Abstract
Several improved techniques for isoelectric focusing of isoenzymes in polyacrylamide gel slabs were developed. Using these techniques, three commercial sources of horseradish peroxidase were each examined with three commercial sources of carrier ampholytes to determine their respective isoenzyme profiles.
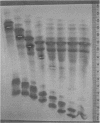
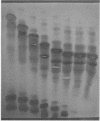
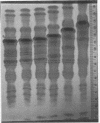
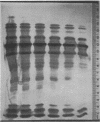
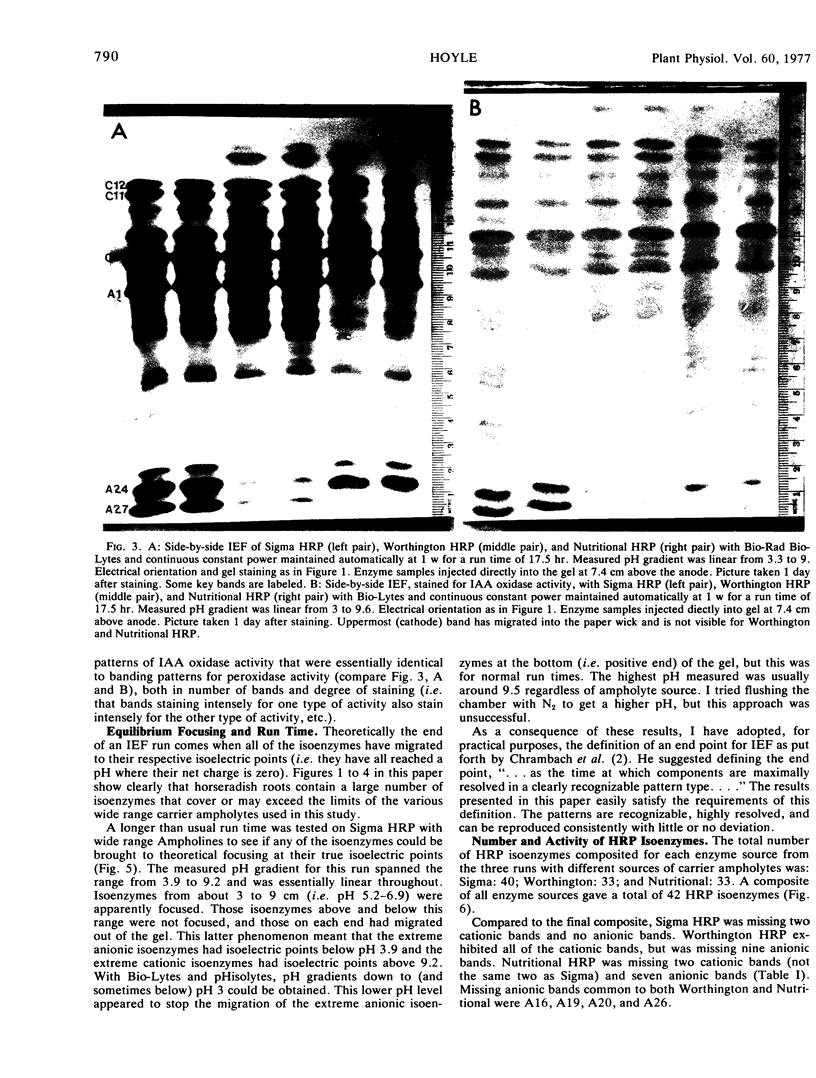
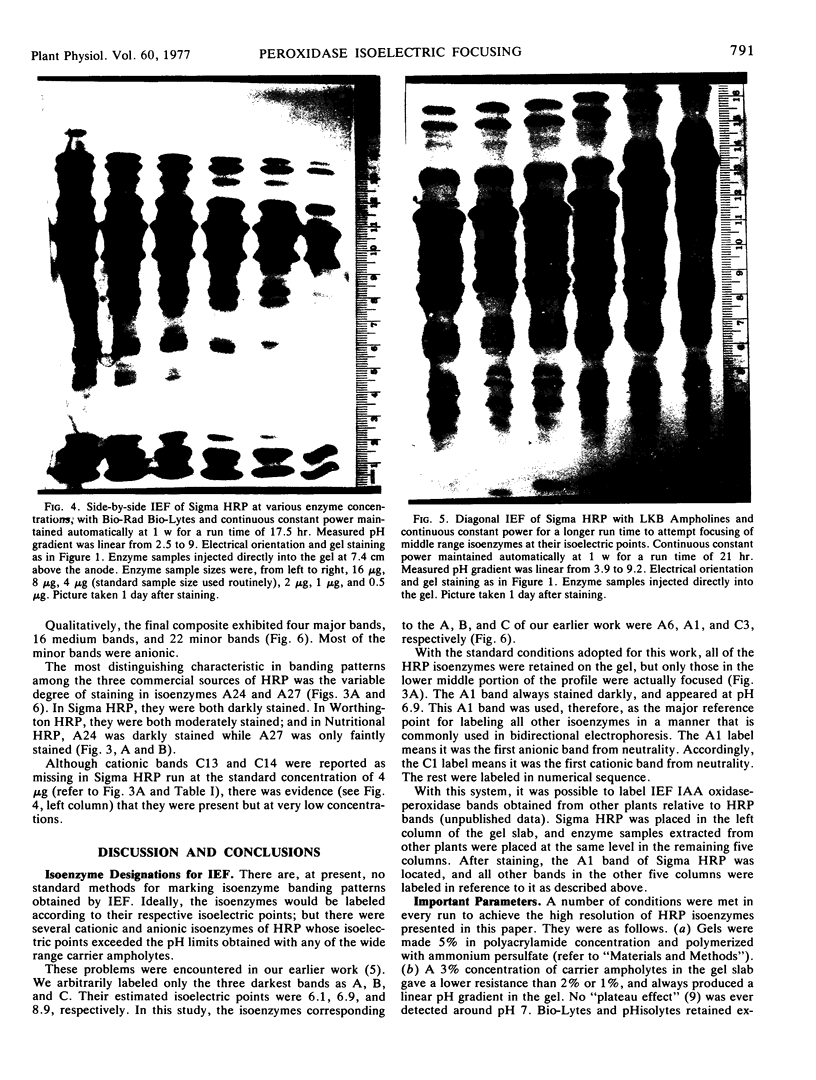
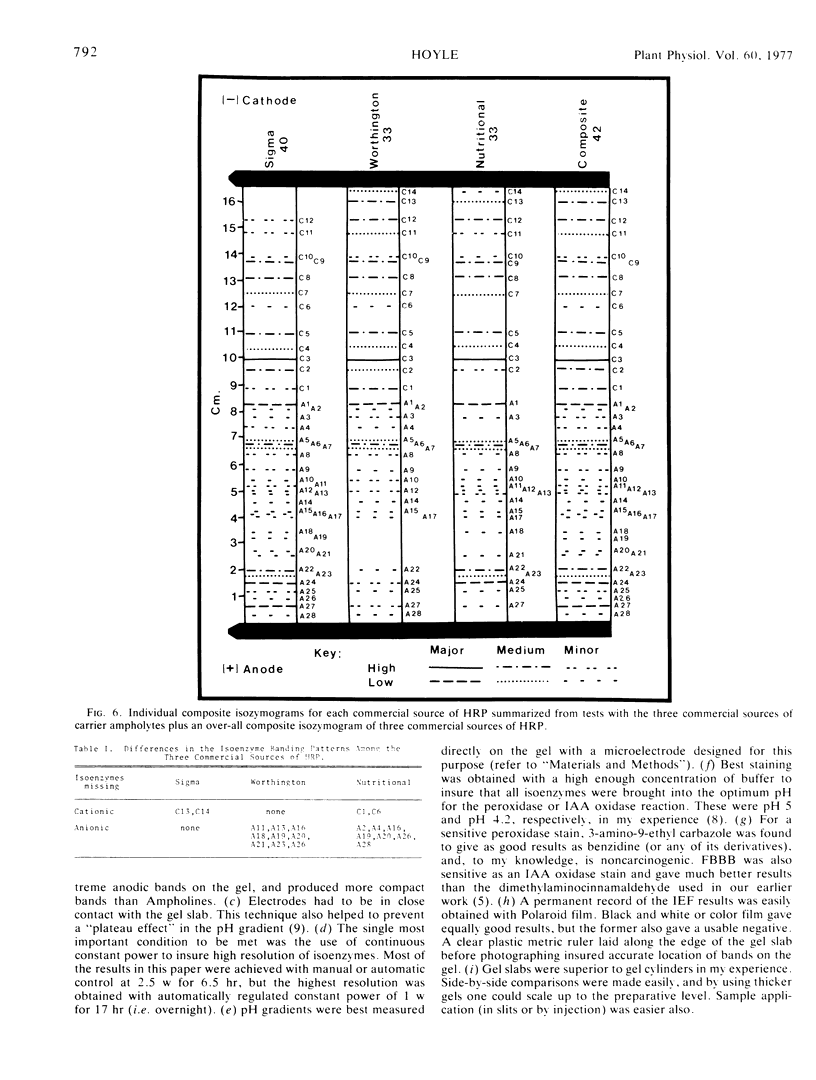
A much higher degree of isoenzyme resolution was obtained than reported previously. A composite of all of the various tests gave a total of 42 peroxidase isoenzymes. Commercial sources of horseradish peroxidase showed many similarities in their isoenzyme patterns, but their differences were sufficient to recognize each source easily. Isoenzyme patterns for all three sources spanned the entire pH range with all three sources of wide range carrier ampholytes. Only a few isoenzymes stained darkly. Most isoenzymes stained moderately