Abstract
The pod wall of pea (Pisum sativum L.) was shown to contain two distinct photosynthetic layers. The outer, comprising chlorenchyma of the mesocarp, captured CO2 from the outside atmosphere; the inner, a chloroplast-containing epidermis lining the pod gas cavity, was involved in photoassimilation of the CO2 released from respiring seeds.
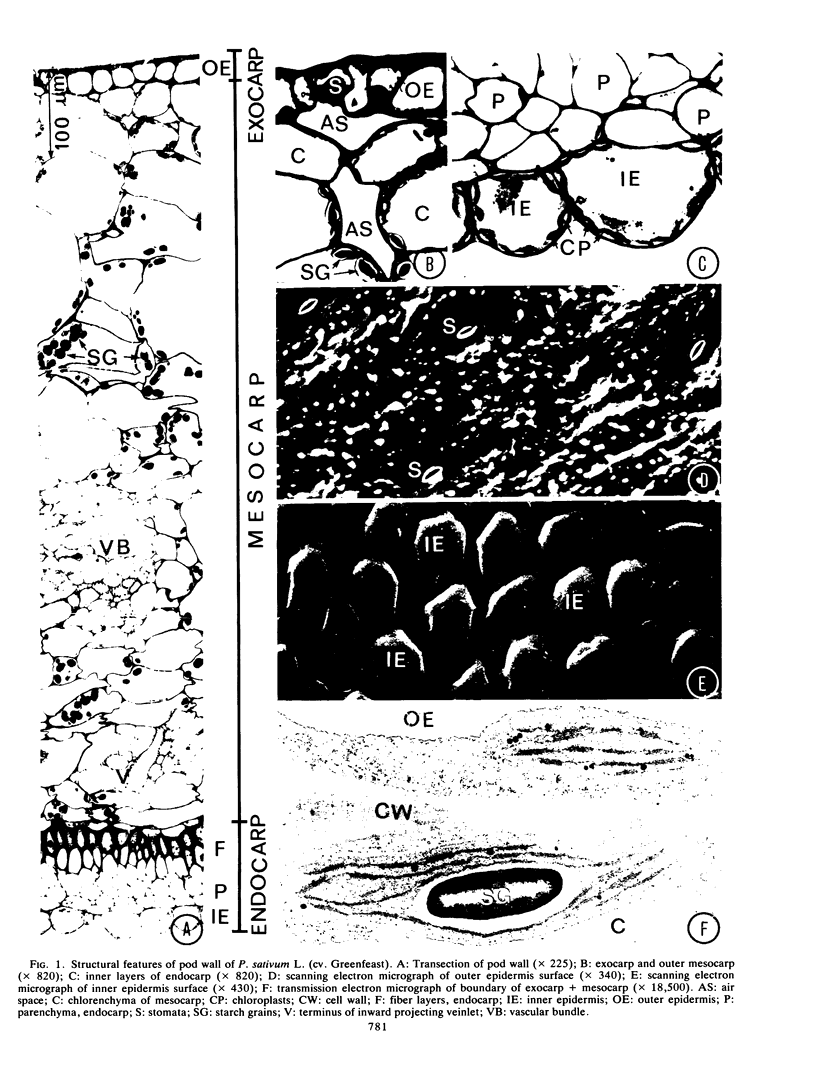
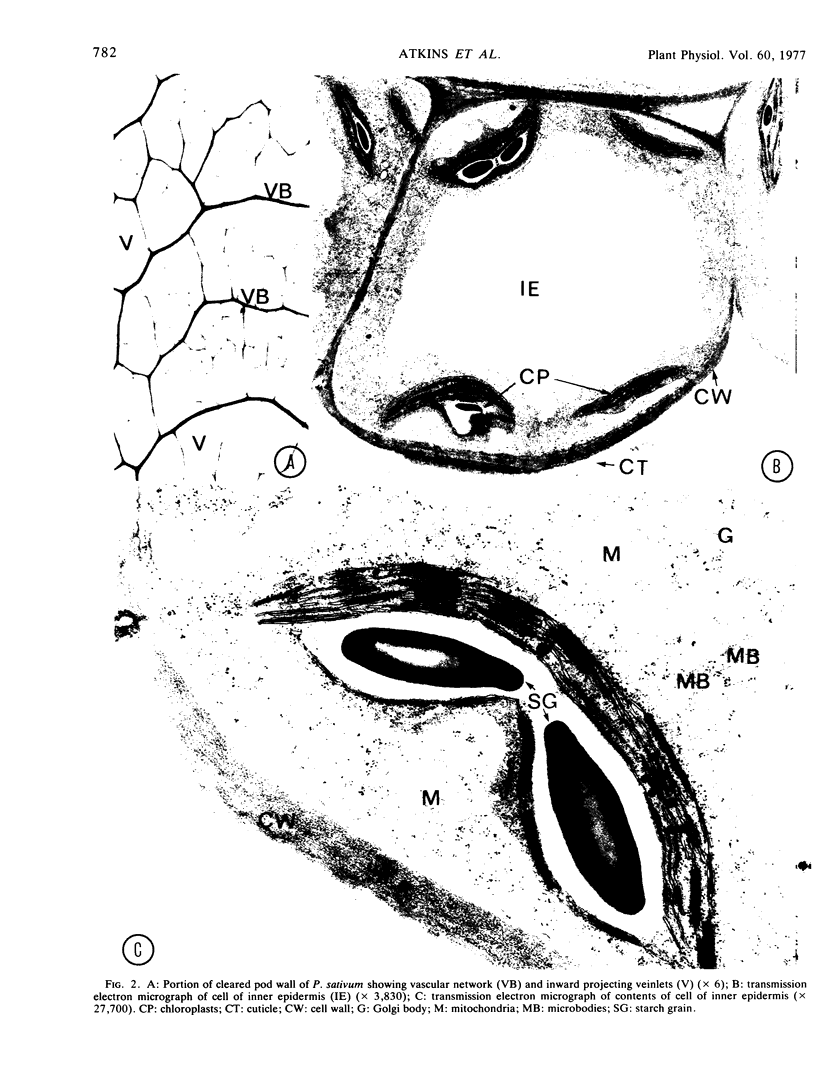
Structural features of the pod included the thick cuticle and stomata of the outer epidermis, the inward projecting veinlets of the vascular network in the mesocarp, the sparsity of air spaces, the fiber and parenchyma layers of the endocarp, and the abundant chloroplasts, thin cuticle, and rounded outer contours of cells of the inner epidermis.
