Abstract
The nature of long day inhibition of flowering in the short day plant Xanthium strumarium L. was studied by correlating the flowering response with the translocation of 14C-assimilates from induced leaves or parts thereof to the shoot tips.
In contrast to an earlier study by Gibby and Salisbury (Plant Physiol 1971 47: 784-789) no inhibitory effect of an immature leaf in long day on the flower-promoting effect of an induced leaf was detected. When the stimulus moved in the basipetal direction, mature leaves in long day were inhibitory to flowering and at the same time reduced the amount of 14C-photosynthate that accumulated in the receptor buds.
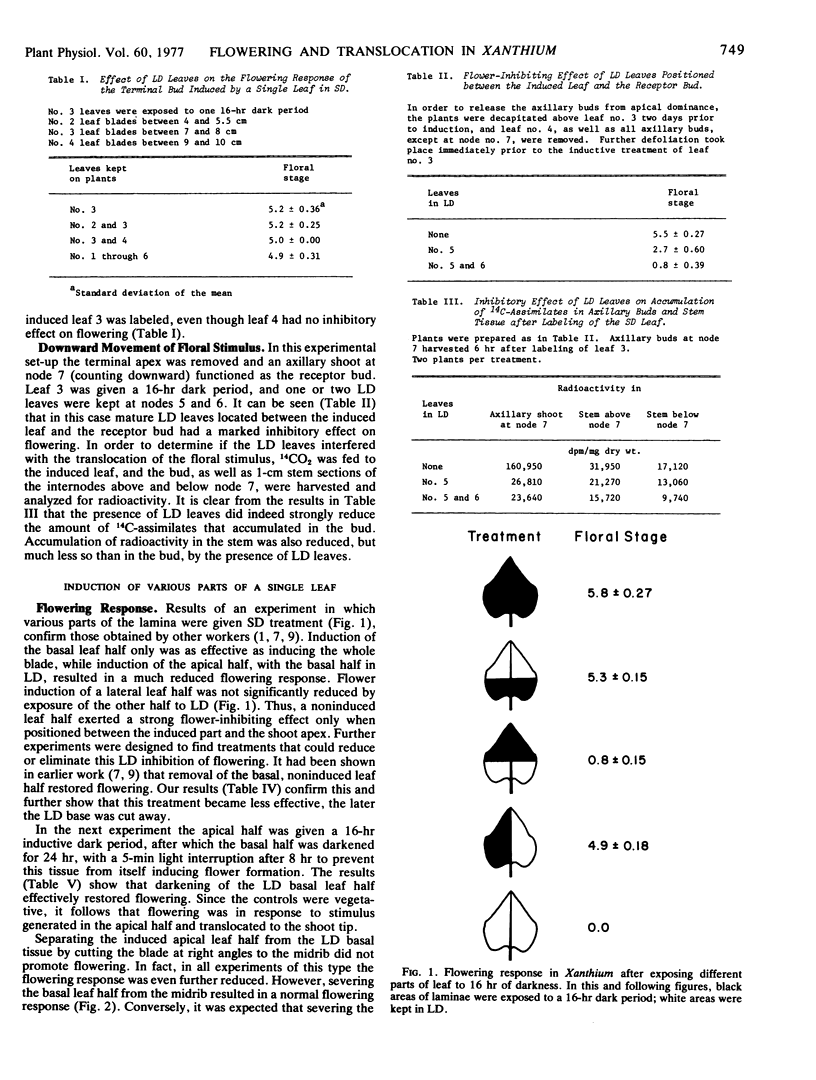
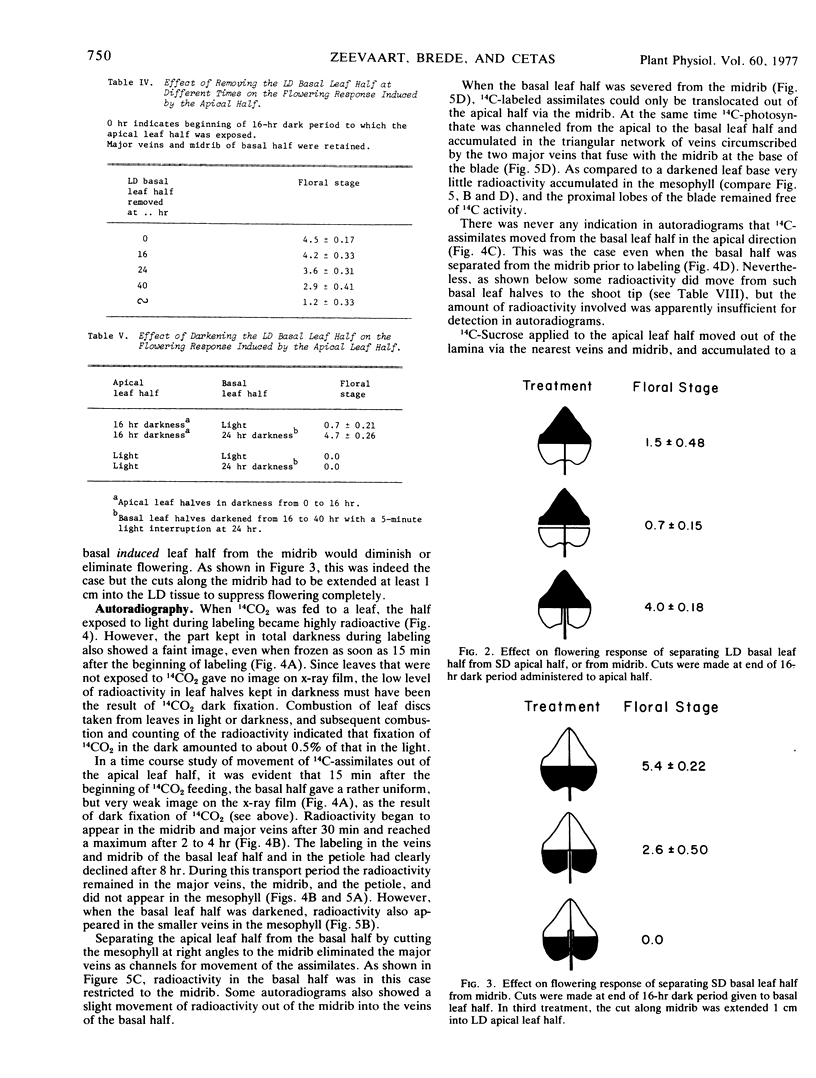
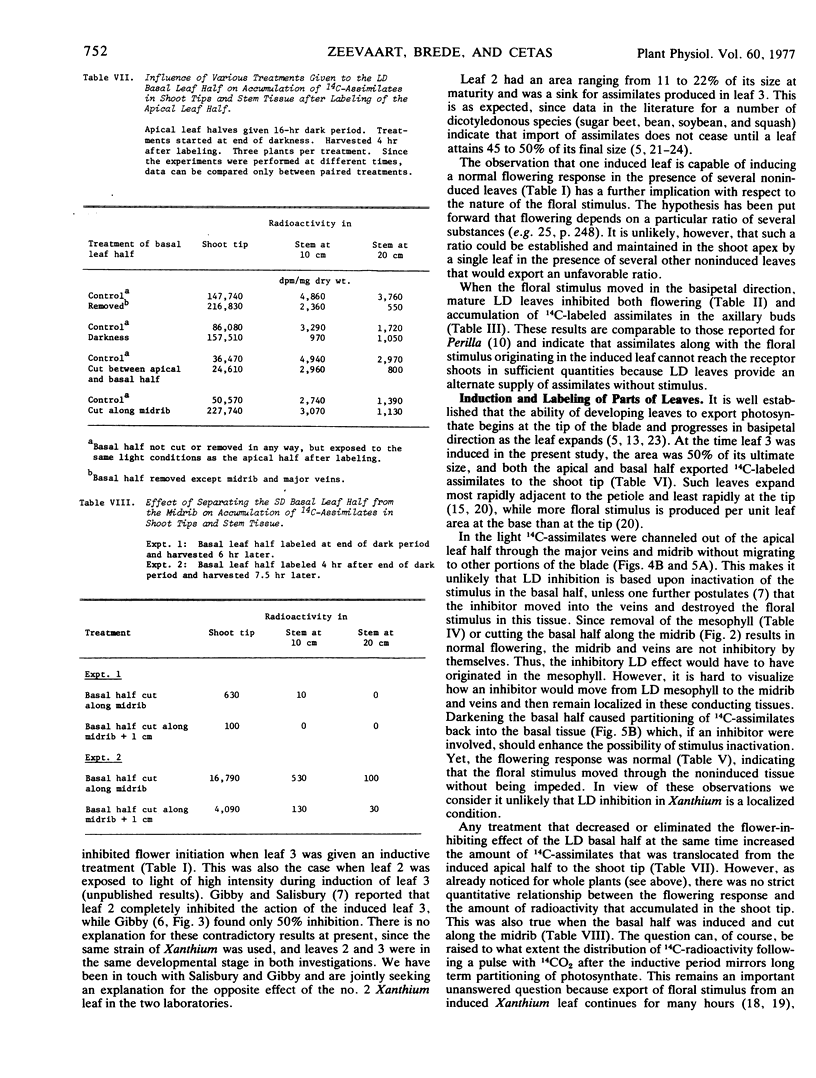
Inhibition of flowering was observed when the apical half of a single leaf was induced and the basal half kept under long day conditions. Induction of a lateral leaf half, with the other half remaining in long day, resulted in a normal flowering response. More 14C-photosynthate was translocated to the shoot tip from the basal than from the apical leaf half.
Removal of the noninduced basal leaf half except for the midrib and major veins, cutting the basal half along the midrib, or keeping the basal half in darkness, resulted in normal flowering. In all three treatments the amounts of 14C-assimilates that accumulated in the shoot tips also increased, presumably because competing export of nonlabeled assimilates from the basal leaf halves was diminished or eliminated.