to lightly, but all were well resolved. Gels stained for indoleacetic acid oxidase activity showed the same pattern as gels stained for peroxidase activity. This was true for all three commerial sources of the enzyme. The isoenzyme patterns obtained in each run were entirely reproducible, and linear pH gradients were obtained in all cases. Limitations in the pH range of the wide range carrier ampholytes relative to the isoelectric points of the extreme anodic and cathodic isoenzymes led to the adoption of a modified definition of focusing time. In addition, the labeling of isoenzymes commonly used in electrophoresis was adapted for labeling isoenzymes resolved by isoelectric focusing.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chrambach A., Doerr P., Finlayson G. R., Miles L. E., Sherins R., Rodbard D. Instability of pH gradients formed by isoelectric focusing in polyacrylamide gel. Ann N Y Acad Sci. 1973 Jun 15;209:44–64. doi: 10.1111/j.1749-6632.1973.tb47518.x. [DOI] [PubMed] [Google Scholar]
- Delincée H., Radola B. J. Thin-layer isoelectric focusing on Sephadex layers of horseradish peroxidase. Biochim Biophys Acta. 1970 Feb 17;200(2):404–407. doi: 10.1016/0005-2795(70)90183-2. [DOI] [PubMed] [Google Scholar]
- Gove J. P., Hoyle M. C. The isozymic similarity of indoleacetic Acid oxidase to peroxidase in birch and horseradish. Plant Physiol. 1975 Nov;56(5):684–687. doi: 10.1104/pp.56.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle M. C. Indoleacetic Acid oxidase: a dual catalytic enzyme? Plant Physiol. 1972 Jul;50(1):15–18. doi: 10.1104/pp.50.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righetti P. G., Drysdale J. W. Isoelectric focusing in gels. J Chromatogr. 1974 Sep 25;98(2):271–321. doi: 10.1016/s0021-9673(00)92076-4. [DOI] [PubMed] [Google Scholar]
- Shaw C. R., Prasad R. Starch gel electrophoresis of enzymes--a compilation of recipes. Biochem Genet. 1970 Apr;4(2):297–320. doi: 10.1007/BF00485780. [DOI] [PubMed] [Google Scholar]
- Vance C. P., Anderson J. O., Sherwood R. T. Soluble and cell wall peroxidases in reed canarygrass in relation to disease resistance and localized lignin formation. Plant Physiol. 1976 Jun;57(6):920–922. doi: 10.1104/pp.57.6.920. [DOI] [PMC free article] [PubMed] [Google Scholar]