the kinetics of some respiratory enzymes of mitochondria. Arch Biochem Biophys. 1971 Jan;142(1):83–90. doi: 10.1016/0003-9861(71)90261-x. [DOI] [PubMed] [Google Scholar]
- Raison J. K., McMurchie E. J. Two temperature-induced changes in mitochondrial membranes detected by spin labelling and enzyme kinetics. Biochim Biophys Acta. 1974 Sep 6;363(2):135–140. doi: 10.1016/0005-2736(74)90053-4. [DOI] [PubMed] [Google Scholar]
- Raison J. K. The influence of temperature-induced phase changes on the kinetics of respiratory and other membrane-associated enzyme systems. J Bioenerg. 1973 Jan;4(1):285–309. doi: 10.1007/BF01516063. [DOI] [PubMed] [Google Scholar]
- Samimy C., Lamotte C. E. Anomalous Temperature Dependence of Seedling Development in Some Soybean (Glycine max [L.] Merr.) Cultivars: Role of Ethylene. Plant Physiol. 1976 Dec;58(6):786–789. doi: 10.1104/pp.58.6.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Asahi T. Biochemical Properties of Mitochondrial Membrane from Dry Pea Seeds and Changes in the Properties during Imbibition. Plant Physiol. 1975 Dec;56(6):816–820. doi: 10.1104/pp.56.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. F., Rinne R. W. Effect of freezing and cold storage on phospholipids in developing soybean cotyledons. Plant Physiol. 1976 Feb;57(2):270–273. doi: 10.1104/pp.57.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeylemaker W. P., Jansen H., Veeger C., Slater E. C. Studies on succinate dehydrogenase. VII. The effect of temperature on the succinate oxidation. Biochim Biophys Acta. 1971 Jul 21;242(1):14–22. doi: 10.1016/0005-2744(71)90083-0. [DOI] [PubMed] [Google Scholar]