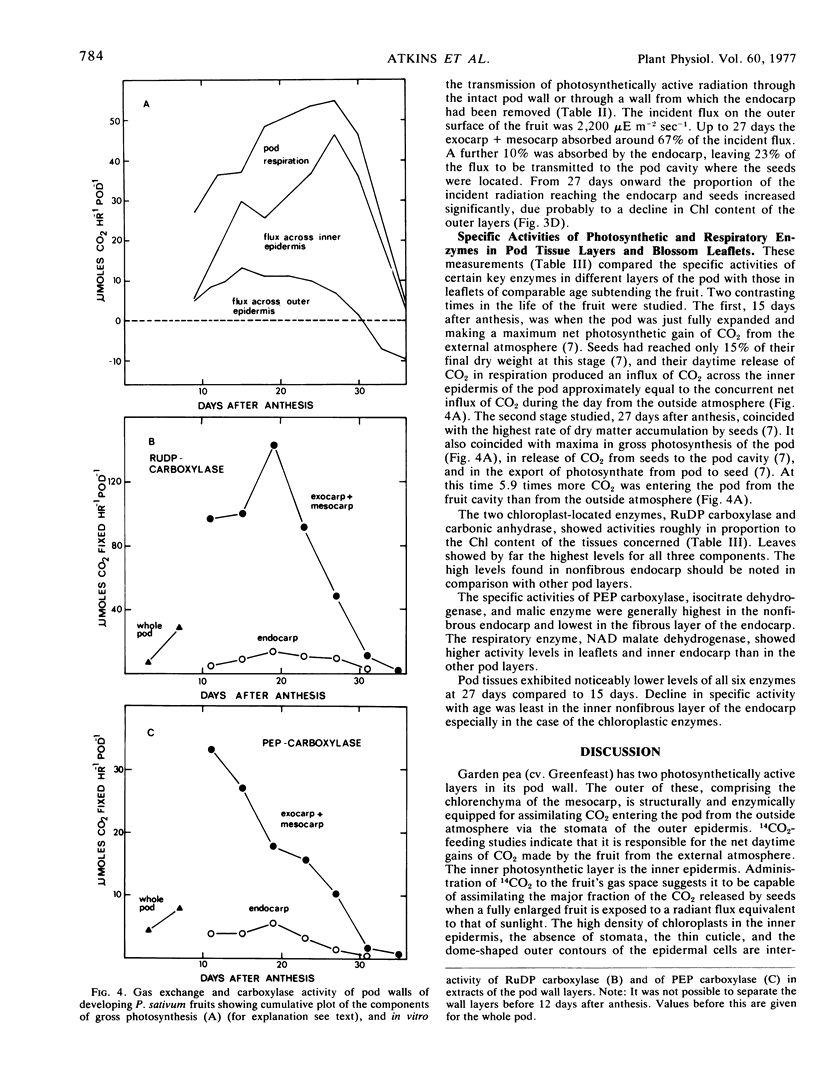
The inner epidermis showed high specific activities of ribulose 1,5-diphosphate (RuDP) carboxylase (EC 4.1.1.39) and phosphoenolpyruvate (PEP) carboxylase (EC 4.1.1.31), contained up to 20% of the pod's chlorophyll, and was capable of fixing 66% of the CO2 released during the photoperiod to the pod gas space by the seeds of a fully grown fruit.
The in vitro carboxylation capacity of the pod exceeded the estimated gross photosynthesis of the fruit for all but the last few days of development. Chlorophyll content and carboxylation activity declined more markedly in the outer photosynthetic layers than in the inner epidermis.
The ratio of activities of RuDP carboxylase to PEP carboxylase in pod extracts varied from 2.4:1 to 12:1 as against 48:1 to 156:1 in extracts of leaves.
Structural and physiological properties of the pod were related to its capacity to conserve respired CO2 and provide photosynthate to developing seeds.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins C. A., Patterson B. D., Graham D. Plant Carbonic Anhydrases: I. Distribution of Types among Species. Plant Physiol. 1972 Aug;50(2):214–217. doi: 10.1104/pp.50.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinn A. M., Atkins C. A., Pate J. S. Significance of photosynthetic and respiratory exchanges in the carbon economy of the developing pea fruit. Plant Physiol. 1977 Sep;60(3):412–418. doi: 10.1104/pp.60.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quebedeaux B., Chollet R. Growth and Development of Soybean (Glycine max [L.] Merr.) Pods: CO(2) Exchange and Enzyme Studies. Plant Physiol. 1975 Apr;55(4):745–748. doi: 10.1104/pp.55.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]