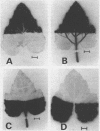
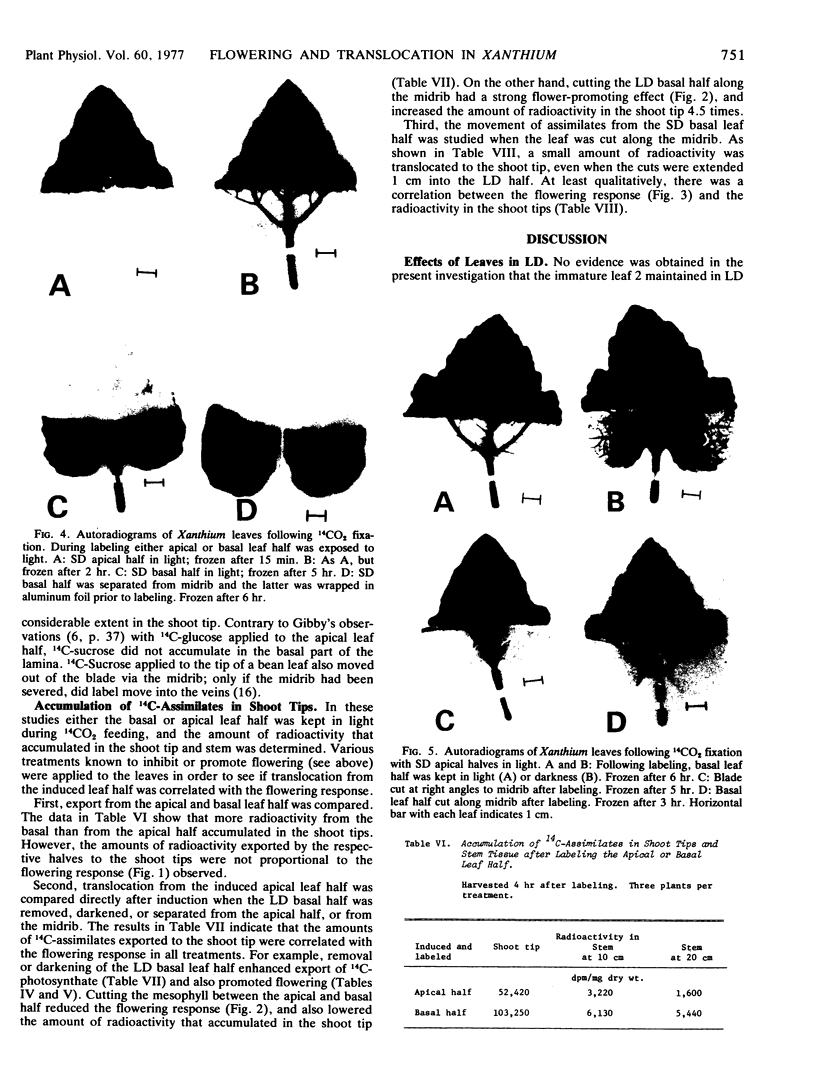
Autoradiography indicated that 14C-assimilates produced in the apical half were channeled through the basal half in the major veins and midrib to the petiole without partitioning back into the mesophyll. Since the veins and midrib in the basal half were by themselves not inhibitory to flowering, it is unlikely that the floral stimulus was inactivated in the long day tissue. When the basal half was labeled with 14CO2, there was no indication in autoradiograms that 14C-labeled assimilates moved in the blade in the apical direction.
As a whole these results demonstrate that transmission of the floral stimulus and translocation of photosynthate are correlated, so that at least part of the long day inhibition in Xanthium can be explained in terms of translocation effects. However, the involvement of a transmissible inhibitor produced in long day tissue cannot be ruled out.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fellows R. J., Geiger D. R. Structural and Physiological Changes in Sugar Beet Leaves during Sink to Source Conversion. Plant Physiol. 1974 Dec;54(6):877–885. doi: 10.1104/pp.54.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibby D. D., Salisbury F. B. Participation of Long-Day Inhibition in Flowering of Xanthium strumarium L. Plant Physiol. 1971 Jun;47(6):784–789. doi: 10.1104/pp.47.6.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. W., Zeevaart J. A. Floral stimulus movement in perilla and flower inhibition caused by noninduced leaves. Plant Physiol. 1973 Apr;51(4):727–738. doi: 10.1104/pp.51.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang A., Chailakhyan M. K., Frolova I. A. Promotion and inhibition of flower formation in a dayneutral plant in grafts with a short-day plant and a long-day plant. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2412–2416. doi: 10.1073/pnas.74.6.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschke K., Zeevaart J. A. Abscisic Acid Content, Transpiration, and Stomatal Conductance As Related to Leaf Age in Plants of Xanthium strumarium L. Plant Physiol. 1976 Aug;58(2):169–174. doi: 10.1104/pp.58.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle N. E. Bioassay of Floral Stimulus in Xanthium. Plant Physiol. 1965 Mar;40(2):261–267. doi: 10.1104/pp.40.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle N. E. Persistence & transport of flowering stimulus in Xanthium. Plant Physiol. 1961 Sep;36(5):656–662. doi: 10.1104/pp.36.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]